Abstract
Using amendments is a cost-effective method to soil cadmium (Cd) remediation, whereas knowledge about how different amendments and rates affect remediation efficiency remains limited. This study aimed to evaluate the impacts of different types and amounts of amendments on soil Cd immobilization and its uptake by plants. Biochar (BC), zeolite (ZE), humic acid (HA), superphosphate (SP), lime (L), and sodium sulfide (SS) were applied at three rates (low, medium, and high) ranging from 0.5 to 5%. The concentration of CaCl2-extractable Cd was considerably affected by the amendments, except HA, and the high doses achieved better immobilization effects than the low doses did. The addition of amendments decreased weak acid soluble Cd by 4.1–44.0% but slightly increased the fractions of oxidizable and residual Cd. These amendments (except BC and HA dose of 1%) decreased Cd accumulation in grains by 1.3–68.8% and (except SP) in roots by 16.3–65.5% compared with the control. The SP efficiently immobilized Cd but posed a potential soil acidification risk. Moreover, SS treatment increased the soil electrical conductivity (EC) value and restricted the growth of wheat, possibly due to high-salt stress. BC, ZE, and L exerted significant effects on the reduction in available Cd as the application rate increased. These amendments enhanced Cd immobilization mainly by changing Cd availability in soil and influencing its redistribution in different fractions in soil and root uptake by plants. This study concluded that BC-5%, ZE-1%, and L-0.5% can be used for Cd immobilization in acidic or neutral soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil cadmium (Cd) pollution has become a serious concern in many regions worldwide (Li et al. 2014). In China, a survey on soil heavy metal pollution reported that Cd is the most widespread contaminant in soils (MEE 2014). Cd usually enters the environment through many pathways, including atmospheric deposition, mining, and fertilization, which could cause its accumulation in soils and organisms (Khan et al. 2015; Xu et al. 2015). It contains carcinogens and toxins endanger human health through the food chain even at low Cd concentrations due to the long biological half-life (10–30 years) of this element (Cocarta et al. 2016; Nordberg 2009). Thus, efficient remediation of Cd-contaminated soil is urgently required for safe food production and safe utilization of soil.
Remediation technologies can be divided into two categories. One reduces soil’s total heavy metal concentration by the engineering measures and phytoremediation (Al Chami et al. 2015; Wuana et al. 2010). The other category reduces the mobility and availability of heavy metals by changing the existing forms of metals in soil through various techniques, including vitrification and in situ chemical immobilization (Guo et al. 2006; Ok et al. 2010). Among such techniques, in situ immobilization is considered as a feasible and effective approach to remediate heavy metal-contaminated soils due to its advantages of high efficiency, simple operation, and low cost (Shaheen and Rinklebe 2015). It helps transform the available forms of metals into a stable solid phase through sorption, precipitation, complexation, ion exchange, or redox in soils, thus reducing the mobility, availability, and toxicity of heavy metals (Bashir et al. 2018; Mahar et al. 2018).
In situ Cd immobilization involves various inorganic materials, such as liming material (Mahar et al. 2018), phosphorus-containing material (Sarwar et al. 2010), mineral material (Wen et al. 2016), and organic amendments, including biochar (BC) (Oziegbe et al. 2019), manure compost (Ahmad et al. 2015), and humic substances (He et al. 2019). Many studies have investigated how different amendments affect available Cd and its uptake by plants. He et al. (2019) observed that the addition of lime (L) decreased Cd availability in soil and its uptake by crops due to the increased soil pH. The rock phosphate significantly reduced Cd accumulation in water spinach (Ipomoea aquatic) by enhancing Cd immobilization, and zeolite (ZE), and BC decreased soil Cd availability by sorption of Cd on their surface (Bashir et al. 2018). However, organic fertilizer application possibly increases the available Cd in moderate Cd-contaminated soil because of the low humification degree (Guo et al. 2018). Sulfur, when applied as an amendment, inhibits the growth of Chinese cabbage in Cd-polluted soil due to considerable acidification induced by sulfur (Mahar et al. 2016). BC as a soil amendment offered significant reduction in Cd mobility in soil and its uptake by plants (He et al. 2017). Nevertheless, the excellent sorption capability of BC may deactivate heavy metals and compete for nutrients with plants, reducing their bioavailability (Kuppusamy et al. 2016). Although the potential benefits of amendment have been confirmed by several studies as above discussed, the potential risk and hazards have not been fully understood. The different types of amendments on remediation of Cd-contaminated soil still need to thoroughly evaluate their effectiveness of immobilizing Cd.
Other studies have shown that the immobilizing effect of amendments on heavy metals in soils varies with different application rates, and such a variation might exert negative effects on soil–plant ecosystems (Mahar et al. 2016; Mignardi et al. 2012). Phosphorus materials have been widely used in Cd-contaminated soil remediation (Basta and McGowen 2004), but their overuse results in phosphorus losses to the environment and reduced uptake of essential trace elements by crops (Boisson et al. 1999; Park et al. 2011a). Excess usage of alkaline materials gives rise to an increased risk of soil alkalinity, which eventually destroys the soil structure and the balance of ions and influences plant growth (Kumpiene et al. 2008). BC contains special chemical elements that are generally beneficial to most species, however, in such cases, phytotoxcity of BC prevails and it will vary depending on the application rates (Downie et al. 2012). The higher application rates of amendments for available heavy metals control would affect the economics of crop yield. Optimization of the application rates of amendments can improve remediation efficiency, reduce the negative effects on soil–plant ecosystems, and decrease costs (Guo et al. 2018). The effects of amendments applied at various rates on Cd immobilization need further attention before large-scale application, but they are not well documented.
The different amendments at various rates were separately added to a Cd-polluted soil to evaluate their effectiveness of immobilizing Cd, geochemical fractionation of Cd, and potential immobilization mechanism through a pot experiment on spring wheat (Triticum aestivum L.). This work will be helpful for comprehensively evaluating the effectiveness of various amendments immobilizing Cd and contribute to their rational use and the development of combined amendments for regulating Cd with other heavy metals compound contaminated soil.
Materials and Methods
Soil Collection and Amendment Characterization
Soil samples were collected from a conventional agricultural field in Zhangshi’s irrigation area (123°3′E, 41°38′N) in Liaoning Province, China. The soil pollution in this area was mainly Cd pollution caused by sewage irrigation from 1950s to 1980s (Xiong et al. 2004). Topsoil (0–20 cm) samples were ground to pass through a 2 mm nylon mesh, and the basic physicochemical properties and available Cd concentrations were analyzed. The soil basic properties were 6.46 pH (neutral but the acid side) (1:2.5 H2O), 0.11 mS cm−1 EC, 14.37 g kg−1 of organic carbon, 1.94 g kg−1 of total nitrogen, 1.68 g kg−1 of total phosphorus, and 13.06 g kg−1 of total potassium. The total and available Cd concentrations in soils were 0.86 and 0.015 mg kg−1, respectively. The total Cd was substantially higher than the environmental quality standards for agricultural soils of China (GB15618-2018; pH < 6.5; 0.4 mg kg−1) according to the Ministry of Ecology and Environment of the People’s Republic of China. Three inorganic materials (L, superphosphate (SP), and sodium sulfide (SS)), two organic materials (BC and humic acid (HA)) and one mineral material (ZE) were selected as soil amendments. SP (pH = 2.40) and SS (pH = 12.99) were purchased from Beijing Puyihua Technology Co., Ltd, Beijing, China, and L (pH = 12.26) and ZE (<10 μm, pH = 10.40) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd, Shanghai, China. HA (pH = 3.95) was from Beijing Xiangyun Xingye Technology Co., Ltd, Beijing, China. BC (pH = 7.95) was obtained from maize straw through pyrolysis under anoxic conditions at 400 °C for 1 h by Shenyang Agricultural University, Shenyang, China. Total Cd concentrations were 0.20, 0.15, 0.02, 0.04, and 0.06 mg kg−1 in BC, ZE, HA, SP, and L, respectively. The descriptions of the soil and amendments were summarized in Tables S1 and S2.
Experimental Design
A pot experiment was conducted from April 2017 to July 2017 (~100 days) at Shenyang Experimental Station of Ecology (41°32′N latitude, 123°23′E longitude), Chinese Academy of Sciences, Liaoning Province, China. The effect of amendments on the immobilization and bioavailability of Cd to wheat was investigated in a greenhouse using Cd-contaminated soil. BC, ZE, and HA were individually added to the soil at 1%, 2.5%, and 5% of soil (w/w), respectively. The application rates of individual SP, L, and SS were 0.5%, 1%, and 2% of soil (w/w), respectively. No amendment was applied in the control soil (CK). The three application rates were denoted as low, medium, and high according to the amount of each amendment. The soil and amendments were mixed completely according to the designed ratio and placed in plastic pots (1 kg per pot). A randomized complete block design composed of 19 treatments (six amendments with three application rates and one control) with three replicates was adopted. The treated soils were incubated at room temperature for 2 weeks before planting to achieve equilibration. The wheat seeds were planted and then thinned to ten seedlings in each pot after 2 weeks. Soil moisture during the entire growth period was maintained to 80% of the water holding capacity by weighting and replenishing distilled water every week in spring and every 3 days in summer.
After maturity, the plant samples were collected together with the soil samples then separated into grain, straw, and root. The root samples were washed with tap water and rinsed completely with deionized water for four times. All plant sample components were oven-dried at 70 °C for 48 h, weighted, and pulverized into powder in an agate grinder ball mill for further analysis. The collected soils were air-dried for testing of soil pH and available Cd. A subsample of the soils was ground to pass through a 0.149 mm sieve for soil Cd speciation analysis.
Sample Analysis
Soil pH and EC were determined at soil-to-water ratios of 1:2.5 and 1:5 (W/V), respectively, by using an automated pH and EC meter. Soil organic carbon and total nitrogen were measured with the Elementar Vario EL III elemental analyzer (Elementar Corporation, Germany). Soil total phosphorus and total potassium were determined according to the procedure of Lu (2000). Soil total Cd was determined after digesting soil with HNO3–HClO4–HF (v:v:v = 4:1:1) using an inductively coupled plasma optical emission spectrometer (ICP-OES). The available Cd was extracted with 0.01 M of CaCl2 according to the protocol of Houben et al. (2013). Briefly, 2 g of the soil sample was extracted by 20 ml of 0.01 M CaCl2 solution. The slurry was shaken continuously at 220 rpm for 2 h at 25 °C then filtered using quantitative filter paper.
Sequential extraction was performed according to the modified Community Bureau of Reference extraction scheme to evaluate the effects of amendments on soil Cd speciation (Rauret et al. 1999; Yang et al. 2013). Cd was divided into four chemical fractions. The extraction steps were as follows: acid soluble fraction (0.11 M CH3COOH, shake for 16 h), bound to reducibles (0.1 M NH2OH·HCl, shake for 16 h), bound to oxidizables (30% H2O2, heating for 1 h at 85 °C ± 2 °C then 1 M NH4OAc, shake for 16 h), and residual Cd.
Plant samples were digested using HNO3–HClO4 (v:v = 4:1) (Lu 2000). The Cd concentration of the extraction solution was determined by ICP-OES. The analytical wavelength of Cd was 214.439 nm. Reagent blanks and a standard reference material (soil: GBW07406; maize: GBW10012) obtained from the National Institute of Standards and Technology were also used for analysis to monitor analytical accuracy and precision. The average recovery ranged from 95 to 102% for plants and from 94 to 105% for soil.
Statistical Analysis
The translocation factor (TF) and immobilization efficiency (IE) of Cd were calculated using the formula (Ali et al. 2013; Qayyum et al. 2017):
where Cdgrain (mg kg−1) and Cdroot (mg kg−1) represent the Cd concentrations in the grain and root, respectively.
All data were expressed as the mean values of three replicates. The significant difference between treatments was checked via one-way analysis of variance at the p < 0.05 level through SPSS 23.0. The correlation was analyzed with the Pearson test (two tailed). In addition, OriginPro software (version 2017) was utilized to make graphs.
Results
Effects of Amendments on Soil pH, EC, and Grain Yield
Soil pH was affected by the amendment types and application rates (Fig. 1a). The soil pH values in the treatments of BC, ZE, L, and SS were significantly higher than those of the control, except in the BC treatment at low level, and the trend was further enhanced with increasing application rate. The increments in soil pH ranged from 0.2 to 2.0 units, and the largest pH was observed in the L treatment. By contrast, soil pH declined with SP additions, where it further decreased with increasing application rate.
Effects of amendments on soil pH (a), EC (b), and grain yield (c). CK control, NG no growth. Low, medium, and high indicate that the amendments were applied at low, medium, and high doses, respectively. The error bars are the standard deviation of the means (n = 3), and the different lowercase letters indicate a significant difference at p < 0.05 among treatments
SS had the greatest effect on soil EC (3.6–10.6 mS/cm), followed by L, SP, and ZE treatments (Fig. 1b). The soil EC values were enhanced with increasing application rates of SS, L, SP, and ZE. No significant differences were observed among BC, HA, and CK treatments in terms of soil EC, but the EC value decreased numerically with increasing HA application rate.
The different amendments exhibited different effects on wheat grain yield, among which the application of BC, ZE, and SP increased the wheat grain yield and further enhanced it with an elevated application rate (Fig. 1c). The highest grain yields were achieved in the treatments receiving L at low and medium rates, whereas the yields were markedly reduced with the high application rate of L. HA application slightly decreased the wheat grain yield, but the difference between CK and HA treatments was not significant. The addition of SS considerably inhibited the growth of wheat, which is probably due to the high soil EC, resulting in nil yields.
Effects of Amendments on CaCl2-Extractable Cd Concentration
The concentration of CaCl2-extractable Cd in the soil decreased with the application of BC, ZE, SP, and L and gradually declined with increasing rates of these amendments (Fig. 2a). CaCl2-extractable Cd decreased by 36.2%, 88.7%, 17.8%, and 74.8% on the average with the application of BC, ZE, SP, and L, respectively, compared with the CK treatment. Medium and high application rates induced a reduction in CaCl2-extractable Cd by 55.1% and 63.8% on the average in the above treatments, respectively. Compared with the control, HA addition increased CaCl2-extractable Cd. SS addition increased CaCl2-extractable Cd at a low application rate and decreased it with increasing application rate of SS, especially at the high application rate.
Effect of amendments on CaCl2-extractable Cd concentration (a) and relationships between CaCl2-extractable Cd and soil pH (b). Low, medium, and high indicate that the amendments were applied at low, medium, and high doses, respectively. The error bars are the standard deviation of the means (n = 3), and the different lowercase letters indicate a significant difference at p < 0.05 among treatments
Correlation between pH and available Cd is presented in Fig. 2b. CaCl2-extractable Cd was significantly negatively correlated with soil pH at wheat harvesting stage (r = −0.757, p < 0.001), indicating that soil pH played a key role in reducing the CaCl2-extractable Cd concentration.
The IE calculated via single extraction with 0.01 M of CaCl2 is commonly used to evaluate the bioavailability of metals in soil (Table 1). The highest IE of amendments in soil Cd was obtained in the ZE treatment (86.45–90.06%) followed by L, BC, and SP treatments. IE was enhanced with increasing application rate in ZE, L, BC, and SP treatments. In the treatments receiving SS, IE was negative at the low application rate and reversed to positive as the application rate increased. However, HA addition induced a consistently negative IE regardless of the application rate.
Effects of Amendments on Soil Cd Distribution
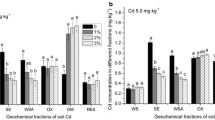
The distribution of Cd in four fractions is presented in Fig. 3. The fractions of Cd in the soil followed the sequence residual > reducible and oxidizable > weak acid soluble. In the control treatment, the residual Cd was dominant and accounted for 52.0% of the total Cd, followed by reducible with 22.8%, oxidizable with 13.9%, and weak acid soluble with 11.3%.
The largest reductions in soil weak acid soluble Cd fraction were observed in L and SP treatments, followed by SS, BC, and ZE treatments; on the average, the values decreased to 6.3%, 6.4%, 9.1%, 9.5%, and 9.5% of the total Cd in these treatments, respectively. The percentage of reducible Cd decreased to 20.2%, 21.0%, and 22.4% on the average in the soil treated with L, SP, and SS, respectively, whereas it increased to 24.4% and 23.3% in the soils treated with BC and ZE, respectively. The oxidizable Cd fraction increased to 20.1%, 18.6%, 16.2%, 15.7%, and 15.6% of the total Cd due to L, SP, ZE, SS, and BC application, respectively. In the HA treatment, the weak acid soluble and reducible Cd fractions slightly decreased, and the proportions of oxidizable and residual Cd fractions slightly increased.
The proportion of weak acid soluble Cd decreased in all amended soils and further declined with increasing dosages of amendments compared with CK. Correlation analysis showed that the weak acid extractable Cd was significantly and negatively correlated with oxidizable and residual Cd, with correlation coefficients of 0.788 (p < 0.01) and 0.297 (p < 0.05), respectively (Table 2). This result indicates that the amendments decreased the available forms of Cd and converted Cd into less available forms.
Effects of Amendments on Cd Uptake by Wheat and Translocation
The Cd concentrations in the roots significantly decreased within the range of 23.7–27.2% in BC, 29.5–33.0% in ZE, 16.3–37.4% in HA, and 48.2–65.5% in L amended soils compared with the control. The Cd uptake in the roots increased with increasing SP application rate, especially for the high rate. In general, a positive correlation existed between Cd in roots and CaCl2-extractable Cd across the amended soils (r = 0.458, p < 0.01). The decreasing effects of amendments on Cd uptake by wheat root were conspicuous in all cases with the exception of SP, and the effects were enhanced with increasing application rate.
The Cd uptake in straw decreased within the range of 41.1–65.3% in SP and 42.1–69.2% in L compared with the untreated soil (Fig. 4b). The concentration of Cd in straw significantly decreased by 34.4%, 41.1%, and 39.7% with ZE at 2.5% and 5% and HA at 5% level, respectively, over the control. However, no significant difference was observed in Cd uptake between BC and the control treatments.
Wheat root (a), straw (b), and grain (c) Cd concentration, and translocation factor (d) of root to grain. Low, medium, and high indicate that the amendments were applied at low, medium, and high doses, respectively. The error bars are the standard deviation of the means (n = 3), and the different lowercase letters indicate a significant difference at p < 0.05 among treatments
All amendment incorporations caused a significant decrease in grain Cd, except for BC and HA at low and medium doses (Fig. 4c). TFroot-grain significantly declined only in the SP treatment (Fig. 4d). A significant positive correlation was found between wheat grain and root Cd concentrations (r = 0.762, p < 0.05), whereas the TFroot-grain values were not markedly correlated with wheat grain Cd (p > 0.05) in all amended soils (except SP). These results indicate that the reduction of grain Cd was primarily attributed to the decrease in root Cd uptake rather than Cd translocation and redistribution among different wheat parts, with the exception of the SP treatment. This finding represents the differences in the mechanisms of various amendments in reducing grain Cd concentrations.
Discussion
This research evaluated and compared the effects of different amendments at different application rates on Cd immobilization in Cd-contaminated soil and its uptake by plant. In the present study, these amendments substantially affected the soil properties, especially soil pH (Fig. 1). In additionally, CaCl2-extractable Cd and weak acid fraction Cd were significantly negatively correlated with soil pH (Fig. 3 and Table 2). These results indicated that soil pH might play a key role in controlling Cd availability and mobility by influencing heavy metal immobilization processes in soil, such as adsorption, desorption, precipitation, and dissolution (Harter 1983; Liang et al. 2017; Peng et al. 2009; Xu et al. 2018). A decrease in soil pH generally mobilizes heavy metals in soil (Chuan et al. 1996), whereas soil pH increases because adding amendments stabilizes Cd in soil through Cd reciprocal distribution among different fractions (Park et al. 2011b). Moreover, an increase in soil pH usually leads to the production of numerous negatively charged sorption sites, the formation of hydroxyl groups of metal cations, and the precipitation of Cd2+ as Cd(OH)2 or CdCO3, thereby decreasing soil Cd availability and promoting the conversion of Cd from available forms to less available or stable forms caused by amendment inputs (Ruttens et al. 2010; Yuan et al. 2015).
Cd was substantially immobilized with BC addition, and the immobilization was further enhanced with increasing dosage. It is possible that the increase in soil pH caused by the addition of BC resulted in Cd immobilization by precipitation and adsorption. The pH of BC was 7.95 in this study (Table S2). In addition, the surface area and diameter average may affect the adsorption of Cd (Table S3), in some cases, the functional groups on the BC surface benefit Cd adsorption (Lu et al. 2014). The FTIR spectra indicated that peaks at 3437 cm−1, 2910 cm−1, 2864 cm−1, 1616 cm−1, 1385 cm−1, and 1100 cm−1 were associated with the BC (Fig. S1). The peak at 3437 cm−1 represents the stretching vibration of water molecule hydroxyl, and that at 1616 cm−1 represents the vibration of aromatic C=O and C=C. The absorption peak of 1385 cm−1 is associated with the bending vibration of phenolic hydroxyl (Yan 2018). The decline in Cd availability caused by the BC input may also be an important reason for the increased grain yield and decreased Cd uptake by plants (Park et al. 2011b). In this study, BC-5% achieved the highest grain yield and the lowest Cd availability and concentration in soil and grain and among the three ratios, showing that the application of BC-5% to the soil had a good remediation effect.
The available Cd contents were considerably decreased by the addition of ZE, which reduced the exchangeable Cd concentration (Wen et al. 2016), possibly due to more adsorption and precipitation by increasing the soil pH and utilizing the special aluminum silicate network structure on the surface of ZE (Shaheen and Rinklebe 2015; Shi et al. 2009). In addition, no significant differences were observed in the availability of Cd among the three application rates of ZE, suggesting that a similar desired effect can be achieved with a low amount of ZE, low cost, and minimal soil disturbance (Guo et al. 2018). Among all treatments, the lowest mobilization and availability of Cd were obtained in the ZE treatment, alleviating Cd toxicity to wheat. This claim was proven by the decrease in Cd uptake by plants and the increase in grain yield. According to the passivation effect on available forms of Cd and its uptake by plant, ZE-1% was the appropriate application rate.
In the present study, no significant differences were found in soil pH, wheat grain, and CaCl2-extractable Cd regardless of the application of HA or the different application rates. This finding might be explained by relatively low humification degree of HA (Walker et al. 2004). Guo et al. (2018) also found that the application of organic fertilizer with incomplete humification did not reduce CaCl2-extractable Cd in mild and moderate Cd-contaminated soils under rice–wheat rotation. Another reason could be that the initial effective adsorption or complexation of Cd induced by HA addition can be reactivated and remobilized into soils with the microbial degradation of HA over time (Evangelou et al. 2004; Park et al. 2011c). Thus, the degree of humification of HA should be considered when HA is used as a remediation amendment.
The soil pH values decreased with increasing dosage of SP due to its acidity, indicating that it could cause further soil acidification (Melamed et al. 2003; Park et al. 2011a). However, it did not improve Cd mobility, even at high application rates, which suggested that the effect of phosphorus-containing amendments on Cd immobilization in soil is a rather complicated process (Basta et al. 2001). The immobilization mechanism mainly included the following: (1) Cd ions were adsorbed directly on the phosphate surface; (2) the increase in soil negative charge enhanced soil adsorption for available Cd; and (3) Cd precipitated with phosphate in soil solution, forming Cd(H2PO4)2, Cd3(PO4)2, and Cd5H2(PO4)4·4H2O (CotterHowells and Caporn 1996; Matusik et al. 2008; Raicevic et al. 2005). Moreover, Waterlot et al. (2011) reported that the isomorphous replacement between Cd2+ and Ca2+ reduced Cd availability due to the close radius of Cd2+ (0.097 nm) and Ca2+ (0.094 nm). Sarwar et al. (2010) indicated that not all phosphate materials could be used as amendments for Cd immobilization due to the different properties, some of these materials increased the solubility of Cd because of the decline in soil pH. Therefore, phosphate materials for Cd-contaminated soil remediation must be carefully selected.
The reduction in Cd toxicity and the improvement in P supply promoted wheat growth in the treatments receiving SP (Bashir et al. 2018). Thus, SP could be used as an amendment for Cd immobilization in Cd-polluted soils with low fertility. Interestingly, the application of SP increased Cd concentration in roots but significantly decreased it in straw and grain (Fig. 4). For the increase in Cd in roots, the precipitated and absorbed Cd might be dissolved in rhizosphere soil, which enhanced the Cd uptake in roots (Shaheen and Rinklebe 2015). Moreover, Ca2+/H+ antiporter gene expression caused by excessive soluble Ca promoted the accumulation of Cd in roots (Hirschi 1999). For the decrease in Cd in grains, it can be attributed to the fact that SP addition inhibits the translocation of Cd from root to grain in the wheat by forming Cd-phosphorus complexes and binding Cd to cell wall components in the root (Qiu et al. 2011). Jiang et al. (2007) also suggested that P could hinder the translocation of Cd in maize because of increments in electronegative ions, such as HPO42− and H2PO4−, which can absorb Cd2+ onto cell walls with them by various mechanisms, including adsorption, complexation, and precipitation, thereby reducing the transfer capability of Cd in plants. This behavior may have accounted for the decline in TFroot-grain with increasing SP addition (Fig. 4d). SP application not only reduced Cd availability in soil but also influenced the physiological responses of crops to Cd, thereby altering the distribution of Cd in different parts of the plant and consequently alleviating its toxicity for consumers.
As an alkaline material, L mainly modulates soil pH to change the availability of heavy metals (Guo et al. 2018). The presence of high divalent Ca2+ in L-treated soil is the cause of soil pH increase (Impellitteri and Scheckel 2006), which can reduce Cd solubility and mobility by precipitation and adsorption (Shaheen and Rinklebe 2015). Plant growth can benefit from the reduction of available Cd (Kim et al. 2017). However, in this study, the wheat yield decreased significantly at the 2% application dose compared with the application rates of L at 0.5% and 1%, possibly due to the soil alkalinity caused by high L doses. Mahar et al. (2016) indicated that excessive addition of CaO (5 and 10%) decreased the biomass of Chinese cabbage in Cd-contaminated soils. Thus, the application rate of L should be optimized in consideration of contaminated soil properties and the desired aims to avoid secondary ecological issues. Overall, 0.5% L is a suitable amount for remediating Cd contamination in the tested soil.
The increase in CaCl2-extractable Cd could be attributed to the increase in salinity caused by SS applied at the low rate (Acosta et al. 2011; Raiesi and Sadeghi 2019; Zhang et al. 2016). However, the availability of Cd decreased as the dosage further increased due to the following: (1) the formation of cadmium sulfide induced by SS application and (2) OH− was produced during the dissolution of SS, resulting in the formation of cadmium hydroxide (Lewis 2010). Soil EC is a vital factor for quantifying soluble salts and evaluating soil ecosystem quality (Mahar et al. 2018). The application of SS significant increased soil EC by 9–25-fold compared with the control (Fig. 1b), suggesting high soluble salts in soil, which likely led to the inhibition of wheat growth. The increase in soil EC and sodium ion concentration caused by SS application led to salt pressure (Zhang et al. 2018), which affected water and nutrient uptake by crops. Therefore, SS could not be a suitable amendment for mitigation of Cd pollution, especially in saline–alkali soil.
In this research, L, ZE, and BC exerted a better effect on reducing Cd availability compared with the other amendments (Table 1), indicating that they were suitable for immobilizing Cd in Cd-contaminated acid or neutral soils. Although the effects of amendments on the reduction in Cd in wheat grain became increasingly significant with the increase in application rate, an inappropriate application rate of the amendments would induce secondary ecological issues and subsequently affect crop growth. According to the effects of amendments on crop grain yield and Cd immobilization and the potential benefits expected, the recommendable application rates of BC, ZE, and L in soil are 5%, 1%, and 0.5% for Cd immobilization in acidic or neutral soils, respectively. However, the IE in soil after the amendment application must be monitored and evaluated under actual field conditions. Furthermore, although SP led to soil acidification to some extent, it could regulate the transport of Cd in various parts of the plant and provide nutrients to crops simultaneously (Bashir et al. 2018; Qiu et al. 2011). Thus, it can be combined with alkaline amendment materials to mitigate the risk caused by single amendment and remediate combined soil pollution (Guo et al. 2018). Further studies are necessary to explore the immobilization effectiveness of combined amendments in Cd-contaminated soil, although the present study has provided some information on optimizing the types and application rates of single amendment.
Conclusion
Our results showed that Cd availability varied with the different types and rates of amendment applications, indicating the changes in CaCl2-extractable Cd and the redistribution of Cd in four fractions under different treatments. Compared with the control, all amendments decreased Cd uptake in roots (except for SP) and grains because they decreased Cd solubility and availability and converted Cd from available forms to less available or stable forms. SS treatment increased the soil EC value, which was associated with the stunted growth of wheat. The application of SP led to soil acidification to some extent. Therefore, SS and SP amendments are inappropriate for Cd immobilization in saline–alkali and acidic soils, respectively. Investigation of the comprehensive effects of the amendments on Cd immobilization, plant growth, and their potential risks indicates that BC-5%, ZE-1%, and L-0.5% are recommended for immobilization of Cd in acidic or neutral soils. These amendments influenced Cd immobilization by changing the availability of Cd in soil, redistributing Cd among different fractions in soil, and affecting root uptake by plants. Further study is necessary to investigate the remediation effects of combined application of various amendments on Cd and other heavy metals compound contaminated soil.
References
Acosta JA, Jansen B, Kalbitz K, Faz A, Martinez-Martinez S (2011) Salinity increases mobility of heavy metals in soils. Chemosphere 85:1318–1324
Ahmad I, Akhtar MJ, Zahir ZA, Mitter B (2015) Organic amendments: effects on cereals growth and cadmium remediation. Int J Environ Sci Technol 12:2919–2928
Al Chami Z, Amer N, Al Bitar L, Cavoski I (2015) Potential use of Sorghum bicolor and Carthamus tinctorius in phytoremediation of nickel, lead and zinc. Int J Environ Sci Technol 12:3957–3970
Ali S et al. (2013) The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol Environ Saf 89:66–72
Bashir S, Zhu J, Fu QL, Hu HQ (2018) Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 194:579–587
Basta NT, Gradwohl R, Snethen KL, Schroder JL (2001) Chemical immobilization of lead, zinc, and cadmium in smelter-contaminated soils using biosolids and rock phosphate. J Environ Qual 30:1222–1230
Basta NT, McGowen SL (2004) Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ Pollut 127:73–82
Boisson J, Ruttens A, Mench M, Vangronsveld J (1999) Evaluation of hydroxyapatite as a metal immobilizing soil additive for the remediation of polluted soils. Part 1. Influence of hydroxyapatite on metal exchangeability in soil, plant growth and plant metal accumulation. Met Accumul Environ Pollut 104:225–233
Chuan MC, Shu GY, Liu JC (1996) Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water Air Soil Pollut 90:543–556
Cocarta DM, Neamtu S, Deac AMR (2016) Carcinogenic risk evaluation for human health risk assessment from soils contaminated with heavy metals. Int J Environ Sci Technol 13:2025–2036
CotterHowells J, Caporn S (1996) Remediation of contaminated land by formation of heavy metal phosphates. Appl Geochem 11:335–342
Downie A, Munroe P, Cowie A, Van Zwieten L, Lau DMJ (2012) Biochar as a geoengineering climate solution: hazard identification and risk management. Crit Rev Environ Sci Technol 42:225–250
Evangelou MWH, Daghan H, Schaeffer A (2004) The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 57:207–213
Guo FY, Ding CF, Zhou ZG, Huang GX, Wang XX (2018) Effects of combined amendments on crop yield and cadmium uptake in two cadmium contaminated soils under rice-wheat rotation. Ecotoxicol Environ Saf 148:303–310
Guo GL, Zhou QX, Ma LQ (2006) Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: a review. Environ Monit Assess 116:513–528
Harter RD (1983) Effect of soil-pH on adsorption of lead, copper, zinc, and nickel. Soil Sci Soc Am J 47:47–51
He DY et al. (2019) Effects of soil amendments applied on cadmium availability, soil enzyme activity, and plant uptake in contaminated purple soil. Sci Total Environ 654:1364–1371
He TY et al. (2017) Effects of biochar on cadmium accumulation in rice and cadmium fractions of soil: a three-year pot experiment. BioResources 12:622–642
Hirschi KD (1999) Expression of arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell 11:2113–2122
Houben D, Evrard L, Sonnet P (2013) Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 92:1450–1457
Impellitteri CA, Scheckel KG (2006) The distribution, solid-phase speciation, and desorption/dissolution of As in waste iron-based drinking water treatment residuals. Chemosphere 64:875–880
Jiang HM, Yang JC, Zhang JF (2007) Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ Pollut 147:750–756
Khan A, Khan S, Khan MA, Qamar Z, Waqas M (2015) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res 22:13772–13799
Kim SC, Hong YK, Oh SJ, Oh SM, Lee SP, Kim DH, Yang JE (2017) Effect of chemical amendments on remediation of potentially toxic trace elements (PTEs) and soil quality improvement in paddy fields. Environ Geochem Health 39:345–352
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manag 28:215–225
Kuppusamy S, Thavamani P, Megharaj M, Venkateswarlu K, Naidu RJEi (2016) Agronomic and remedial benefits and risks of applying biochar to soil: current knowledge and future research directions. Environ Int 87:1–12
Lewis AE (2010) Review of metal sulphide precipitation. Hydrometallurgy 104:222–234
Li ZY, Ma ZW, van der Kuijp TJ, Yuan ZW, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468:843–853
Liang J et al. (2017) Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 181:281–288
Lu KP et al. (2014) Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agr Ecosyst Environ 191:124–132
Lu RK (2000) Soil agricultural chemical analysis and method. China Agricultural Science and Technology Press, Beijing
Mahar A et al. (2016) Impact of CaO, fly ash, sulfur and Na2S on the (im)mobilization and phytoavailability of Cd, Cu and Pb in contaminated soil. Ecotoxicol Environ Saf 134:116–123
Mahar A et al. (2018) (Im)mobilization of soil heavy metals using CaO, FA, sulfur, and Na2S: a 1-year incubation study. J Int J Environ Sci 15:607–620
Matusik J, Bajda T, Manecki M (2008) Immobilization of aqueous cadmium by addition of phosphates. J Hazard Mater 152:1332–1339
MEE (2014) China soil pollution survey communique in Chinese. Ministry of Ecology and Environment of the People’s Republic of China. http://www.mee.gov.cn/gkml/sthjbgw/qt/201404/t20140417_270670.htm
Melamed R, Cao XD, Chen M, Ma LQ (2003) Field assessment of lead immobilization in a contaminated soil after phosphate application. Sci Total Environ 305:117–127
Mignardi S, Corami A, Ferrini V (2012) Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 86:354–360
Nordberg GF (2009) Historical perspectives on cadmium toxicology. Toxicol Appl Pharm 238:192–200
Ok YS et al. (2010) Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ Earth Sci 61:1301–1308
Oziegbe O, Aladesanmi OT, Awotoye OO (2019) Effect of biochar on the nutrient contents and metal recovery efficiency in sorghum planted on landfill soils. Int J Environ Sci Technol 16:2259–2270
Park JH, Bolan N, Megharaj M, Naidu R (2011a) Comparative value of phosphate sources on the immobilization of lead, and leaching of lead and phosphorus in lead contaminated soils. Sci Total Environ 409:853–860
Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011b) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451
Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung JW (2011c) Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J Hazard Mater 185:549–574
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640
Qayyum MF et al. (2017) Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 174:515–523
Qiu Q, Wang YT, Yang ZY, Yuan JG (2011) Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem Toxicol 49:2260–2267
Raicevic S, Kaludjerovic-Radoicic T, Zouboulis AI (2005) In situ stabilization of toxic metals in polluted soils using phosphates: theoretical prediction and experimental verification. J Hazard Mater 117:41–53
Raiesi F, Sadeghi E (2019) Interactive effect of salinity and cadmium toxicity on soil microbial properties and enzyme activities. Ecotoxicol Environ Saf 168:221–229
Rauret G, Lopez-Sanchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Ruttens A et al. (2010) Long-term sustainability of metal immobilization by soil amendments: Cyclonic ashes versus lime addition. Environ Pollut 158:1428–1434
Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, Bibi S, Farid G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 90:925–937
Shaheen SM, Rinklebe J (2015) Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol Eng 74:319–326
Shi WY, Shao HB, Li H, Shao MA, Du S (2009) Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J Hazard Mater 170:1–6
Walker DJ, Clemente R, Bernal MP (2004) Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere 57:215–224
Waterlot C, Pruvot C, Ciesielski H, Douay F (2011) Effects of a phosphorus amendment and the pH of water used for watering on the mobility and phytoavailability of Cd, Pb and Zn in highly contaminated kitchen garden soils. Ecol Eng 37:1081–1093
Wen J, Yi YJ, Zeng GM (2016) Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J Environ Manag 178:63–69
Wuana RA, Okieimen FE, Imborvungu JA (2010) Removal of heavy metals from a contaminated soil using organic chelating acids. Int J Environ Sci Technol 7:485–496
Xiong X et al. (2004) Cadmium contamination of soils of the Shenyang Zhangshi irrigation area, China: An historical perspective. Bull Environ Contam Toxicol 73:270–275
Xu C, Xiang Q, Zhu HH, Wang S, Zhu QH, Huang DY, Zhang YZ (2018) Effect of biochar from peanut shell on speciation and availability of lead and zinc in an acidic paddy soil. Ecotoxicol Environ Saf 164:554–561
Xu YG, Yu WT, Ma Q, Zhou H (2015) Potential risk of cadmium in a soil-plant system as a result of long-term (10 years) pig manure application. Plant Soil Environ 61:352–357
Yan SH (2018) Research on the physicochemical properties and stability of biochar pyrolysis at different condition. Master Thesis. Shenyang Agricultural University
Yang SL, Zhou DQ, Yu HY, Wei R, Pan B (2013) Distribution and speciation of metals (Cu, Zn, Cd, and Pb) in agricultural and non-agricultural soils near a stream upriver from the Pearl River, China. Environ Pollut 177:64–70
Yuan XZ et al. (2015) Speciation and environmental risk assessment of heavy metal in bio-oil from liquefaction/pyrolysis of sewage sludge. Chemosphere 120:645–652
Zhang CJ, Sale PWG, Tang CX (2016) Cadmium uptake by Carpobrotus rossii (Haw.) Schwantes under different saline conditions. Environ Sci Pollut Res 23:13480–13488
Zhang HX, Tian Y, Guan B, Zhou DW, Sun ZW, Baskin CC (2018) The best salt solution parameter to describe seed/seedling responses to saline and sodic salts. Plant Soil 426:313–325
Funding
This work was financially supported by the strategic Priority Research Program of the Chinese Academy of Sciences (XDA23060503), National Key Technology Research and Development Program of China (2015BAD05B01), and Doctoral Research Fund of Liaoning Province, China (20180540071).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Zhou, C., Yuan, H., Ning, C. et al. Evaluation of Different Types and Amounts of Amendments on Soil Cd Immobilization and its Uptake to Wheat. Environmental Management 65, 818–828 (2020). https://doi.org/10.1007/s00267-020-01287-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-020-01287-4