Abstract
Background
Dorsal nasal augmentation is an essential part of injection rhinoplasty on the Asian nose. Aesthetic physicians require detailed knowledge of the nasal anatomy to accurately and safely inject filler.
Methods
One hundred and thirty-five histological cross sections were examined from 45 longitudinal strips of soft tissue harvested from the midline of the nose, beginning from the glabella to the nasal tip. Muscles and nasal cartilage were used as landmarks for vascular identification.
Results
At the nasal tip, a midline longitudinal columellar artery with a diameter of 0.21 ± 0.09 mm was noted in 14 cadavers (31.1 %). At the infratip, subcutaneous tissue contained cavernous tissue similar to that of the nasal mucosa. The feeding arteries of these dilated veins formed arteriovenous shunts, into which retrograde injection of filler may be possible. All of the nasal arteries present were identified as subcutaneous arteries. They coursed mainly in the superficial layer of the subcutaneous tissues, with smaller branches forming subdermal plexuses. A substantial arterial anastomosis occurred at the supratip region, in which the artery lay in the middle of the subcutaneous tissue at the level of the major alar cartilages. These arteries had a diameter ranging between 0.4 and 0.9 mm and were found in 29 of 45 specimens (64.4 %). This was at the level midway between the rhinion above the supratip and the infratip. This anastomotic artery also crossed the midline at the rhinion superficial to the origin of the procerus on the lower end of the nasal bone. Here the arterial diameter ranged between 0.1 and 0.3 mm, which was not large enough to cause arterial emboli. Fascicular cross sections of the nasalis muscle directly covered the entire upper lateral cartilage. The subdermal tissue contained few layers of fat cells along with the occasional small artery. The procerus arose from the nasal bone and was continuous with the nasalis in 16 cadavers (35.6 %). There was fatty areolar tissue between the procerus and the periosteal layer and no significant arteries present. The procerus ascended beyond the brow to insert into the frontalis muscle with very few cutaneous insertions. The supratrochlear vessels and accompanying nerve were occasionally found on the surface of the frontalis muscle.
Conclusion
Most nasal arteries found in the midline are subcutaneous arteries. Filler should be injected deeply to avoid vascular injury leading to compromised perfusion at the dorsum or filler emboli at the nasal tip.
Level of Evidence V
This journal requires that the authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although surgical rhinoplasty has evolved into a minimally invasive procedure, in which the surgeon can correct the dorsum and perform tip refinement using a hidden incision within a single nostril [1]; injection rhinoplasty is more beneficial both from the patients’ and physicians’ viewpoints with regard to being less expensive, non-invasive, reversible and having no downtime [2, 3]. Asian injection rhinoplasty is generally performed using two procedure techniques both with different purposes. The most common is utilised for augmentation, whilst the other technique is required for support.
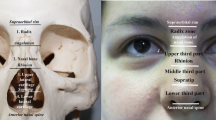
The first procedure is often used at the midline of the upper part of the nose for subsequent augmentation of the nasal dorsum and radix (Fig. 1). The second injection technique creates support for the columella and nasal tip. The dorsal augmentation technique can be performed in two ways. The first is direct injection perpendicular to the skin using a sharp needle. The other approach is indirect injection entering from both the glabella and the infratip using a long blunt cannula to create a straight dorsum (Fig. 1a) [4]. The direct approach usually needs multiple injections to cover the length of the nasal dorsum and results in more precise filler placement with minimal shape distortion or deviation. This method will leave serial puncture holes on the nasal dorsum for a few days, particularly if large particle hyaluronic acid filler is used to imitate the nasal bones. Blunt cannula injection requires more skill and experience to palpate the position of the cannula tip and estimate the amount of filler that has been injected at the target area. Rapid oedema of the soft tissue and bleeding from the intercanthal veins in some patients may confound the visual results, and thus wrongly assume that the correct amount of filler has been placed. Both the sharp needle and blunt cannula are recommended to place filler deep on the preperiosteal layer of the nasal bone in the midline [2, 4, 5]. Subdermal threading using a sharp needle is the common technique used for other areas, but for the nose this technique has been reported to cause injury to the subdermal vasculature [6]. Cannula deviation from the midline during the infratip approach requires more filler product to ensure both sides are even and also carries a higher risk of vascular complications [7].
Injection rhinoplasty. a, b Demonstration of dorsal augmentation from a lateral and oblique view. Three green needles indicate direct injection perpendicular to the skin using a sharp needle. Upper and lower black cannulae illustrate cannula injection along the gliding plane under the superficial musculoaponeurotic system (sub-SMAS plane) as an indirect injection technique. c, d Illustration of recommended technique. c Craniocaudal deep injection into the preperiosteal or subperiosteal plane to avoid vascular injury and migration of filler by gravity. This trajectory is limited by the prominent superciliary ridge. d For correction of the dorsal hump, infratip injection may not reach the curved nasal bone. An entry point at the upper lateral cartilage or the nasian allows correct needle insertion
Furthermore, volume augmentation may be used to correct asymmetry and deformity. Camouflage of a dorsal hump deformity and twisted nose in Asians is sometimes performed under certain circumstances [2, 3, 5, 8]. It is different from the injection rhinoplasty procedure for Caucasians [2], in that it rarely increases the size of an Asian nose, because the midline column of filler in dorsal augmentation creates a longitudinal ridge making the nose look smaller in comparison to the previous flat dorsum.
The other procedure increases projection of the nasal tip and overall support of the lower nose. This is usually done by placing filler between the fibrous tissues joining the medial crura of the lower lateral cartilages [2]. This technique allows a firm encapsulation of the filler within confined fibrous and cartilaginous structures, resulting in stable support of the columella and projection of the nasal tip. Some physicians may add filler directly at the nasal tip, just beneath the skin to adjust the shape of the tip projection and smooth irregularities [2, 4, 5, 7]. If this technique does not create enough projection, placement of filler deep into the anterior nasal spine will add more elevation and increase the nasolabial angle for a more aesthetically pleasing look [4]. Care must be taken not to inject filler directly into the wall of the nasal or oral cavity and to ensure that leakage of filler into the nasal septum and bulging into the oral mucosa does not occur. Injection at the alar base is not a part of the nasal augmentation procedure, but can obliterate a deep alar crease and smooth the upper part of a sagging nasolabial fold.
Motivated by excellent results, patients regularly visit aesthetic clinics for filler treatment. Minor complications such as erythema, swelling and ecchymosis usually occur immediately after filler placement. These complications are commonplace, last a few days and therefore acceptable for the patient. However, two rare but threatening complications need serious consideration. These severe complications of the injection rhinoplasty are blindness and skin infarction [6, 9–11]. Nasal augmentation is second to glabellar injection as the most common cause of blindness following filler injections, accounting for 25 % of the 98 cases of ocular complications [12, 13]. Blindness occurs when filler is accidentally injected retrogradely into the proximal segment of the ophthalmic artery, where the retinal and choroidal arteries branch off. Inadvertent cannulation or leakage of filler droplets into a torn arterial wall within the cutaneous retaining ligaments or fibrotic scar tissue that keep it open are the suspected causes of blindness. Vascular wall injury with prolonged constriction and/or thrombosis can compromise the vasculature, leading to tissue infarction (Fig. 2). Although the nasal arterial vasculature is well described [14], information on the size, depths and planes of the arterial structures is still lacking and is therefore of limited use in a clinical setting. This study investigated the midline anatomical architecture of the nose and the positions of the significant nasal arteries from longitudinal histology sections to determine safe planes for injections.
High pressure injection into the superficial temporal, supratrochlear, supraorbital or lateral nasal arteries could force filler droplets into the ophthalmic artery through anastomoses at the superior nasal corner of the orbit (white arrows). If the filler droplets enter the origin of the ophthalmic artery or the internal carotid artery, visual and neurological symptoms are likely to develop
Materials and Methods
The whole of the midline specimens of the nasal soft tissues were harvested from fifty formaldehyde-embalmed cadavers for investigation of the midline nasal arteries. These midline longitudinal strips of 5 mm width were taken using a sharp surgical knife and a periosteal elevator beginning from the glabella between the medial eyebrows to the nasal tip, excluding the columella (Fig. 3). At the rhinion, part of the septal cartilage was harvested together with the specimens for use as a landmark. Each strip was then divided into three pieces of equal length for easier handling and processing of the histological longitudinal sections using haematoxylin and eosin stain (H&E stain). The specimens were cut at 9 µm thickness to preserve the cartilage in the sections. The cross sections of the specimens were inspected and searched for an anastomotic artery across the midline. To determine the risk of vascular complications, diameters of the midline arteries and thickness from the skin surface of the mobile soft tissues were measured using a digital scale bar in the microscope software ZEN lite (Carl Zeiss, Jena, Germany).
Results
Five specimens were excluded because of extensive oedema and maceration. Among the 50 specimens harvested, 45 were used, 5 discarded and each was divided into 3 areas. The thickness of the soft tissue from the skin to the frontal bone at the glabella was 7.2 ± 2.1 mm and it narrowed down to 3.1 ± 1.8 mm at the radix and over the nasal bone. Thickness of tissue increased to 5.7 ± 3.4 mm above the lateral and major alar cartilages. The midline specimens could be divided into three regions according to tissue type and thickness. The upper part was the glabella overlying the frontal bone where the maximum depression was at the radix (Fig. 4). The second part was the bony dorsum, where the soft tissue of the nasal dorsum was thinner than the lower part of the nose in the midline (Fig. 5). The third part was the cartilaginous dorsum situated at the lower half of the nose (Fig. 6). This third section included the nasal tip in which the soft tissue was thick and round. The junction between the lateral part of the septal cartilage and the major alar cartilage could not be observed from the skin, only from the histology cross sections.
The lower third segment of the nasal tip. The nasal tip is the highest risk part for injection rhinoplasty, due to the frequent presence of a sizeable lateral nasal artery at the lower nose. The artery usually anastomoses with the artery on the opposite side or the dorsal nasal artery from the ophthalmic artery
The transverse part of the nasalis muscle appeared in both upper and lower parts of the nose (Figs. 5, 6), even though in gross dissections the muscle covered only the side of the nose up to where the midline nasal fascia interposed. In histological cross sections, the muscle existed in the midline as cross sections of muscle fascicles arranged longitudinally over the upper lateral cartilage. The muscle occupied the space between the dermis and the perichondrium. It covered the entire lateral cartilage without any interposing fatty layer and in 21 cadavers (46.7 %); it extended downward beyond the alar cartilage. The subdermal tissue contained few layers of fat cells and occasionally some small arteries.
The procerus arose from the nasal bone and was continuous with the nasalis without any demarcation in 16 cadavers (35.6 %) (Fig. 4, Fig. 5). The procerus was thicker at its origin on the nasal bone and became thinner over the frontal bone. There was fatty areolar tissue between the procerus and the periosteal layer of the frontal bone and no significant arteries. The thin longitudinal fibres of the procerus ascended beyond the upper margin of the brow to insert into the frontalis muscle, with very few cutaneous insertions in 4 cadavers (8.8 %). In 6 cadavers (13.3 %) the procerus muscle ended in the subcutaneous layer below the glabella and did not extend above the brow level. This might explain why someone has a constant midline vertical glabella wrinkle without the action of the procerus muscle. In contrast to the preperiosteal layer, the muscle might be accompanied by the supratrochlear nerve and vessels on the superficial surface in 12 cadavers (26.7 %). In these cases, the subcutaneous layer of the glabella has a high risk of vascular compromise.
At the lower part of the nose, especially at the nasal tip, there was a longitudinal columellar artery with a diameter of 0.21 ± 0.09 mm coursing from the upper lip to join the lateral nasal artery in 14 cadavers (31.1 %) (Fig. 7). At the infratip, subcutaneous tissue contained cavernous tissue similar to the nasal mucosa (Fig. 6). In 11 cadavers (24.4 %), the cavernous tissue entered the tip area. The feeding arteries of these dilated veins may form arteriovenous anastomoses in which retrograde injection of filler into the artery may occur.
Four pieces of the lower third segment. The columellar artery is present in the pieces a and d (arrow heads) at the infratip subdermis. The lateral nasal artery anastomosis is in the subcutaneous tissue of the nasal tip in pieces a, b and c (arrows). Cavernous tissue at the infratip (delicate vascular structures in the subcutaneous tissue below the arrow head) is demonstrated by piece e, confirmed by CD31 immunohistochemistry staining for vascular endothelial lining. The right two pieces, d and e, are derived from the same paraffin block specimen
Based on the results of the current study, all the arteries in the nose were subcutaneous arteries (Table 1). When these arteries entered the nose, they coursed mainly at the superficial layer of the subcutaneous tissue with smaller branches travelling more superficially, supplying the skin as subdermal plexuses. A substantial arterial anastomosis occurred at the supratip region, where the artery lay in the middle of the fatty layer of the subcutaneous tissue at the level of the major alar cartilages. Arteries with a diameter ranging between 0.4 and 0.9 mm were found in 29 of 45 specimens (64.4 %) (Fig. 6, 7) midway between the rhinion above the supratip and the infratip. This anastomotic artery also crossed the midline at the rhinion superficial to the origin of the procerus on the lower end of the nasal bone. The arterial diameter ranged between 0.1 and 0.3 mm and was not large enough to cause arterial emboli.
Discussion
We had successful experience of inserting a 27G cannula into the superficial temporal artery, with a diameter of 1 mm, in fresh cadavers. It was difficult to enter the arterial lumen of smaller arteries in the subcutaneous tissue without local fibrotic scarring. Therefore, inadvertent cannulation into a 0.4–0.9 mm artery using a small cannula in clinical practice seems highly unlikely.
Based on our previous research of the midline nasal anastomotic artery, asymmetric facial arteries are frequent, with a dominant larger facial artery on one side. Without a significant angular artery off the smaller facial artery, the dominant facial artery from the lower face, or the ophthalmic artery from the orbit, usually has a large supratip artery running across the midline to anastomose and supplies blood to the other side of the face that contains the underdeveloped facial artery. In systematic data collection of the current study, we expected to find a sizeable artery with a diameter larger than 1 mm at any level of the nasal midline, but cadaveric examination of formalin-embalmed nasal specimens in the current investigation revealed none.
From this study we can now understand the rare incidence of blindness from nasal augmentation. In this series of cadavers we did not find a sizeable artery large enough for a cannula of 0.4 mm diameter to enter into the arterial lumen and cause embolic complication. Thus the incidence of a large enough artery crossing the midline is certainly rare and less than one in forty-five. Even though most patients are safe to be injected, there are a few that are at higher risk for filler complications. The relatively high risk area is identified at the subcutaneous plane of the supratip compared to other areas. During treatment, we are unable to identify those who have a significant supratip anastomosis. When blindness occurs, it threatens patients, aesthetic surgeons and all aesthetic practitioners. Thus relevant anatomical knowledge is important to prevent that complication.
Although midline nasal specimens in the current study did not contain any obvious large arteries, during dissection for preparation of teaching material in more than a hundred soft-embalmed cadavers with latex injection in the arterial system, we occasionally encountered a large midline nasal artery with a diameter of 1.2–1.5 mm (Fig. 8). The chance of encountering a large artery crossing the midline may vary between individuals, depending on a variety of factors. In clinical practice, geriatric patients may have smaller arteries, therefore teenagers could have a higher chance of arterial emboli. In this cadaveric study, swelling of tissue, vascular constriction during transfer, storage and formalin embalming may permanently collapse and shrink the arteries. This may influence the diameter of the arterial cross sections. How the data are collected is also important, a collapsed artery may look larger during dissection and look smaller than the actual size in an oblique histological section. Nevertheless, this study locates the most dangerous zone at the supratip, midway between the skin and the alar cartilage (Fig. 6, 7, 8).
Vasoconstriction in formalin-embalmed cadavers eliminates the chance of encountering the sizeable artery we occasionally found in the soft-embalmed cadavers with arterial latex injection. With regards to the effect of vasoconstriction, we encourage using adrenaline in local anaesthetic agents to decrease the arterial size. This practice certainly decreases the chance of inadvertently cannulating the artery within the nasal subcutaneous tissue during a cannula injection.
Cannula insertion from the infratip should glide along the fibrous articulation between the medial crura of the major alar cartilages and between the upper lateral cartilages and suture between the nasal bones to prevent vascular complication (Fig. 1a, b) [4]. This trajectory with a higher entry point over the cartilaginous part could be performed safely using a sharp needle under local anaesthesia to enter into the subperiosteal layer of the nasal bone (Fig. 1d) [15].
The tendency of insufficient vasculature is high at the upper part of the nose, while the lower part, especially at the alar rim, presents a combined risk of arterial emboli and insufficiency (Fig. 9b). In the nose, multi-plane injection recommended by some authors [4, 7] is prohibited due to the high chance of vascular complications, especially in the subcutaneous tissue plane. At the glabella, multi-plane injection is well tolerated but not safe at the medial orbital rim. Injection at the nasal tip can be done safely at the perichondrium (Fig. 9c). Superficial subdermal injection at the nasal tip carries a delayed risk of post-injection erythema caused by hypersensitivity reaction, superficial venous thrombophlebitis and alar infarction from subdermal arterial compression [2, 9].
Summary of the danger areas during nasal injections. a The infratip (square) is highly vascular as it contains cavernous tissue that continues from the nasal submucosa containing an arteriovenous shunt. b Three vascular danger points where filler can escape into the arterial lumen; the rhinion where the dorsal nasal anastomosis is occasionally found, the supratip where significant lateral nasal anastomoses form, and the infratip in which the columellar artery enters from below and cavernous tissue infiltrates. The supratip (middle arrow) poses the highest risk. c Recommendation of needle trajectory for tip refinement. Even though an artery is injured by the needle, symptomatic emboli could be avoided by retrograde injection of a small droplet at the time of withdrawal
Comparison between three levels of deep plane injection for dorsal nasal augmentation shows that injection at the bony dorsum is more difficult than at the glabella and the cartilaginous dorsum. The soft tissue overlying the nasal bones is thinner than the glabella and the cartilaginous dorsum and the dorsal nasal arteries are delicate. These limitations may not allow for the large volume injections required to create a very high dorsum (Fig. 4, 5, 6). We recommend using a sharp needle with manual bending to facilitate insertion from the glabella and adding subperiosteal injection with pre-tunnelling after the preperiosteal injections (Fig. 1). This is the only way to apply a large amount of filler to augment the dorsum with minimal compromise to the vascular system of the overlying soft tissue. Elevation of the periosteum will stimulate new bone formation over the nasal bones creating a permanent higher dorsum [15]. The physician should keep in mind that over stretching of the nasal soft tissue will compromise tissue perfusion, thus use of a cold pack is avoided in such cases. The patient should be observed for an hour in the office and ask for self-observation and awareness for 6 h. If signs of poor tissue perfusion such as bleaching, discoloration and excruciating pain at the treatment site or beyond the area along the local arterial distribution persist for more than an hour, hyaluronidase should be injected for degradation of the filler at the dorsal augmentation area and the area of local arterial distribution.
In some patients, the nasal bones are flat and the upper lateral cartilages are thin and do not project anteriorly, dorsal augmentation is not possible when the patient asks for a very high dorsum after the patient has already received the first or even second injections from other clinics. This is because the injection will fill the pocket of filler creating high pressure in an area with limited coverage of soft tissue resulting in reflux of filler through the needle hole.
When the dorsal hump is to be corrected, needle insertion from the infratip is not recommended because the limitation of the skin may not allow the needle to enter the periosteum of the nasal bone. A closer insertion point is needed to access the correct plane (Fig. 1c, d).
For correction of a bulbous round nose tip and improving tip definition, the cannula should be inserted craniocaudally from the bony or cartilaginous dorsum and enter the subcutaneous plane of the nasal tip just under the dermis (Fig. 9c). Injection should be performed slowly with occasional aspiration due to the presence, in some individuals, of cavernous tissue in the subcutaneous tissue similar to that on the mucosa side of the nasal cavity (Fig. 9a). Occlusion of the venous plexus may bring about redness of the nasal tip from thrombophlebitis. However, nasal tip erythema is usually caused by enterococcal contamination leading to infection. Over tightness of tissue from filler injection is avoided to prevent tissue infarction.
General recommendation: When an aesthetic physician assesses a patient and designs a specific treatment, general recommendations for injection rhinoplasty are as follows. Needle entry points should be at least 5 mm apart from the injection area to avoid retrograde filler leakage from the needle hole in the event that repeated injections cause a compressed pocket of filler in the tissue. A cannula is the preferred instrument for deep, craniocaudal insertion into the preperiosteal areolar sub-SMAS (superficial musculoaponeurotic system) plane to avoid vascular injury and migration of filler by gravity (Fig. 1c, 9c). After positioning the needle, the syringe is aspired before injection to eliminate any intravascular placement. Retrograde injection is performed to minimise the pressure at the cannula tip while simultaneously controlling the effect of the filler to create volume. The needle entry point should be positioned cranial to the concavity, with the skin elevated and the needle inserted deep into the sub-SMAS, just superior to the periosteum or perichondrium.
Conclusion
Most nasal arteries found in the midline are subcutaneous arteries. Filler should be injected deeply to avoid vascular injury causing compromised perfusion at the dorsum or filler emboli at the nasal tip.
References
Harrison DH (2013) Reflections on the open and closed rhinoplasty. J Plast Reconstr Aesthet Surg 66:1356–1359
Schuster B (2015) Injection rhinoplasty with hyaluronic acid and calcium hydroxyapatite: A retrospective survey investigating outcome and complication rates. Facial Plast Surg 31:301–307
Cassuto D (2009) The use of Dermicol-P35 dermal filler for nonsurgical rhinoplasty. Aesthet Surg J 29:S22–S24
Han X, Han X, Hu J, Cheng L, Li F (2015) Multiplane hyaluronic acid (EME) in female Chinese rhinoplasty using blunt and sharp needle technique. J Plast Reconstr Aesthet Surg. 68:1504–1509
Baptista C, Nguyen PSA, Desouches C, Magalon G, Bardot J, Casanova D (2013) Correction of sequelae of rhinoplasty by lipofilling. J Plast Reconstr Aesthet Surg 66:805–811
Humphrey CD, Arkins JP, Dayan SH (2009) Soft tissue fillers in the nose. Aesthet Surg J 29:477–484
Kurkjian TJ, Ahmad J, Rohrich RJ (2014) Soft-tissue fillers in rhinoplasty. Plast Reconstr Surg 133:21e–126e
Piggott JR, Yazdani A (2011) Hyaluronic acid used for the correction of nasal deviation in an 18-year-old Middle Eastern man. Can J Plast Surg 19:156–158
Pavicic T, Funt D (2013) Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol 13:295
Kim SN, Byun DS, Park JH, Han SW, Baik JS, Kim JY (2014) Panophthalmoplegia and vision loss after cosmetic nasal dorsum injection. J Clin Neurosci 21:678–680
DeLorenzi C (2013) Complications of injectable fillers, part I. Aesthetic Surg J 33:561–575
Beleznay K, Carruthers JD, Humphrey S, Jones D (2015) Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg 41:1097–1117
Li X, Du L, Lu JJ (2015) A novel hypothesis of visual loss secondary to cosmetic facial filler injection. Ann Plast Surg 75(3):258–260
Saban Y, Andretto AC, Bouaziz D, Polselli R (2012) Nasal arterial vasculature: medical and surgical applications. Arch Facial Plast Surg 14:429–436
Mashiko T, Takanobu M, Harushi K, Harunosuke D, Kentaro K, Shinichiro K, Kahori K, Akira O, Kuni Y, Yoshimura K (2013) Semipermanent volumization by an absorbable filler. Plast Reconstr Surg Glob Open 1:1–11
Acknowledgments
Miss Yasmina M E Sahraoui from the University of Liverpool kindly revised this manuscript. Dr. Depicha Jindatip and Mrs. Vanida Buasorn kindly supported all histology cross sections and vascular endothelial staining.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Tansatit, T., Moon, HJ., Rungsawang, C. et al. Safe Planes for Injection Rhinoplasty: A Histological Analysis of Midline Longitudinal Sections of the Asian Nose. Aesth Plast Surg 40, 236–244 (2016). https://doi.org/10.1007/s00266-016-0621-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-016-0621-1