Abstract
Background
Dual-plane techniques offer excellent pocket locations for breast augmentation. Traditional techniques require incisions in the inframammary or periareolar crease, which are rarely accepted in the authors’ department because of visible scars on the breast. Therefore, the authors developed a transaxillary approach for dual-plane procedures using an endoscope.
Methods
During a period of 36 months between April 2009 and March 2012, 89 consecutive patients with small breasts were treated surgically. They underwent transaxillary types 2 or 3 dual-plane breast augmentation as outpatients. For the axillary endoscopic subglandular tunneling approach (AESTA), a long subglandular tunnel was created along the lateral portion of the pectoralis major muscle to reach the nipple–areolar complex. The type 2 dual-plane technique was applied in 67 patients, and the type 3 technique was used in 22 patients.
Results
The mean age of the patients was 37.5 years (range 31–48 years), and the mean postoperative follow-up period was 11 months (range 7–42 months). Good surgical outcomes were obtained, and the procedure was reproducible.
Conclusions
The use of AESTA allowed the authors to achieve types 2 and 3 dual-plane breast augmentation through a transaxillary incision. They believe that AESTA can yield constant and satisfactory outcomes similar to the inframammary and periareolar approaches.
Level of Evidence V
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dual-plane breast augmentation is a popular procedure that combines retromammary and partial retropectoral pocket locations. Types 2 and 3 dual-plane procedures, which are divided according to the extent of dissection at the parenchyma–muscle interface, differ from the conventional subpectoral plane procedures in that they precisely divide the origins of the pectoralis major muscle across the inframammary fold and dissect the subglandular material from the parenchyma–muscle interface [1]. These planes offer the advantages of subglandular and subpectoral breast augmentation. They also mitigate the inherent shortcomings of these methods [2].
However, types 2 and 3 dual-plane augmentations generally use incisions in the periareolar or inframammary crease, which leaves traces of surgery on the breast and may produce hypertrophic scars. Scars are of critical importance for Asian women who want to undergo breast surgery. They prefer the transaxillary approach despite the aesthetic advantages of dual-plane breast augmentation.
We used types 2 and 3 dual-plane procedures through the periareolar approach for 4 years because patients more frequently accept periareolar scars than inframammary scars. However, patients show a definite reluctance to undergo the periareolar approach because of unsightly scars. Therefore, we attempted types 2 and 3 dual-plane breast augmentation procedures through the transaxillary approach with the aid of endoscopy via subglandular tunneling. These procedures were successful.
Patients and Methods
We retrospectively reviewed the cases of 89 patients who underwent transaxillary endoscopic breast augmentation using type 2 or 3 dual-plane procedure performed by a single surgeon. A complete documented medical evaluation, including appropriate screening for preexisting breast disease and risk factors, was performed before the surgery. Only patients who underwent primary breast augmentation were included in the study.
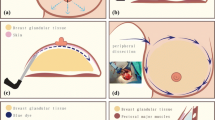
The type 2 dual-plane procedure was performed for patients with breasts that had highly mobile parenchyma. The type 3 dual-plane procedure was performed for patients with glandular ptosis and constricted lower pole breasts [2]. Preoperative surface landmarks including the lateral border of the pectoralis major muscle, a new inframammary fold, and the upper limit of the parenchyma–muscle interface were designed with the patient in the upright position (Fig. 1). In Fig. 1, the subglandular tunneling site is indicated between the red dots on lines C and D. Patients next were asked to raise both arms over the head with the hands clasping, which reflected the ideal position of the nipple and relative movement of the nipple–areolar complex after augmentation.
Preoperative design for axillary endoscopic subglandular tunneling. The red line represents a newly planned inframammary fold. Black line A is the lateral border of the pectoralis major muscle and corresponds to dotted line D. Black line B is the upper border of the separated parenchyma–muscle interface. Dotted lines C and D are parallel to each other. A subglandular tunnel is situated between the two dotted lines and is 2.5 cm wide in the subfascial plane. Dotted line D is the lateral aspect of the subglandular tunnel
Surgical Technique
The procedure was performed using general anesthesia, with antibiotic prophylaxis provided 30 min before the surgery. After protection of the nipples using a shield of DuoDERM Extra Thin (ConvaTec, Skillman, NJ, USA) or Tegaderm (3M Healthcare, Neuss, Germany), the patient was placed in the supine position with the arms abducted 90°. Mepivacaine 10 mL, which has fewer cardiac side effects than bupivacaine [3, 4], was administered to each breast for intercostal nerve block. A mixture including 200 mL of normal saline, 20 mL of mepivacaine, and 0.5 mL of epinephrine was injected into the planned dissection plane to reduce postoperative pain and decrease the anesthetic depth.
An appropriate 4-cm incision was made to overlap precisely the natural skin creases behind the anterior axillary fold. The lateral pectoral fascia was incised using electrocautery to identify the border of the pectoralis major muscle under the fascia. This procedure was performed under direct vision with the help of army–navy retractors, and a pocket was created using an endoscope to visualize the operative field. This direct vision throughout the procedure enables surgeons to keep a bloodless field, resulting in fewer complications. An 11-mm 10° operating endoscope (Richard Wolf, Knittlingen, Germany) and a 15-mm operating tube (Richard Wolf) were used to visualize the operative field.
With the aid of the endoscope, meticulous dissection was performed along the lateral portion of the pectoralis muscle to make a suitable subglandular tunnel in the subfascial plane as planned preoperatively (Fig. 2a). The dissection area was the space between lines C and D (Fig. 1). The lateral margin of the subglandular tunnel was the lateral border of the pectoralis major muscle, and the medial margin was parallel to the lateral margin. The width of the tunnel was about 2.5 cm.
Axillary endoscopic subglandular transaxillary tunneling. a The white dot represents a long retractor. The subglandular tunnel is created over the pectoralis major muscle. The width of the tunnel corresponds to the width of the retractor. b The tunnel reaches the nipple–areolar complex planned in line B shown in Fig. 1. The white dot represents the breast parenchyma
Subglandular tunneling was continued up to the nipple–areolar complex (Fig. 2b). Within the nipple–areolar complex, separation of the parenchyma–muscle interface was extended from the medial parenchyma to the lateral margin of the pectoralis major muscle up to a new inframammary fold as wide as initially planned (Fig. 3). The dissection limits around the nipple–areolar complex differed between the types 2 and 3 dual-plane procedures. With the type 2 dual-plane technique, the parenchyma–muscle interface was separated up to the inferior edge of the nipple–areolar complex.
Further dissection of the superior nipple–areolar complex was performed for the type 3 dual-plane procedure. Proper subglandular tunneling was followed by submuscular endoscopic dissection as in the conventional method (Fig. 4a). In the submuscular plane, the pectoralis major muscle was cut free from this medial point to the lateral muscle border, approximately at the 4–7 o’clock position in the right breast and at the 5–8 o’clock position in the left breast, with meticulous electrocauterization. During muscle detachment, caution must be observed to preserve the pectoralis fascia from the muscle, which was easily seen on the top of the endoscopic view after contraction of the cut pectoralis muscle (Fig. 4b).
a Submuscular dissection through the same axillary incision performed as in the conventional manner. The black dot is the pectoralis major muscle, and the white dot is the pectoralis minor muscle. b Detachment of the pectoralis major muscle origins. The black dot is an intact superficial pectoral fascia supporting the subglandular fat layer. The white dot represents the end of the contracted pectoralis major muscle after cutting
The pocket was irrigated with 500 mL of Adam’s solution [5] (a mixture of normal saline 1,000 mL, betadine 100 mL, cephazolin 1 g, and gentamicin 80 mg) through a soft-tipped catheter. In the next step, hemostasis was achieved with the aid of the endoscope and bipolar cauterization. Any restrictions caused by incomplete division of the pectoralis major muscle were released, and a new inframammary fold was assessed.
A round, textured silicone gel implant (Allergan, Irvine, CA, USA) was inserted via the transaxillary incision in all the patients. Successful placement of the implant was evaluated by endoscopy (Fig. 5). An additional adjustment of its position was possible by modifying the separation limits of the interface between the breast parenchyma and the pectoralis muscle.
a Endoscopic view of the subglandular tunnel after dissection for type 2 dual-plane breast augmentation. The subglandular fat layer is elevated with a retractor (black dot), and the contracted pectoralis major muscle is seen at the bottom (white dot). The rib and intercostal muscle are seen between subglandular fat and the cut pectoralis major muscle. c Implant insertion in type 2 dual-plane breast augmentation. b Endoscopic view of the subglandular tunnel after dissection for type 2 dual-plane breast augmentation. d Implant insertion in type 2 dual-plane breast augmentation. Because the implant is covered with muscle at a level above the nipple–areolar complex, it appears more buried in fat and muscle than in type 3 dual-plane breast augmentation
The incision wound was closed using 4-0 PDS for subcutaneous tissue and 6-0 nylon for skin. An indwelling suction drain with the valve closed was inserted, if needed, before compression dressing. An elastic compressive brassiere was placed after elastic plaster was applied on the axillary and upper and lower portions of the breast (Fig. 6). The catheter was maintained for 1–3 days with the valve open until the daily amount of drainage was less than 20 mL. On postoperative day 4, an elastic bandage was applied for 3 weeks instead of the previously used compressive brassiere and elastic plaster. All the patients were evaluated by pre- and postoperative photographic analysis and investigated regarding operative satisfaction.
Results
Between April 2009 and March 2012, 89 patients with small and ptotic breasts underwent transaxillary endoscopic dual-plane breast augmentation procedures in our department. The mean age of the patients was 37.5 years (range 31–48 years), and the mean postoperative follow-up period was 11 months (range 7–42 months). Altogether, 67 patients (75 %) underwent the type 2 dual-plane procedure, and 22 patients (25 %) underwent the type 3 dual-plane procedure. A round, textured, silicone gel implant (Allergan) was used in all cases. The implant size had a range of 225–290 mL (average 260 mL). The mean operating time was 130 min (range 100–180 min). All the patients returned to daily life activities within 1 week.
Complications occurred unilaterally for only five patients (5.6 %; Table 1). One patient who had unilateral Baker type 2 capsular contracture was treated by simple capsulotomy through an endoscopic approach with good results. A second patient, who experienced unilateral Baker type 3 capsular contracture, was almost completely improved by a change of the implant after a new space was made over the surface of its anterior capsule.
Three additional patients experienced hematoma 12–15 h after surgery. In each case, the hematoma was removed, and the pocket was sufficiently irrigated with Adam’s solution [5] on postoperative day 1. We found a pumping perforator below the nipple [6] in one patient and coagulated the perforator with a spatula-shaped endoscopic dissector. The bleeding was successfully controlled, and the patient recovered without any significant sequelae.
Of the 89 patients, 84 (94 %) without complications were satisfied with the aesthetic outcome, with no scars on the breast (Figs. 7, 8). Photographic analyses of all the patients were performed using standardized postoperative photographs.
Discussion
Endoscopic transaxillary breast augmentation procedures have been discussed in many articles [7–10]. The procedure was initially used for subglandular augmentation [10] but has been improved. It currently is applied to submuscular or subfascial breast augmentation [8, 9]. Various endoscopic procedures, such as the muscle-splitting technique [11] and type 2 dual-plane breast augmentation [12], have been attempted and have become practical.
Although endoscopic breast augmentation has some disadvantages (e.g., specific instruments are required, and the learning curve is steep), the procedure has been regarded as one of the most favorable and popular surgical options because it also has obvious advantages (e.g., it produces invisible scars, and meticulous dissection is possible). In particular, our patients want to avoid scars on their anterior chest, even though the scar is not easily recognized and no one would know that the breasts had been treated with surgery.
Another critical consideration is that creation of any scars on the breast still is taboo in our society. The axillary scar after application of the transaxillary approach also is visible, especially if there are wound-healing problems. We take great care when we suture the incision and manage the sutured site so that a hypertrophic scar is highly unlikely. Although the axillary scar is visible and permanent, it is sufficiently hidden to be inconspicuous.
For some breast surgeons, endoscopes may be an ancillary instrument in clinical practice. However, for Korean surgeons, endoscopes are a main device used particularly in transaxillary breast augmentation performed through any plane. In fact, the endoscopic technique may be challenging even for skilled surgeons performing dual-plane breast augmentation procedures because only type 1 dual-plane breast augmentation performed endoscopically has been feasible. Types 2 and 3 dual-plane breast augmentation procedures are difficult to perform endoscopically [2]. We have made an effort to dissect types 2 and 3 dual planes precisely through a transaxillary incision using the axillary endoscopic subglandular tunneling approach (AESTA).
With AESTA, the pectoralis fascia is cut, followed by extended dissection along the lateral portion of the pectoralis major muscle to create a subglandular tunnel at the level of the subfascial plane. Line B in Fig. 1 determines which of the dual plane procedures (type 2 or 3) will be used. Line B is located at the nipple level in the type 2 dual-plane procedure and at a level above the nipple in the type 3 dual-plane procedure. There are few anatomic alterations on the anterior surface of the pectoralis major muscle above the nipple because only a 2.5-cm-wide subglandular tunnel exists on the lateral portion of the pectoralis major muscle from the axilla to the nipple.
Surgeons should not dissect the lateral margin of the pectoralis major muscle at the point of superficial and deep pectoral fascia intermingling because preservation at this point can avoid excessive lateral elevation of the pectoralis major muscle after implant insertion. Release of the pectoralis major muscle origins and separation of the parenchyma–muscle interface trigger proximal contraction of the muscle. The distal portion of the pectoralis major muscle is proximally displaced due to contraction of the muscle, and the free end of the muscle tends to roll over due to the vector of force generated and adherence to tissue during implant insertion.
An additional adjustment after implant insertion is achieved by further separation of the parenchyma–muscle interface above the nipple. Surgeons also can evaluate the extent of dissection to determine whether the implant should be placed in the type 2 or 3 dual plane through endoscopy.
An 11-mm 10° endoscope presents an excellent operative field on the monitor sufficient for visualizing the whole pocket [13]. Although most surgeons regard types 2 and 3 dual-plane breast augmentation procedures through the transaxillary approach as impractical, the feasibility of these procedures was verified with our endoscopic technique. Although three of our patients experienced hematomas during our early learning stage (due to immature technical skills), all were detected 12–15 h after surgery and removed on postoperative day 1. In two cases, no active bleeding occurred, but in one case, the pumping perforator below the nipple [6] was a main bleeder. After that experience, close attention was given to coagulating the perforator during the endoscopic dissection. The patients with hematoma were followed for more than 1 year, and no capsular contracture occurred. Our results showed only two capsular contractures. The follow-up period was relatively short for reporting a reliable occurrence rate, so continuous follow-up assessment has been scheduled.
Conclusion
The AESTA technique was developed because most Asian women want to avoid inframammary and periareolar scars. The study results showed clearly that the dual-plane procedure through the inframammary approach is efficient. Types 2 and 3 dual-plane breast augmentation procedures also can be successfully performed through the transaxillary approach with the aid of endoscopy. Our technique allows surgeons to leave inconspicuous scars after types 2 and 3 dual-plane breast augmentation procedures.
References
Alpert BS, Lalonde DH (2008) MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg 121:1–7
Tebbetts JB (2001) Dual-plane breast augmentation: optimizing implant–soft tissue relationships in a wide range of breast types. Plast Reconstr Surg 107:1255–1272
Copeland SE, Ladd LA, Gu XQ, Mather LE (2008) The effect of general anesthesia on the central nervous and cardiovascular system toxicity of local anesthetics. Anesth Analg 106:1140–1149
Liu P, Feldman HS, Covino BM, Giasi R, Covino BG (1982) Acute cardiovascular toxicity of intravenous amide local anesthetics in anesthetized ventilated dogs. Anesth Analg 61:317–322
Adams WP Jr, Conner WC, Barton FE Jr, Rohrich RJ (2000) Optimizing breast pocket irrigation: an in vitro study and clinical implications. Plast Reconstr Surg 105:334–338
Tebbetts JB (2002) Achieving a predictable 24-h return to normal activities after breast augmentation: Part II. Patient preparation, refined surgical techniques, and instrumentation. Plast Reconstr Surg 109:293–305
Ho LC (1993) Endoscopic-assisted transaxillary augmentation mammaplasty. Br J Plast Surg 46:332–336
Serra-Renom J, Garrido MF, Yoon T (2005) Augmentation mammaplasty with anatomic soft, cohesive silicone implant using the transaxillary approach at a subfascial level with endoscopic assistance. Plast Reconstr Surg 116:640–645
Momeni A, Padron NT, Bannasch H, Borges J, Bjorn Stark G (2006) Endoscopic transaxillary subpectoral augmentation mammaplasty: a safe and predictable procedure. J Plast Reconstr Aesthet Surg 59:1076–1081
Villafane O, Garcia-Tutor E, Taggart I (2000) Endoscopic transaxillary subglandular breast augmentation using silicone gel textured implants. Aesthet Plast Surg 24:212–215
Stumpfle RL, Pereirs-Lima LF, Valiati AA, Mazzini GS (2012) Transaxillary muscle-splitting breast augmentation: experience with 160 cases. Aesthet Plast Surg 36:343–348
Luan J, Mu D, Mu L (2009) Transaxillary dual-plane augmentation mammaplasty: experience with 98 breasts. J Plast Reconstr Aesthet Surg 62:1459–1463
Tebbetts JB (2006) Axillary endoscopic breast augmentation: processes derived from a 28-year experience to optimize outcomes. Plast Reconstr Surg 118:53S–80S
Acknowledgments
This work was supported by a 2012 Inje University Research Grant.
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.H., Yoon, W.J. Axillary Endoscopic Subglandular Tunneling Approach for Types 2 and 3 Dual-Plane Breast Augmentation. Aesth Plast Surg 38, 521–527 (2014). https://doi.org/10.1007/s00266-014-0306-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-014-0306-6