Abstract
Ambotuy
Favoring males’ specific sexual signals, female preferences play a major role in frogs’ evolutionary process by selecting traits linked to those signals. However, the factors constraining and determining those preferences are scarcely explored in an evolutionary background. Here, through a phylogenetic comparative approach we check whether anuran species phylogenetic proximity and calling site predicts female preferences for dominant frequency and whether those preferences influence species sexual size dimorphism. Our hypotheses are as follows: 1) closer species have similar females’ preferences related to the dominant frequency of the partners’ calls; 2) the calling site influences sound propagation and consequently the preference of females for the dominant frequency of the males’ calls; and 3) the preference for calls with low dominant frequency influences the size of the males and consequent reduction of the biSased dimorphism for females. We did not find support for our hypotheses, neither for the influence of phylogenetic proximity nor for calling site determining these preferences. Moreover, female preferences did not impact on species sexual size dimorphism. Besides shedding light into our hypotheses, this study represents a considerable advance on evolutionary studies of female preferences in anura, which still lacks broad species comparative approaches. Furthermore, we suggest future studies to expand knowledge regarding frogs’ female preferences.
Significance statement
This study advances our comprehension of female preferences in frogs by investigating the factors that shape these preferences and their implications for species sexual size dimorphism. Utilizing phylogenetic comparative methods, an approach rarely used in the context of anuran female preferences, this study represents a significant step in applying broad comparative approaches in this field. Highlighting the complex nature of mate choice and its relationship to morphology, soundscape, and phylogeny, we present important insights into evolutionary hypotheses related to female preferences. Lastly, we provide advice on how future studies could further explore this topic in a broader comparative framework while also discussing the limitations of available data on anuran mating preferences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female preferences play a major role on animal’s sexual selection by determining mate choices based on sexual signals (Andersson and Simmons 2006). Favoring a specific trait variant, mating preferences might result in important evolutionary changes in male morphology, behavior, and on species extinction or adaptation in contexts of environmental change (Houde and Endler 1990; Ptacek 2000). Many studies have focused on hypotheses to explain evolution of specific mating preferences (e.g., good genes (Zahavi 1975), direct benefits (Williams 1966), and Fisher’s process (Fisher 1930)). However, more efforts are needed to understand how female choice acts on sexual selection to understand its role in species adaptation (Candolin and Heuschele 2008).
Anura is one of the most well-studied taxa in female preference research (Gerhardt and Huber 2002). Anuran females choose their mates based on several characteristics, such as territory quality (Wells 1977; Howard 1978), parental ability (Márquez 1993), morphology (Robertson 1986; Zhu et al. 2016), and specially by their acoustic features (Gerhardt and Huber 2002). Experimental playback approaches have already shown that females from several species might choose their mates based on call properties like dominant frequency, calling rate, and call duration (Gerhardt and Huber 2002; Bee 2008; Moreno-Gómez et al. 2015; Yu et al. 2020). Specifically, the dominant frequency (i.e., the frequency in the call where most energy is concentrated (Köhler et al. 2017)) is widely used by anuran females to choose their mates (Wollerman et al. 1998; Lardner and Lakim 2004; Zhu et al. 2016). Directly determined by males’ body size (Gingras et al. 2013a), this characteristic is used by several animals in their mate choice process, including anura (Fitch and Hauser 2003; Reby and McComb 2003).
As in other animal groups (Colleye et al. 2011; Arato and Fitch 2021), closely related anurans are known to have similar call characteristics, especially those related to morphological characters, such as dominant frequency (Hepp et al. 2017). Compliant with that, using phylogenetic comparative tools, studies have shown that phylogenetic close anurans share many of their call’s characteristics (Goicoechea et al. 2010; Gingras et al. 2013b). Furthermore, considering the idea that species have pre-existent preferences inherent to signal perception neurological aspects (Ryan and Rand 1993), this acoustic resemblance could also reflect on similar preferences between related species. Yet, this topic is still a gap in the evolutionary knowledge of anura.
Choosing a mate represents a cost in terms of predation risk, time, and energy (Milinski and Bakker 1992; Godin and Briggs 1996). Therefore, the capacity of females to correctly evaluate a mate plays a major role in the cost–benefit balance of mating decision processes (Wiley 1994). Acoustic signals in nature are subject to the influence of environmental factors (e.g., topography, air temperature, wind speed, or vegetation cover) that constrain the efficiency of signal transmission, affecting its quality and range (Wiley and Richards 1978). Accordingly, studies have explored how frog species microhabitat and calling site affect sound propagation (Goutte et al. 2016, 2018; Tonini et al. 2020). Calling from herbaceous vegetation or trees represents an advantage in terms of signal propagation, as those sites provide longer signal propagation distance due to their height while also facing few physical barriers and therefore, diminished excess attenuation (Marten and Marler 1977; Wiley and Richards 1978; Römer 1992). In contrast, acoustic signals emitted from the ground are more susceptible to signal attenuation, since part of the reflected signal energy is absorbed by the soil (Marten and Marler 1977; Forrest 1994). While signal reflection is better in water than in ground, attenuation is still dependent on water depth and on the interaction between water and water-surface, which increases signal attenuation for specific frequency windows (Forrest 1994). Moreover, sound production in water might be problematic as depth also affects species ability to float, limiting their vocal sac inflation, which impacts calls attractiveness (Halfwerk et al 2017). These constraints on sound production and propagation imply that frog females are subject to errors while interpretating signals coming from calling males (Forrest 1994; Bee and Micheyl 2008). Thus, females from species in which males typically call from disadvantageous sites in terms of signal transmission properties and production would receive a less reliable signal, not being able to correctly evaluate mates and exert their mating preferences.
Sexual selection can strongly influence sexual size dimorphism, by both increasing and decreasing differences in the size of the sexes (Andersson and Iwasa 1996). However, the relationships between sexual selection and sexual size dimorphism in anurans were only explored considering competition between males as a proxy for sexual selection (Shine 1979; Han and Fu 2013; Pincheira-Donoso et al. 2020). On the other hand, female choice is an alternative to explore the relationship between sexual selection and sexual size dimorphism, as a previous meta-analysis on call frequency and male body size preferences in anurans as observed that females, in general, prefer males that vocalize with a low dominant frequency, that indicate larger males (McLean et al. 2012). In this sense, as female choice can indirectly affect male size through the selection of specific call frequencies, this preference could affect sexual size dimorphism.
Despite the well-established knowledge on female preferences for call frequencies in anurans (Marquez 1995; Rosso et al. 2006; Schrode et al. 2012), large-scale approaches on their evolutionary determinants are still scarce. Therefore, assessing those relationships turns out to be a relevant matter in terms of ecological and evolutionary knowledge for anura. Thus, using phylogenetic comparative tools (Felsenstein 1985) we test the following hypotheses: (1) preference for call frequency is a phylogenetically conservative trait, and thus females of closely related species share similar preferences for dominant frequency; (2) male calling site influences sexual preferences, and thus females do not exhibit preferences for dominant frequency in species with males calling from poor sound propagation sites (i.e., water and ground in comparison to perched, see methodology); and (3) species in which females exhibit preferences for lower dominant frequency have sexual size dimorphism less skewed or skewed toward males.
Methods
Female frequency preference data
We retrieved female frequency preference (FFP) through a systematic literature search using Scopus and Web of Science database. The search included articles in English containing the following terms combination in their abstract, keywords or title: (Anura* OR frog* OR treefrog* OR toad* OR (all anuran families)) AND (“sexual select*” OR “mat* choic*” OR “mat* preference*” OR “female choic*” OR “female preference*” OR “assortative mat*” OR “mat* success” OR “reproductive success”). Our survey includes records published until January, 2022, starting from 1970 for Scopus and from 1945 for Web of Science. We identified three types of FFP in 30 species: preference for lower dominant frequencies, preference for average dominant frequencies, and absence of preference. In a few species (3 cases), three or more studies had divergent results regarding FFP (species), in those cases we considered the most consensual result between them.
Calling site data
We determined calling site using a previous classification by Tonini et al. (2020), that divides anuran species into three groups according to their preferred calling site: water (6), ground (16), and perched (8). For a few species of our FFP dataset which were not included in that classification (3), we searched scientific literature using Google Scholar and AmphibiaWeb platforms to classify their calling site.
Sexual size dimorphism data
To calculate sexual size dimorphism (SSD), we retrieved maximum body size (snout-vent length, SVL) for males and females from the Pincheira-Donoso et al. (2020) and additional literature search. Maximum SVL was used because it is indicative of the potential size of taxon with indeterminate growth, due to allometric limitations (Levy and Heald 2015). For duplicate species in the database, we used the highest SVL found. The SSD calculation was done as follows: ln (male maximum SVL/female maximum SVL). This proportion is common in literature, allowing a simple interpretation of values (Smith 1999). Thus, SSD is equal to zero in species with males and females of the same size, and takes negative values in species with larger females and positive values in species with larger males.
Comparative analyses
All our analytical procedures were based on 1000 phylogenetic trees for the amphibians and all 30 species in our data were matched on the Jetz and Pyron (2018) phylogeny. Species names of our data sources (above) were paired with those defined in the available phylogenies and nomenclature standardized accordingly.
To test phylogenetic patterns in FFP shape, we use the fit Discrete function, which is suitable for fitting models that involve discrete traits and phylogenetic trees. We tested all available evolutionary tree transformation models (Brownian, ACDC, lambda, kappa, delta, and white noise) and selected the one with the lowest average AICc (considering the 1000 phylogenetic trees) as the best evolutionary model for our data.
To test the relationship between FFP and calling site, we used phylogenetic GLM models, with the phyloGLM function, computing 100 bootstraps and using maximum penalized likelihood estimation. Here, we only used the presence or absence of FFP. This model was performed 1000 times (once for each phylogenetic tree) and we used the average coefficients to account for the phylogenetic uncertainty.
To test the relationship between SSD and FFP, we performed a phylogenetic linear model, with the “phylolm” function. FFP are categorized as lower dominant frequencies, preference for average dominant frequencies, and absence of preference. Here, we also use the average coefficients obtained from the 1000 trees. All analytic and phylogenetic procedures were performed in the software R v4.3.0 (R Core Team 2022), using the packages phytools (Revell 2012), phylolm (Tung Ho and Ané 2014), and geiger (Harmon et al. 2008).
Results
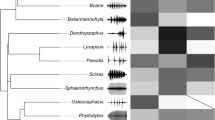
We obtained data for FFP for 30 species from 12 different families (see Supplementary Data). While almost half our species show preferences for lower values of dominant frequency (~ 53%), a considerable number prefer average values (20%) or do not show any preferences for call frequency (~ 27%). No species show preferences for higher values of dominant frequency. Figures 1, 2, and 3 present the distribution of studied traits (FFP, calling site, and SSD) mapped onto the phylogeny.
Species phylogenetic relationships and studied traits: female frequency preference (FFP), calling site and sexual size dimorphism (SSD), following one of the 1000 simulated trees by Jetz and Pyron (2018). Absence of dots represents data absence
Relationship between female frequency preference (FFP) and sexual size dimorphism (SSD) in anuran species. The box horizontal lines represent the interval between 25, 50 (median), and 75% percentiles. The vertical line represents the highest and lowest values excluding outliers (observations which fall outside by 1.5 times the interquartile range). Violin plots represent data distribution
Following the AICc criteria, the white-noise model had the best fit to the evolution of FFP in all our phylogenies (99.9% of trees; mean of AICc = 67.746; Table 1), coherent with Pagel’s lambda close to 0. As this model converts the tree into a star phylogeny, losing all traces of shared ancestry, we do not support the relation between phylogenetic proximity and FFP.
We do not find support for calling site influence on female frogs’ preference for dominant frequency (Table 2). Also, we find no effect of the FFP on species SSD (Table 2).
Discussion
Overview
Using phylogenetic comparative methods, we did not find support for our hypothesis that females from close related species share similar preferences for dominant frequency. Furthermore, we did not find support for the influence of calling site on determining these preferences. Finally, according to the data available, female preference did not seem to influence sexual size dimorphism. Besides shedding light into our hypotheses, this study also raises relevant questions regarding data availability, usage, and future research topics.
Phylogenetic signal
Contrary to our expectations, FFP is not a phylogenetically conserved trait across frogs’ evolutionary history according to the data available for female preferences in the study group. Female preference is probably a trait with high levels of phenotypic plasticity and might be affected by several factors, such as temperature, social aggregations size, and species interaction (Gerhardt 1978; Höbel and Gerhardt 2003; Tanner and Bee 2019). Thermal coupling, for example, occurs when in face of a temperature change, both the signals emitted by conspecifics and the preference are changed in the same direction (Gerhardt 1978). However, there are cases, as seen in Hyla cinerea (Schneider 1799), where only the signal or the preference is altered by temperature, which impairs the identification and evaluation of the signals emitted by conspecifics (Gerhardt and Mudry 1980). Considering background noise from chorus, females of Dendropsophus ebraccatus (Cope, 1874) do not exhibit preferences for dominant frequency when there is a high level of chorus noise (Wollerman and Wiley 2002). Furthermore, in sympatry, species interactions might modify female frequency preference to avoid hybridation (Márquez and Bosch 1997).
Males’ call frequencies are not the only characteristic driving females’ mating decisions. For example, female preferences for temporal characteristics like call rate and call duration have been documented for several anuran species (Bosch and Márquez 2005; Dawson and Ryan 2009; Richardson et al. 2010; Laird et al. 2016). Mate choice may also be related to characteristics that involve direct benefits for the female, such as males’ territories or nests (Wells 1977; Höbel 2000; da Rocha et al. 2018). It might also be related to the good gene model of female preference so that attributes such as color can indicate males that have higher genetic quality (Maan and Cummings 2009). Studies have shown that females of some frog species from our dataset also express preferences for male characteristics other than call frequency (Backwell and Passmore 1990; Bosch and Márquez 2001; Baugh and Ryan 2011; Richards-Zawacki et al. 2012). For instance, Oophaga pumilio (Schmidt, 1857) females choose their mates based on visual cues related to males’ colors (Summers et al. 1999; Maan and Cummings 2009). Furthermore, studies with Engystomops pustulosus (Cope, 1864) suggest that male attractiveness should be a multidimensional characteristic, in which females choose their mates based on the evaluation of several characteristics simultaneously and not exclusively through their dominant frequency (Ryan and Rand 2003; Baugh et al. 2008). The phenotypic plasticity of female preferences as well as the preference for other characteristics might explain why according to our results, closely related taxa do not share female frequency preferences. However, it is important to highlight that despite being a considerable effort in comparison to other studies in the area while embracing 30 species with described FFP, this represents only a very small sample of all anura diversity (i.e., approximately 7500 species (Frost 2020)).
Calling site
Females’ signal perception and interpretation might not be such a limiting and costly factor for mating choice as we expected. As predicted by sound propagation properties, specific calling sites might represent real challenges to signal transmission (Wiley and Richards 1978; Richards and Wiley 1980; Hardt and Benedict 2020). However, the acoustic adaptation hypothesis (Morton 1975; Wiley and Richards 1978) predicts that species’ acoustic signals should evolve to optimize their transmission in their habitats. For instance, while comparing species calling from water and non-floating ones, Muñoz et al. (2020) found that water species had lower dominant frequencies, an advantage in terms of signal propagation distance. Moreover, animals might develop behavioral strategies to minimize signal degradation like increasing their call amplitude and duration (Brumm and Slater 2006; Ey et al. 2009). Finally, receivers are also expected to evolve their cognitive systems in order to interpret those degraded signals (Naguib and Wiley 2001). Thus, if both senders and receivers are adapted to minimize the impact of acoustic signal degradation, females should be still able to exert and maintain their preferences regardless of environment signal propagation constraints.
In contrast with FFP, species calling site data is a lot more representative (i.e., Tonini et al. (2020) database classifies 2176 species according to their calling sites). However, we should consider that using different scales on habitat classification may represent different aspects of signal propagation. For instance, Zimmerman (1983) tests the effect of habitat on frogs’ acoustic signal evolution using a binary classification (open and closed habitats), while Goutte et al. (2018) uses a continuous measure of canopy cover to represent habitat propagation properties. While the calling site classification we use embrace several species, it is not necessarily the best representation of the environmental constraints on signal propagation and therefore female choosiness.
Female preferences and SSD
We found no evidence that female preference for call frequency influences SSD in anurans. Probably, other factors, such as parental care, fecundity selection, and different life history traits between sexes, act more intensely in the direction of SSD. For instance, it is known that the presence of parental care might decrease SSD in anurans (Han and Fu 2013) and larger female size is favored by fecundity selection, since larger females have greater fecundity (Han and Fu 2013; Nali et al. 2014). Considering that anurans have indeterminate growth, female anurans have greater longevity, age, and size compared to males, and much of the SSD variation in anurans can be explained by the age difference between the sexes (Monnet and Cherry 2002). Furthermore, even if female frequency preferences do not appear to play a role in SSD, other aspects related to sexual selection might play a role.
Concluding remarks
In this study we explored some of the evolutionary relationships determining and constraining female preferences for call frequency in anura. Moreover, we also explore the influence of those preferences on sexual size dimorphism. Through phylogenetic comparative methods we showed that, contrary to our expectations, related species do not share dominant frequency preferences and these preferences are not determined by species calling sites. Additionally, we found no effect of female preferences on SSD, which might indicate that other factors play a more important role than sexual selection in determining SSD.
Despite of the large dataset gathered for this study, which represent a considerable advance on evolutionary studies of female preferences in anura, data availability is still scarce and might be a limitation to identify robust patterns. Perhaps expanding the data on FFP to more species and using different scales of species calling habitat could lead to different results. In addition, other aspects of sexual selection must be explored to understand its role on SSD. Lastly, we recommend future studies to also explore other possibly relevant variables, such as species mating systems, operational sex ratio, and reproductive pattern.
Data availability
Data generated or analyzed during this study comes from a literature survey; therefore, in addition to the data used, we provide complete references for the studies from which they are derived in the supplementary data.
References
Andersson M, Iwasa Y (1996) Sexual selection. Trends Ecol Evol 11:53–58. https://doi.org/10.1016/0169-5347(96)81042-1
Andersson M, Simmons LW (2006) Sexual selection and mate choice. Trends Ecol Evol 21:296–302. https://doi.org/10.1016/j.tree.2006.03.015
Arato J, Fitch WT (2021) Phylogenetic signal in the vocalizations of vocal learning and vocal non-learning birds. Phil Trans R Soc B 376:20200241
Backwell PRY, Passmore NI (1990) Suitable approach perches affect female phonotaxis in an arboreal frog. Herpetologica 46:11–14
Baugh AT, Ryan MJ (2011) The relative value of call embellishment in túngara frogs. Behav Ecol Sociobiol 65:359–367. https://doi.org/10.1007/s00265-010-1053-6
Baugh AT, Akre KL, Ryan MJ (2008) Categorical perception of a natural, multivariate signal: mating call recognition in túngara frogs. P Natl Acad Sci USA 105:8985–8988
Bee MA (2008) Parallel female preferences for call duration in a diploid ancestor of an allotetraploid treefrog. Anim Behav 76:845–853. https://doi.org/10.1016/j.anbehav.2008.01.029
Bee MA, Micheyl C (2008) The cocktail party problem: what is it? How can it be solved? And why should animal behaviorists study it? J Comp Psychol 122:235–251. https://doi.org/10.1037/0735-7036.122.3.235
Bosch J, Márquez R (2001) Call timing in male-male acoustical interactions and female choice in the midwife toad Alytes obstetricans. Copeia 2001:169–177. https://doi.org/10.1643/0045-8511(2001)001[0169:ctimma]2.0.co;2
Bosch J, Márquez R (2005) Female preference intensities on different call characteristics and symmetry of preference above and below the mean in the Iberian midwife toad Alytes cisternasii. Ethology 111:323–333. https://doi.org/10.1111/j.1439-0310.2004.01058.x
Brumm H, Slater PJB (2006) Animals can vary signal amplitude with receiver distance: evidence from zebra finch song. Anim Behav 72:699–705. https://doi.org/10.1016/j.anbehav.2006.01.020
Candolin U, Heuschele J (2008) Is sexual selection beneficial during adaptation to environmental change? Trends Ecol Evol 23:446–452. https://doi.org/10.1016/j.tree.2008.04.008
Colleye O, Vandewalle P, Lanterbecq D, Lecchini D, Parmentier E (2011) Interspecific variation of calls in clownfishes: degree of similarity in closely related species. BMC Evol Biol 11:365. https://doi.org/10.1186/1471-2148-11-365
Cope ED (1864) Contributions to the herpetology of tropical America. Proc Acad Natl Sci Phila 16:166–181
Cope ED (1874) Description of some species of reptiles obtained by Dr. John F. Bransford, Assistant Surgeon United States Navy, while attached to the Nicaraguan Surveying Expedition in 1873. Proceedings of the Academy of Natural Sciences of Philadelphia 26:64–72
da Rocha SMC, Lima AP, Kaefer IL (2018) Territory size as a main driver of male-mating success in an Amazonian nurse frog (Allobates paleovarzensis, Dendrobatoidea). Acta Ethol 21:51–57. https://doi.org/10.1007/s10211-017-0280-5
Dawson B, Ryan MJ (2009) Early experience leads to changes in the advertisement calls of male Physalaemus pustulosus. Copeia 2009:221–226. https://doi.org/10.1643/CE-07-254
Ey E, Rahn C, Hammerschmidt K, Fischer J (2009) Wild female olive baboons adapt their grunt vocalizations to environmental conditions. Ethology 115:493–503
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford
Fitch WT, Hauser MD (2003) Unpacking “honesty”: vertebrate vocal production and evolution of acoustic signals. Acoust Commun 65–137. https://doi.org/10.1007/0-387-22762-8_3
Forrest TG (1994) From sender to receiver: propogation and environmental effects on acoustic signals. Am Zool 34:644–654. https://doi.org/10.1093/icb/34.6.644
Frost DR (2020) Amphibian species of the world: an online reference, version 6.0. In: American Museum of Natural History, New York, USA, http://research.amnh.org/vz/herpetology/amphibia/%5Cnhttp://research.amnh.org/herpetology/amphibia/index.html
Gerhardt HC (1978) Temperature coupling in the vocal communication system of the gray tree frog, Hyla versicolor. Science 199:992–994. https://doi.org/10.1126/science.199.4332.992
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. The University of Chicago Press, Chicago
Gerhardt HC, Mudry KM (1980) Temperature effects on frequency preferences and mating call frequencies in the green treefrog, Hyla cinerea (Anura: Hylidae). J Comp Physiol 137:1–6. https://doi.org/10.1007/BF00656911
Gingras B, Boeckle M, Herbst CT, Fitch WT (2013a) Call acoustics reflect body size across four clades of anurans. J Zool 289(2):143–150. https://doi.org/10.1111/j.1469-7998.2012.00973.x
Gingras B, Mohandesan E, Boko D, Fitch WT (2013b) Phylogenetic signal in the acoustic parameters of the advertisement calls of four clades of anurans. BMC Evol Biol 13:134. https://doi.org/10.1186/1471-2148-13-134
Godin J-GJ, Briggs SE (1996) Female mate choice under predation risk in the guppy. Anim Behav 51:117–130. https://doi.org/10.1006/anbe.1996.0010
Goicoechea N, De La Riva I, Padial JM (2010) Recovering phylogenetic signal from frog mating calls. Zool Scr 39:141–154. https://doi.org/10.1111/j.1463-6409.2009.00413.x
Goutte S, Dubois A, Howard SD, Marquez R, Rowley JJL, Dehling JM, Grandcolas P, Rongchuan X, Legendre F (2016) Environmental constraints and call evolution in torrent-dwelling frogs. Evolution 70:811–826. https://doi.org/10.1111/evo.12903
Goutte S, Dubois A, Howard SD, Márquez R, Rowley JJL, Dehling JM, Grandcolas P, Xiong RC, Legendre F (2018) How the environment shapes animal signals: a test of the acoustic adaptation hypothesis in frogs. J Evol Biol 31:148–158. https://doi.org/10.1111/jeb.13210
Halfwerk W, Smit JAH, Loning H, Lea AM, Geipel I, Ellers J, Ryan MJ (2017) Environmental conditions limit attractiveness of a complex sexual signal in the túngara frog. Nat Commun 8:1891. https://doi.org/10.1038/s41467-017-02067-1
Han X, Fu J (2013) Does life history shape sexual size dimorphism in anurans? A Comparative Analysis BMC Evol Biol 13:27. https://doi.org/10.1186/1471-2148-13-27
Hardt B, Benedict L (2020) Can you hear me now? A review of signal transmission and experimental evidence for the acoustic adaptation hypothesis. Bioacoustics 30:716–742. https://doi.org/10.1080/09524622.2020.1858448
Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W (2008) GEIGER: investigating evolutionary radiations. Bioinformatics 24:129–131. https://doi.org/10.1093/bioinformatics/btm538
Hepp F, Lourenço ACC, Pombal JP (2017) Bioacoustics of four Scinax species and a review of acoustic traits in the Scinax catharinae species group (Amphibia: Anura: Hylidae). Salamandra 53:212–230
Höbel G (2000) Reproductive ecology of Hyla rosenbergi in Costa Rica. Herpetologica 56:446–454
Höbel G, Gerhardt HC (2003) Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea). Evolution 57:894–904. https://doi.org/10.1111/j.0014-3820.2003.tb00300.x
Houde AE, Endler JA (1990) Correlated evolution of female mating preferences and male color patterns in the guppy Poecilia reticulata. Science 248:1405–1408. https://www.jstor.org/stable/2874454
Howard RD (1978) The evolution of mating strategies in bullfrogs, Rana catesbeiana. Evolution 32:850–871. https://doi.org/10.2307/2407499
Jetz W, Pyron RA (2018) The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life. Nat Ecol Evol 2:850–858. https://doi.org/10.1038/s41559-018-0515-5
Köhler J, Jansen M, Rodríguez A, Kok PJR, Toledo LF, Emmrich M, Glaw F, Haddad CFB, Rödel M-O, Vences M (2017) The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa 4251:1–124
Laird KL, Clements P, Hunter KL, Taylor RC (2016) Multimodal signaling improves mating success in the green tree frog (Hyla cinerea), but may not help small males. Behav Ecol Sociobiol 70:1517–1525. https://doi.org/10.1007/s00265-016-2160-9
Lardner B, Lakim MB (2004) Female call preferences in tree-hole frogs: why are there so many unattractive males? Anim Behav 68:265–272. https://doi.org/10.1016/j.anbehav.2004.05.003
Levy DL, Heald R (2015) Biological scaling problems and solutions in amphibians. CSH Perspect Biol 8:a019166. https://doi.org/10.1101/cshperspect.a019166
Maan ME, Cummings ME (2009) Sexual dimorphism and directional sexual selection on aposematic signals in a poison frog. P Natl Acad Sci USA 106:19072–19077. https://doi.org/10.1073/pnas.0903327106
Márquez R (1993) Male reproductive success in two midwife toads, Alytes obstetricans and A. cisternasii. Behav Ecol Sociobiol 32:283–291. https://doi.org/10.1007/BF00166518
Márquez R, Bosch J (1997) Male advertisement call and female preference in sympatric and allopatric midwife toads. Anim Behav 54:1333–1345. https://doi.org/10.1006/anbe.1997.0529
Marquez R (1995) Female choice in the midwife toads (Alytes obstetricans and A. cisternasii). Behaviour 91:1689–1699. https://doi.org/10.1017/CBO9781107415324.004
Marten K, Marler P (1977) Sound transmission and its significance for animal vocalization. I Temperate Habitats Behav Ecol Sociobiol 2:271–290. https://doi.org/10.1007/BF00299740
McLean MJ, Bishop PJ, Nakagawa S (2012) Male quality, signal reliability and female choice: assessing the expectations of inter-sexual selection. J Evol Biol 25:1513–1520. https://doi.org/10.1111/j.1420-9101.2012.02533.x
Milinski M, Bakker TCM (1992) Costs influence sequential mate choice in sticklebacks, Gasterosteus aculeatus. Proc R Soc Lond B 250:229–233. https://doi.org/10.1098/rspb.1992.0153
Monnet J-M, Cherry MI (2002) Sexual size dimorphism in anurans. Proc R Soc Lond B 269:2301–2307. https://doi.org/10.1098/rspb.2002.2170
Moreno-Gómez FN, Bacigalupe LD, Silva-Escobar AA, Soto-Gamboa M (2015) Female and male phonotactic responses and the potential effect of sexual selection on the advertisement calls of a frog. Anim Behav 104:79–86. https://doi.org/10.1016/j.anbehav.2015.03.010
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34
Muñoz MI, Goutte S, Ellers J, Halfwerk W (2020) Environmental and morphological constraints interact to drive the evolution of communication signals in frogs. bioRxiv. https://doi.org/10.1101/2020.04.18.047936
Naguib M, Wiley RH (2001) Estimating the distance to a source of sound: mechanisms and adaptations for long-range communication. Anim Behav 62:825–837. https://doi.org/10.1006/anbe.2001.1860
Nali RC, Zamudio KR, Haddad CFB, Prado CPA (2014) Size-dependent selective mechanisms on males and females and the evolution of sexual size dimorphism in frogs. Am Nat 184:727–740. https://doi.org/10.1086/678455
Peters WCH (1867) Herpetologische Notizen. Monatsberichte der Königlichen Preussische Akademie des Wissenschaften zu Berlin 1867:13–37
Pincheira-Donoso D, Harvey LP, Grattarola F, Jara M, Cotter SC, Tregenza T, Hodgson DJ (2020) The multiple origins of sexual size dimorphism in global amphibians. Glob Ecol Biogeogr 30:443–458. https://doi.org/10.1111/geb.13230
Ptacek MB (2000) The role of mating preferences in shaping interspecific divergence in mating signals in vertebrates. Behav Process 51:111–134. https://doi.org/10.1016/S0376-6357(00)00123-6
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Reby D, McComb K (2003) Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav 65:519–530. https://doi.org/10.1006/anbe.2003.2078
Revell LJ (2012) phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x
Richards DG, Wiley RH (1980) Reverberations and amplitude fluctuations in the propagation of sound in a forest: implications for animal communication. Am Nat 115:381–399
Richardson C, Joly P, Léna J-P, Plénet S, Lengagne T (2010) The challenge of finding a high-quality male: a treefrog solution based on female assessment of male calls. Behaviour 147:1737–1752. https://doi.org/10.1163/000579510X530221
Richards-Zawacki CL, Wang IJ, Summers K (2012) Mate choice and the genetic basis for colour variation in a polymorphic dart frog: inferences from a wild pedigree. Mol Ecol 21:3879–3892. https://doi.org/10.1111/j.1365-294X.2012.05644.x
Robertson JGM (1986) Female choice, male strategies and the role of vocalizations in the Australian frog Uperoleia rugosa. Anim Behav 34:773–784. https://doi.org/10.1016/S0003-3472(86)80061-6
Römer H (1992) Ecological constraints for the evolution of hearing and sound communication in insects. In: Webster DB, Popper AN, Fay RR (eds) The Evolutionary Biology of Hearing. Springer, New York
Rosso A, Castellano S, Giacoma C (2006) Preferences for call spectral properties in Hyla intermedia. Ethology 112:599–607. https://doi.org/10.1111/j.1439-0310.2005.01186.x
Ryan MJ, Rand AS (1993) Sexual selection and signal evolution: the ghost of biases past. Phil Trans R Soc B 340:187–195. https://doi.org/10.1098/rstb.1993.0057
Ryan MJ, Rand AS (2003) Sexual selection in female perceptual space: how female túngara frogs perceive and respond to complex population variation in acoustic mating signals. Evolution 57:2608–2618. https://doi.org/10.1111/j.0014-3820.2003.tb01503.x
Schmidt O (1857) Diagnosen neuer Frösche des zoologischen Cabinets zu Krakau. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Classe 24:10–15
Schneider JG (1799) Historia Amphibiorum Naturalis et Literarariae. Fasciculus Primus. Continens Ranas, Calamitas, Bufones, Salamandras et Hydros in Genera et Species Descriptos Notisque suis Distinctos. Jena: Friederici Frommanni
Schrode KM, Ward JL, Vélez A, Bee MA (2012) Female preferences for spectral call properties in the western genetic lineage of Cope’s gray treefrog (Hyla chrysoscelis). Behav Ecol Sociobiol 66:1595–1606. https://doi.org/10.1007/s00265-012-1413-5
Shine R (1979) Sexual selection and sexual dimorphism in the amphibia. Copeia 1979:297–306. https://doi.org/10.2307/1443418
Smith RJ (1999) Statistics of sexual size dimorphism. J Hum Evol 36:423–458. https://doi.org/10.1006/jhev.1998.0281
Summers K, Symula R, Clough M, Cronin T (1999) Visual mate choice in poison frogs. Proc R Soc Lond B 266:2141–2145
Tanner JC, Bee MA (2019) Within-individual variation in sexual displays: signal or noise? Behav Ecol 30:80–91. https://doi.org/10.1093/beheco/ary165
Tonini JFR, Provete DB, Maciel NM, Morais AR, Goutte S, Toledo LF, Pyron RA (2020) Allometric escape from acoustic constraints is rare for frog calls. Ecol Evol 10:3686–3695. https://doi.org/10.1002/ece3.6155
Tung Ho LS, Ané C (2014) A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst Biol 63:397–408. https://doi.org/10.1093/sysbio/syu005
Wells KD (1977) Territoriality and male mating success in the green frog (Rana clamitans). Ecology 58:750–762
Wiley HR (1994) Errors, exaggeration and deception in animal communication. In: Real L (ed) Behavioral mechanisms in ecology. University of Chicago Press, Chicago, pp 157–189
Wiley RH, Richards DG (1978) Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav Ecol Sociobiol 3:69–94
Williams GC (1966) Adaptation and natural selection. Princeton University Press, Princeton, NJ
Wollerman L, Wiley RH (2002) Background noise from a natural chorus alters female discrimination of male calls in a neotropical frog. Animal Behav 63:15–22. https://doi.org/10.1006/anbe.2001.1885
Wollerman L, Carolina N, Hill C (1998) Stabilizing and directional preferences of female Hyla ebraccata for calls. Anim Behav 55:1619–1630
Yu Y, Hu Y, Zhang Q, Zheng R, Shen B, Kong S, Li K (2020) Female preferences for call properties of giant spiny frog (Quasipaa spinosa). Pak J Zool 52:825–834. https://doi.org/10.17582/journal.pjz/20180503100508
Zahavi A (1975) Mate selection—a selection for a handicap. J Theor Biol 53:205–214. https://doi.org/10.1016/0022-5193(75)90111-3
Zhu B, Wang J, Zhao L, Sun Z, Brauth SE, Tang Y, Cui J (2016) Bigger is not always better: females prefer males of mean body size in Philautus odontotarsus. PLoS ONE 11:e0149879
Zimmerman BL (1983) A comparison of structural features of calls of open and forest habitat frog species in the Central Amazon. Herpetologica 39:235–246
Acknowledgements
We are thankful for the reviewers’ comments and feedback during the submission of this manuscript.
Funding
This work was supported by a Coordenação de Aperfeiçoamento de Pessoal e Nível Superior (CAPES) PhD scholarship to JVB and IM; Global Marie S. Curie Fellowship (EAVESTROP-661408 to DL) granted by the European Commission (Program H2020); Conselho Nacional de Desenvolvimento Científico (CNPq grant number 309894/2017–4 to RPB and 303467/2021–5 to JAFD-F); and Fundação de Âmparo a Pesquisa do Estação de Goiás/Conselho Nacional de Desenvolvimento Científico/Programa de Apoio aos Núcleos de Excelência (FAPEG/CNPq/PRONEX 201710267000507 to RPB). This work was developed in the context of the National Institutes for Science and Technology (INCT) in Ecology, Evolution, and Biodiversity Conservation (CNPq proc. 465610/2014–5 and FAPEG proc. 201810267000023).
Author information
Authors and Affiliations
Contributions
Conceptualization: JVB, IM, DL, and RPB; methodology: JVB, IM, and JAFD-F; formal analysis and investigation: JVB and IM; writing—original draft preparation: JVB and IM; writing—review and editing: JVB, IM, DL, JAFD-F, and RPB.
Corresponding author
Ethics declarations
Ethics approval
This study uses data from a literature survey and, therefore, does not involve any direct observation or manipulation of animals by the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by A. Taylor Baugh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bernardy, J.V., Melo, I., Llusia, D. et al. Female preferences for dominant frequency in frogs: constraints and impact on sexual size dimorphism. Behav Ecol Sociobiol 78, 4 (2024). https://doi.org/10.1007/s00265-023-03418-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03418-3