Abstract
Animals often halt foraging in the presence of predator cues, an ecological phenomenon known as the foraging/predator avoidance tradeoff. Although some have proposed that anxiety may exist in animals exposed to predator cues, few studies have examined whether such interactions lead to anxiety-like behavior in animals other than laboratory rodents and zebrafish. In this experiment, a foraging/predator avoidance tradeoff task was modified using adult male African clawed frogs (Xenopus laevis) and a looming visual predator stimulus to determine if (1) visual predator cues reduce appetitive behavior, (2) visual predator cues lead to predator avoidance behavior, and (3) if visual predator cues alter the abundance of transcripts in the optic tectum known to be modulated in other brain areas during anxiety. Frogs exposed to the predator stimulus did not reduce their food intake, although sweeping, a foraging behavior, was significantly reduced by the predator stimulus. Predator-exposed animals spent significantly more time stationary and entered the predator zone less compared controls. There were no statistically significant changes in relative transcript abundance of anxiety-related peptides between the groups in the optic tectum. Collectively, this tradeoff task was able to induce discrete avoidance and appetitive behaviors that are similar to anxiety-like behaviors observed in other predator avoidance models; however, the effects of visual threats on feeding and anxiety-related transcript abundance requires further study.
Significance statement
Halting foraging activities to increase vigilance and engage avoidance behaviors has been proposed to play an adaptive role in survival of predator encounters in a number of animal species. Some have proposed that anxiety evolved as a state associated with the engagement of avoidance and antipredator behavior. However, few studies have examined whether such foraging/predator avoidance tradeoffs result in anxiety-like behavior in animals other than rodents. We developed a foraging/predator avoidance tradeoff task in an aquatic frog species to determine if the sight of a looming visual threat interferes with feeding and causes anxiety-like behavior in an amphibian. Our data suggest that some, but not other (feeding, for example), behavioral aspects of foraging/predator avoidance tradeoffs mimic anxiety-like behavior when a visual threat is present. These data contribute to a growing body of literature indicating that anxiety may be an adaptive response to predator threats in non-mammalian species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals must make decisions rapidly in nature, often sacrificing one necessary activity for another in the interest of survival. Arguably, the best-studied ecological tradeoffs involve predation and its effects on feeding and/or reproduction (Harris and Carr 2016; Harris 2020; Schiwitz et al. 2020; Băncilă et al. 2021). Predator presence activates stress pathways in prey animals (Harris and Carr 2016) and can rapidly inhibit both foraging and reproduction (Narayan et al. 2013; Duggan et al. 2016; Harris and Carr 2016). While it is common to view anxiety in response to a predator as an adaptive state that prepares the animal for a confrontation and/or escape (Kandel 1983; Morris et al. 2019), there is little empirical evidence supporting an adaptive value of anxiety in terms of subsequent reproductive fitness and survival. However, there have been many studies on the comparative biology of anxiety (Cryan and Sweeney 2011; Kysil et al. 2017; de Abreu et al. 2020), and a reasonable conclusion to draw by inference is that selection pressures have maintained aspects of an anxiety state in most vertebrate groups.
In human studies, anxiety is commonly assessed using self-reporting tools such as the Hamilton Anxiety Rating Scale (Hamilton 1959) or Beck Anxiety Inventory (Beck et al. 1988) Neither of these self-reporting tools can be used with experimental animals, and there are no objective biomarkers that can be used to measure either state (temporary) or trait (pathological, enduring feature of an individual) anxiety state in animal models (Belzung and Griebel 2001). Thus, scientists often use behavioral tests, whose measures may reflect certain types of anxiety-like behavior, to identify so-called anxiogenic stimuli and to test anxiolytic drugs. There are many examples of behavioral assays employing ethologically relevant predators or predator cues (usually visual or olfactory) to elicit fear and/or anxiety-like behavior in controlled laboratory studies using a number of vertebrate model animals (mammals, Blanchard et al. 1990 Merali et al. 2003a; Almada and Coimbra 2015; Harris and Carr 2016; amphibians, Duggan et al. 2016; fishes, Cianca et al. 2013; Stewart et al. 2014). In a few studies, researchers have adapted these ethologically relevant tests of anxiety-like behavior to include an appetitive stimulus, usually food, thus mimicking ecologically relevant foraging/predator avoidance tradeoffs (Muller-Gass et al. 2000; Merali et al. 2003a, b; Duggan et al. 2016).
Laboratory-based food/predator tradeoff tasks for studying anxiety may appear similar in a way to the exploration–exploitation tradeoffs central to the plus minus maze and the light–dark test in rodents (Cryan and Holmes 2005; Campos et al. 2013) but have an important difference in that the food stimulus is to trigger movement. The inclusion of an appetitive stimulus in so-called anxiety tests may be especially important when studying anti-predator behavior in certain non-mammalian species (such as the amphibian, Xenopus laevis) that generally display low overall locomotor activity and may require a food stimulus to encourage them to explore a novel environment (Green 2009; Videlier et al. 2014; Duggan et al. 2016; Kelleher et al. 2018; Prater et al. 2018b). While several anxiety tests have been developed to study anxiety behavior, one particular test of interest for aquatic vertebrates is the overhead looming stimulus test (Cianca et al. 2013), in particular because the adverse behaviors exhibited in this test using zebrafish (Danio rerio) can be reversed by canonical anxiolytics (Cianca et al. 2013).
The optic tectum (an evolutionary homolog of the superior colliculus in mammals) plays a critical role in the behavioral decision making that underlies avoidance and approach behavior. Imaging (Javanmard et al. 1999; Cornwell et al. 2012; Kessler et al. 2012), physiological (de Almeida et al. 2006; Baek et al. 2019), and pharmacological studies (Azevedo et al. 2020) indicate that this brain area is more involved in post-limbic processing of a variety of anxiogenic stimuli than previously thought (Forcelli et al. 2016) and may play a central role in post-traumatic stress disorder (Steuwe et al. 2015; McFadyen et al. 2019). Despite growing evidence for a role of the optic tectum/superior colliculus in mediating anxiety-like behavior, little is known about the tectal neuromodulators and neurotransmitters involved in such control, especially in relation to a predator. Muthuraju et al. (2016) reported that intra-superior collicular injections of a dopamine D2 receptor antagonist in rodents altered fear responses in the elevated plus maze, while Azevedo et al. (2020) reported that the putative anxiolytic drug ACH-000029 reversed stressor-induced changes in c-fos immunoreactivity in the superior colliculus, among other brain areas (Muthuraju et al. 2016; Azevedo et al. 2020). Nothing is known about changes in gene or protein expression in the tectum after exposure to a predator.
Exposure to olfactory predator cues increased latency to approach food in rodents (Merali et al. 2003a, b). Latency to approach food in response to a predator has been assumed to reflect an increase in anxiety, as this increased latency can be attenuated using canonical anxiolytics such as diazepam, chlordiazepoxide, propranolol, and desmethylimipramine (Merali et al. 2003a, b). Recently, Duggan et al. (2016) reported increased latency to approach food, reduced feeding duration, and reduced food intake in a predator avoidance test using juvenile frogs (X. laevis). Decreased entries into and reduced time spent in anxiogenic areas are classic hallmarks of anxiety-like behavior (Liu et al. 2007; Gupta et al. 2014; Serova et al. 2014). Another common response to a predatory stimulus is reduced swimming behavior to avoid detection (Bencan et al. 2009). Anxiolytics can reverse these behaviors, increasing swimming behavior overall and increasing entrances into and time spent in anxiogenic areas (Keers et al. 2012). In the present study, we report on the development of a predator foraging/predator avoidance tradeoff test in sexually mature frogs which, because of their size, are more suitable for analysis of biochemical changes in the tectum associated with ecologically relevant approach and avoidance decision-making. We used a looming predator stimulus, which is ecologically relevant for this species as they are preyed on by a variety of waterfowl (Loveridge 1953; Kopij 1996, 1998). Moreover, looming stimuli specifically activate defensive behaviors initiated in the tectum (Wu et al. 2005; Liu et al. 2011; Huang et al. 2017; Lischinsky and Lin 2019; Zhou et al. 2019). In addition to measuring a suite of approach and avoidance behaviors related to feeding and anxiety in other models, we examined the abundance of transcripts for key anxiety-regulatory genes in the optic tectum. We predicted that there were no significant differences between treatment groups for any of the appetitive and avoidance behaviors measured prior to predator exposure and that exposure to a looming predator would (a) increase the latency to contact food and reduce food intake; (b) decrease swimming behavior, entrances, and time spent in anxiogenic areas; and (c) alter the abundance of anxiety-related gene transcripts in the tectum.
Methods
Animals and care
Adult male Xenopus laevis frogs (n = 23) were obtained commercially (Xenopus Express, Inc., Brooksville, FL, USA). Frogs were maintained in flow through dechlorinated tap water in fiberglass 300-L tanks (178 cm L × 46 cm W × 51 cm D) at a stocking density of 30 frogs per tank and at a temperature of 23 ± 3 °C. Frogs were fed NASCO floating frog brittle three times a week during their acclimation period (7 days). Prior to the test, they were food deprived for an additional 7 d. Frogs were maintained on a 12-h light:12-h dark cycle, and all experiments were conducted in the dark phase under red light. The luminosity in the frog room during the light phase was approximately 350 lx and during testing was approximately 20 lx inside the tank during the dark phase.
Tradeoff task
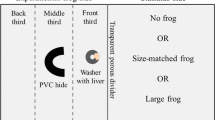
All lighting and temperature conditions were kept consistent with the acclimation phase. Forty-eight hours prior to the tradeoff task, frogs were individually isolated in glass aquaria (30.5 cm × 15.2 cm × 20.3 cm, 38 L) containing 3 L deionized water with 0.33 g/L Instant Ocean (Fig. 1). There were no differences (p = 0.5706, U = 42) in body mass between frogs assigned to the control group (38.3 g ± 4.93 g S.E.M.) versus the experimental (predator treatment) group (33.3 g ± 4.66 g). Twenty-four hours before the task, frogs were moved to the test arena, a glass aquarium (91.4 cm × 30.5 cm × 40.6 cm, 114 L) containing 13 L deionized water with 0.33 g/L Instant Ocean (Fig. 1). The sides of the test arena were covered with black plastic to avoid any possible light interference.
A schematic of the test arena for the tradeoff task. The task was recorded using a camera suspended above the tank. Experiments were conducted in the dark with red night vision lights for recording. The safe and predator areas of the tank were not physically separated, and frogs could swim between both areas. The safe side contained a PVC pipe “hide,” and the predator side contained a food (for both absence and presence of the predator groups). The hide and the food were located 12.7 cm from the ends of the safe and predator sides, respectively. The predator apparatus was located 47 cm from the bottom of the tank and the recording camera was located 58 cm from the bottom of the tank. Figure created by Wendy Beth Jackelow Medical and Scientific Illustration
The test arena was divided into two areas of equal size, a predator zone that consisted of the area directly below the predator stimulus (described below) and the safe zone consisted of the area around the hide, with no physical barrier between the two divisions (Fig. 1). A hide (PVC pipe, 9 cm × 8.3 cm diameter) was placed in the middle of the safe zone also equidistant from the sides of the tank (Fig. 1).
The tradeoff task consisted of three timed periods: a 10-min period with no food or predator stimulus (time period A), a 30-min period with food in the presence or absence of a visual predator stimulus (time period B), and a 10-min period with fresh food but no visual predator stimulus (time period C, to detect any effects on food intake with a longer latency) (Fig. 2). Frogs received approximately 3.5 g fresh chicken liver (Pilgrim’s Pride Corporation, Greenly, CO) as the food stimulus attached to a metal washer (1.9 cm × 5.08 cm) during the time periods B and C. The metal washer prevented the food from floating away from the predator zone during the tradeoff task.
Timeline of the tradeoff task. Animals were first isolated from the group housing 48 h before the testing began. The animals were moved to the test arena for 24 h prior to the test for acclimation. We recorded three simultaneous time periods on the test day: time period A (no stimulus, 10 min), time period B (absence/presence of predator and food, 30 min), and time period C (new food, no predator, 10 min). After time period C frogs were anesthetized, euthanized, and tissue was collected for RNA extraction
The looming predator stimulus (a preserved common snipe, Gallinago gallinago) was mounted on a 32-cm-long wooden dowel connected to a servomotor (Hitec, SubMicroServoU, USA) allowing the head to pivot 90° back and forth every 30 s over the predator zone of the test arena (Fig. 1). The common snipe was chosen because it is a predatory wading bird with a sharp pointed bill that is similarly featured to that of cormorant species indigenous to South Africa and known to prey on X. laevis (Kopij 1996, 1998). The pivoting motion provided a purely visual stimulus that was controlled using a microcontroller (Arduino, Duemilanove, Italy). A similar apparatus was used to measure anxiety-related behavior in zebrafish by Cianca et al. (2013).
Two groups of frogs were tested using the tradeoff task: frogs that were placed in the testing arena with food but no looming predator stimulus (control, n = 10) and a separate group of frogs exposed to food and the looming predator stimulus (predator-exposed group, n = 10). Additional frogs (n = 3) were added to the predator-exposed group to replace frogs that had displayed atypical sedentary behavior (did not move for over two minutes into the recording). A control group with no food or looming predator stimulus was not included due to studies that have revealed a lack of sufficient exploratory locomotion in anurans without an appropriate food stimulus (Avila and Frye 1978; Claas and Dean 2006). For both groups, the behavioral test was continuously recorded and conducted in the dark with two Energizer Vision HD + © red light night vision headlamps placed diagonally above the test tank between 15:00 h and 18:00 h.
For the control animals, the predator zone was identical to the safe zone but the visual predator stimulus did not operate (Fig. 2). Behavior was recorded using a 12-megapixel Samsung Galaxy S9 phone camera recording at 720 P that was located above the tank.
Behavioral analysis
Behavior was scored using JWatcher (Macquarie University, http://galliform.bhs.mq.edu.au/jwatcher/) software as per the accompanying handbook’s instructions (Blumstein and Daniel 2007). All videos were assigned a randomized code, and behavioral scoring was conducted after the live portion of the experiment was over. Behavior was analyzed using an ethogram based on previous literature with modifications (Duggan et al. 2016) (Table 1). Behavioral data was double-blinded and analyzed by three separate individuals without observation from others, and the scoring methods between the observers were correlated using an inter-rater agreement calculated on JWatcher (inter-rater agreement % ≥ 0.84). The distance measurements (entrances and time spent in each zone) were measured manually with a stopwatch. All behavioral measurements were determined per millisecond in the tank or per millisecond of total movement as appropriate.
Tissue collection
Animals were weighed immediately after the behavioral test and anesthetized in 0.5% MS-222 (with equal parts NaHCO3) and euthanized by decapitation. Brains were excised and placed in RNAlater© in a Petri dish, and the optic tecta removed by dissection. Tectal sections were frozen in 10 volumes of RNAlater© in a RNAse free 1.5-mL tubes at − 20 °C overnight and stored at − 80 °C long term.
RNA extraction
Tissue stored frozen in RNAlater was thawed on ice and RNA was extracted using RNAqueous-4PCR DNA-free RNA Isolation for RT-PCR kits (Thermo Fisher) as described previously (Prater et al. 2018a). This included a DNAase step to eliminate genomic DNA. Murine RNase inhibitor (New England Biolabs, 1 unit/1 mL) was added to the freshly extracted RNA and the RNA stored at − 80 °C. RNA quality and concentration were determined using an Experion ™ Automated Electrophoresis System (Bio-Rad Laboratories, Inc.) and Experion™ RNA StdSens Reagents and RNA StdSens Chips (Bio-Rad Laboratories, Inc.). RNA samples with an RNA quality indicator (RQI) > 7 were then reverse transcribed to cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). RNA samples with RQI < 7 were eliminated from analysis. The thermal cycler was set at 25 °C for 10 min, then 37 °C for 120 min, and was completed at 85 °C for 5 min. A control RNA group treated with the High Capacity cDNA Reverse Transcription Kit but not reverse transcriptase was checked by qRT-PCR for a tenfold difference. cDNA was stored at − 20 °C.
Primer design and quantitative real time PCR (qRT-PCR) assay
Primers were constructed for serotonin-1A receptor (5-HT1A) (htr1a), dopamine D1 (drd1), and D2 receptor transcripts (drd2) using PrimerBlast (Table 2). Primers for ribosomal protein L8 (rpl8) and corticotropin releasing factor (crf) were acquired from previous literature (Boorse and Denver 2006; Carr et al. 2013). Rpl8 was used as a reference gene since it has been shown it has a constant and ubiquitous expression in X. laevis (Shi and Liang 1994). GenBank Accession Numbers for the rpl8, crf, htr1a, drd1, and drd2 are as follows U00920.1, S50096.1, NM_001085830.1, XM_018251699, NM_001101742. These genes were selected due to their established role in preclinical models of anxiety and depression (htr1a, Samuels et al. 2016; Mohammad et al. 2016; drd1 and drd2, especially D1-D2 heterodimers, Shen et al. 2015; crf, Tafet and Nemeroff 2020).
Primer and template concentrations were measured using a Nanodrop ND-1000 spectrophotometer. Polymerase chain reaction (PCR) reactions were carried out on 96-well optical plates (Applied Biosystems, Grand Island, NY), consisting of 1 µl diluted cDNA template (200 ng cDNA), 1 µl of forward primer (200 nM), 1 µl of reverse primer (200 nM), 12.5 μl of SYBR green PCR master mix, and nuclease-free water for a total volume of 25 μl. Non-template controls included all other reagents except cDNA templates, which were replaced with nuclease-free water instead. Plates were then centrifuged and loaded onto a CFX96 RT-PCR detection system (Bio-Rad). Amplification efficiency for the primer sets were determined using a tenfold serial dilution (300, 30, 3, 0.3, and 0.03 ng) of the template and calculating the slope of the regression plotting Ct values against the log of the template amount. The equation E = 10(-1/slope) was used to determine the amplification efficiency, of which only primers with above 98% amplification efficiency were used. Cycle threshold values were normalized using the rpl8 reference gene and expressed as a percentage of control values using the ΔΔCt method. The identity of the PCR products was confirmed using gel electrophoresis after RT-PCR and sequencing. There were no changes in rpl8 transcript abundance as a function of treatment group (control, predator-exposed). Cycle threshold averages of 32 and above were not analyzed.
Statistical analysis
The independent variables tested were food intake (periods B and C only, mass consumed/body mass), latency to move, latency to contact food, sweeping, time in contact with food (B and C only), time spent in safe zone, number of entrances to predator zone, percent time swimming toward the safe zone, percent time swimming toward sides, percent time swimming toward predator zone, percent time inactive, and the relative Ct values for the transcript analysis. For all variables except sweeping, directional swimming and the transcript analysis, data were corrected by the exact time in the test period. Directional swimming-dependent variables (toward predator zone, toward safe zone) were divided by total time swimming.
The statistical approach was guided by two broad questions. First, were there initial differences in each independent variable between treatment groups in test period A, before the food looming predator stimulus were introduced? Second, were there treatment (predator, no predator) or time effect (periods B and C) differences in the independent variables? Thus, our general approach was to test for differences between treatment groups in period A using Students two-tailed t-test or the Mann–Whitney test to address the first question. Data for periods B and C were initially analyzed using mixed model analysis of variance (ANOVA) with time as a within-subjects variable and treatment as a between-subjects variable. Data failing to meet the assumptions for parametric tests were transformed using log10 or square root transformations. If these data did not meet parametric requirements, they were analyzed individually by test period using the Kruskal–Wallis H test or the Mann–Whitney test. Effect size is reported for significant results, Cohen’s d test was used to calculate effect size for t-tests (https://www.socscistatistics.com/effectsize/default3.aspx), epsilon squared (\(\varepsilon^{2}\)) was used as an effect size for the Kruskal–Wallis H test (Tomczak and Tomczak 2014), and eta squared (\({\upeta }^{2}\)) for mixed model ANOVA. A summary of the test results for all dependent variables is shown in Supplemental Tables S1–S3. All statistical analyses and graphing were performed with SPSS (v. 25, SPSS Inc., Chicago, IL) and MATLAB (R2020b, MathWorks).
Results
Pretrial baseline activity during test period A
There were no significant differences in any of the behaviors measured between control and predator exposed frogs during period A (Table S1).
The effects of a looming predator stimulus on food consumption
Almost none of the animals ate in period C, therefore we report data analyzed for period B only. Six of ten frogs in the control group consumed some amount of food, and three of ten animals in the predator group consumed food during test period B. Mass of food consumed corrected for body mass data were not distributed normally and transformation by log10 or square root failed to improve this. Overall, there was no statistically significant (U = 39.50, p = 0.436) difference in food intake between control and predator-exposed frogs during test period B.
The effects of a looming predator stimulus on behavior during test periods B and C
Only three out of twenty animals in both treatment levels that contacted food during period C, so we analyzed data for time in contact with food and latency to contact food for period B only. Time spent in contact with food was not different between treatment groups in period B (U(18) = 35.00, p = 0.280) nor was latency to contact food (U(18) = 30.00, p = 0.143).
Untransformed data on sweeps were not normally distributed and did not meet Levene’s test for equal variances, and this was not remedied by transformation. Kruskal–Wallis H tests showed that predator-exposed frogs exhibited significantly fewer forearm sweeps compared to control frogs during test period B (\(\chi^{2}\)(1) = 5.865, p = 0.015, \(\varepsilon^{2}\) = 0.310, Fig. 3A) but not period C (\(\chi^{2}\)(1)=3.180, p = 0.075). There were no differences between treatment levels in period A (Mann–Whitney, U = 43.50, p = 0.631).
Differences in forelimb sweeps (A), entrances into the predator side (B), total time swimming (C), and time inactive (D) in adult male X. laevis in the absence or presence of a predator across all three test periods. Asterisk indicates a statistically significant difference between predator and non-predator condition at that time point. Bars represent the mean ± S.E.M. of 9–10 animals per group. Asterisk indicates predator-exposed group is significantly different from control group, p < 0.05
Frogs in the predator-exposed group spent significantly less total time spent swimming during time period B (corrected for total time, χ2(1) = 14.29, p < 0.001, \(\varepsilon^{2}\) = 0.752) (Fig. 3B) but not during period C (χ2(1) = 0.006, p = 0.940). There were no initial differences between treatment levels in total time spent swimming during test period A (U = 30.50, p = 0.143).
Data for entrances into the predator zone did not meet the assumption for parametric testing even after transformation. Entrances into the predator zone were significantly lower in the predator exposure group during time period B (corrected for total time, χ2(1) = 12.62, p < 0.001, \(\varepsilon^{2}\) = 0.660) (Fig. 3C) but not during period C (χ2(1) = 1.755, p = 0.185). There were no initial differences between treatment levels in entrances to the predator zone during test period A (t(18) = 0.478, p = 0.638).
Frogs in the predator group spent more time inactive relative to controls during period B (χ2(1) = 11.57, p < 0.001, \(\varepsilon^{2}\) = 0.609) (Fig. 3D) but not during period C (χ2(1) = 0.28, p = 0.597). There were no differences between the control and treatment groups during period A (t(18) = − 0.236, p = 0.816).
Time spent in the safe zone data were distributed normally across all three test periods and were analyzed across all three test periods using a mixed model ANOVA with time (A, B, C) as a within-subjects’ factor and treatment (predator, control) as a between-subjects’ factor. There was a significant main effect of time (F2/36 = 3.388, p = 0.045, \(\eta^{2}\) = 0.158) but not treatment (F1/18 = 0.249, p = 0.624, \(\eta^{2}\) = 0.014), and there was not a significant interaction (F2/36 = 1.139, p = 0.331, \(\eta^{2}\) = 0.060) between the two independent variables. All frogs spent significantly more time in the safe zone during period C versus period A as determined by pairwise comparisons (p = 0.028, least significant difference test). There were no differences in any of the directional movement measures (time spent swimming toward the predator zone or the safe zone). Time spent swimming toward the predator zone was analyzed using the Kruskal–Wallis H test as the data failed tests for normality. There was no effect of treatment on time spent swimming to the predator zone during periods B (\(\chi^{2}\)(1) = 0.463, p =0.496) or C (\(\chi^{2}\)(1) = 3.023, p = 0.082). There were no significant main effects (time, F1/36 = 1.016, p = 0.372; treatment, F1/18 = 3.365, p = 0.073) or interaction (F2/36 = 1.827, p = 0.176) on time spent swimming toward the safe zone as determined by mixed model analysis.
Data on latency to move failed normality and equal variance tests even after transformation. There were no effects of treatment during either period B (\(\chi^{2}\)(1) = 1.463, p = 0.226) or period C (\(\chi^{2}\)(1) = 1.287, p = 0.257).
The effects of a looming predator stimulus on relative transcript abundance in the optic tectum
There were no significant changes in the relative abundances of transcripts for crf (t(10) = 0.8466, p = 0.417), drd1 (t(11) = 0.6756, p = 0.5132), drd2 (t(11) = 0.5984, p = 0.5617) and htr1a (t(11) = 1.913, p = 0.0821) in the tecta of frogs exposed to a predator stimulus vs controls (Fig. 4).
Differences in transcript abundance of A corticotropin releasing factor (crf), B dopamine D1 receptor (drd1), C dopamine D2 receptor (drd2), and D serotonin 1a receptor (htr1a) relative to the reference gene, ribosomal protein L8 (rpl8) in the optic tecta of control and predator-exposed X. laevis. Data are represented as mean ± S.E.M. of n = 6–7 animals
Discussion
We modified a foraging/predator avoidance tradeoff task (Duggan et al. 2016) for adult X. laevis in an attempt to study anxiety-like behaviors in this species. Compared to controls, frogs exposed to the predator stimulus (1) reduced forelimb sweeping, (2) spent less time swimming, and (3) reduced travel to the predator side of the tank. There was no change in relative abundance of anxiety-related transcripts in the optic tecta of animals exposed to the predator stimulus compared to control animals. Overall, while the predator stimulus modified discrete aspects of appetitive and approach behavior, adult frogs did not eat less in response to the stimulus. This is interesting given that juvenile X. laevis consistently reduce food intake when exposed to a live predator (Duggan et al. 2016; Islam et al. 2019; Prater et al. 2020). Without more testing, it is unclear if the difference is related to the age, sexual maturity, or size in the test animals or a live predator versus a predator cue(s). Age-dependent difference in anti-predator behavior have been observed in marmots (Lea and Blumstein 2011). Lea and Blumstein (2011) suggest that what drives such differences are actually difference in vulnerability at different life history stages, i.e., because juvenile marmots are inherently at higher risk of predation that would be expected to exhibit greater anti-predator behavior.
Adult X. laevis are adept at capturing immobile or weakly swimming prey by lunging with an open mouth or by creating a partial vacuum using the forelimbs to draw prey into their mouth and then using their hind limbs to tear apart larger chunks of food into smaller manageable portions once captured (Avila and Frye 1978). Previous studies have termed this forelimb movement “scooping or sweeping” behavior, which when combined with lunging to grab the food with their jaws, define basic prey capture behavior in X. laevis (Gray et al. 1997). Predator exposure decreased sweeping behavior in our study, thus reducing movement and likely reducing the risk of detection by predators (Takahara et al. 2012).
Frogs swam less and reduced their entrances into the predator side of the tank in the presence of a predator, possibly as a mechanism to avoid detection (Narayan et al. 2013; Kysil et al. 2017). Remaining stationary is a common behavioral response seen in animals when exposed to an anxiogenic stimuli (Lang et al. 1998; Sayin et al. 2004). Decreasing movement likely reduces the chances of predation as a stationary target in the dark would be harder to detect than one that is constantly moving. Even when predator-exposed animals were moving, they tended to move around in the safe side, or into the safe side, avoiding entering the area below the predator apparatus. This is a common behavior in several anxiety tests involving mice or zebrafish where anxiogenic areas will be avoided or the animals remains as far as possible from those areas (Cianca et al. 2013; Kysil et al. 2017). The optic tectum may play a role in determining anxiogenic versus safe regions in the tank (Dong et al. 2009). In this study, we did not want to stimulate tonic immobility, that is an inability to move in response to an extremely fearful stimulus (Perusini and Fanselow 2015). The fact that there was still some movement during time period B during the active predator exposure, and a return to normal movement in time period C when no predator was present, indicates that tonic immobility did not occur.
In this study, we also noted that the predator-exposed animals did not spend more time in the safe zone of the tank, a common indicator of anxiety in animal studies (Blaser and Rosemberg 2012; Smith et al. 2012; Bourin 2015). This may be due to two facets of frog behavior, their lack of appetitive behavior (which has previously been discussed) and their naturally sedentary behavior coupled with a lack of exploratory behavior (Videlier et al. 2015; Coleman et al. 2019). The primary defensive response of anurans is inactivity, with active defensive movement only being triggered after contact (Videlier et al. 2014). This resulted in animals exposed to the predator stimulus remaining stationary in the side of the tank they were located at the beginning of time period B. We did not move the frogs to a set starting position during the beginning of the test in order to reduce our interference with the animal and to eliminate an additional possible stressor. During predator exposure, frogs tended to remain motionless in the area where they were when the test started, rather than move to the “safe” side to further avoid predation. Animals were provided with a hide, which was large enough to accommodate the frogs, however it was not used often by the frogs in this study, further reinforcing that inactivity is a more prevalent defensive response in frogs compared to hiding or movement away from a predator (Duggan et al. 2016).
There were no significant changes in relative transcript abundance of anxiety-related transcripts in frogs exposed to the predator stimuli. This may be due to the brevity of the study, which may not allow for accumulation of transcripts over a prolonged period (Albert and Fiori 2014). Future studies should inspect the effect of repeated exposure to the foraging tradeoff model and its effect on transcription of anxiety-like transcripts over time. Furthermore, it may be that the predator apparatus was not an overt enough stimulus to induce transcription. Anxiety-like transcripts may be produced by certain conditions, such as ether vapor, and yet not produced at all during other stressors such as shaking stressors or food deprivation (Prater et al. 2018a). Overt and direct threats increase anxiogenic peptide production, whereas more diffuse anxiogenic stimuli may not trigger transcriptional changes in anurans.
Validation of behavioral anxiety assays are based on face validity, predictive validity, and construct validity (Belzung and Griebel 2001; Bourin 2015). Face validity asserts that a model is phenotypically similar, and the response observed to the stimuli should be identical to the behavioral or physiological responses in humans (Rodgers et al. 1997). For this criterion, we noted that frogs that were subjected to the predator stimulus significantly reduced entrances into the anxiogenic environment, similar to human behavior in avoiding entering or staying in anxiety-inducing environments (Pineles et al. 2011; Freeman et al. 2015). The second criterion is predictive validity, which entails that the behavior in question is reversible when the anxiogenic stimuli is removed (Bourin 2015). Predictive validity was evaluated in this foraging tradeoff test during time period C, where the predator apparatus was removed for the predator exposed group and behavior was expected to revert to baseline. For this criterion, we noted that in time period C, animals that had previously been exposed to the predator stimulus in time period B increased their swimming behavior (Fig. 3B). Forelimb sweeping, which was significantly decreased in time period B, reverted back to control levels in period C, reflective of increased foraging behavior once the visual predator stimulus was removed. Finally, the last criterion is construct validity, where there must be a similar rationale underlying the animal behavior model and actual human behavior (Bourin 2015). This criterion is met by anxiety disorders being similarly maladaptive, reducing appetite or reducing an individual’s motivation to engage in daily tasks like eating or approaching a falsely anxiogenic environment (Krohne 2001; Kaye et al. 2004). While humans no longer routinely worry about the threat of predation, the interplay between a beneficial reward and fear of loss are tradeoffs that are an innate part of human life. We see similar risk-reward scenario in social contexts whenever we are applying ourselves towards increasing our resources (important meetings or applying for jobs), we often must put ourselves in anxiogenic environment (public speaking or meeting potential clients) and while the consequences are not nearly as dire as for the frogs, it still a risk-reward scenario that occurs daily (Cheng and McCarthy 2018). For these reasons, we view this novel foraging/predator avoidance trade-off test is sufficient to induce anxiety-like behavior in our model organisms. Future studies will also be conducted to evaluate if anxiolytic drug administration is sufficient to reverse the avoidance behavior and increase the appetitive behavior seen in time period B. These studies would be important in further establishing predictive validity for this test and to confirm that this behavior is representative of anxiety behavior and the awareness and reaction to the predatory stimulus is modulated by homologous anxiety pathways to humans.
Our tradeoff task is ethologically relevant and uses a sustained threat that, based upon the predator imminence theory of fear and anxiety being on the same trait continuum (Perusini and Faneslow 2015) with the degree of anxiety being proportional to predator proximity. Anxiety-like behaviors are often defined by a prolonged state of tension, worry, or apprehension (reflected in this study by decreased entrances to the predator zone by stimulus-exposed animals), while fear-like behaviors are usually phasic and abrupt fight-or-flight responses that are coupled with elevated arousal (Steimer 2002; Perusini and Fanselow 2015). Given that the few significant differences in behavior that we measured fell into the former category, we conclude that they represent anxiety-like behavior on the part of the frog.
Conclusion
In this study, we took the first steps to identify if X. laevis frogs can be used to study the subcortical aspects of predator-induced changes in appetitive and avoidance behavior. Using a novel foraging/predator avoidance tradeoff model in which we provided frogs with food simultaneously with an ecologically relevant predator stimulus, we found that predator exposure reduced swimming behavior and reduced entrances into the predator zone, consistent with predator avoidance. While food consumption was not significantly reduced in animals exposed to the predator stimulus, sweeping, an active foraging behavior, was significantly reduced in predator stimulus-exposed animals, indicative of reduced appetitive behavior and suggesting reduced movement to avoid detection. This study shows that the novel foraging/predator avoidance trade-off test was sufficient to promote avoidance behavior and reduce appetitive behavior; however, the ability of the predator stimulus to induce measurable changes in the transcription of anxiety-related genes requires further testing.
Data availability
Data are available in the “Supplementary information.”
References
Albert PR, Fiori LM (2014) Transcriptional dys-regulation in anxiety and major depression: 5-HT1A gene promoter architecture as a therapeutic opportunity. Curr Pharm Design 20:3738–3750
Almada RC, Coimbra NC (2015) Recruitment of striatonigral disinhibitory and nigrotectal inhibitory GABAergic pathways during the organization of defensive behavior by mice in a dangerous environment with the venomous snake Bothrops alternatus (Reptilia, Viperidae). Synapse 69:299–313
Avila VL, Frye PG (1978) Feeding-behavior of African clawed frog (Xenopus-laevis daudin) - (amphibia, anura, pipidae) - effect of prey type. J Herpetol 12:391–396
Azevedo H, Ferreira M, Mascarello A, Osten P, Guimaraes CRW (2020) The serotonergic and alpha-1 adrenergic receptor modulator ACH-000029 ameliorates anxiety-like behavior in a post-traumatic stress disorder model. Neuropharmacology 164:107912
Baek J, Lee S, Cho T et al (2019) Neural circuits underlying a psychotherapeutic regimen for fear disorders. Nature 566:339–343
Băncilă RI, Plaiasu R, Stanescu F, Schmidt BR, Nae I, Denoel M (2021) Food level and light conditions affect the antipredator behavior in larvae of a stream-breeding amphibian. Behav Ecol Sociobiol 75:36
Beck AT, Brown G, Epstein N, Steer RA (1988) An inventory for measuring clinical anxiety - psychometric properties. J Consult Clin Psychol 56:893–897
Belzung C, Griebel G (2001) Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res 125:141–149
Bencan Z, Sledge D, Levin ED (2009) Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav 94:75–80
Blanchard RJ, Blanchard DC, Rodgers J, Weiss SM (1990) The characterization and modeling of antipredator defensive behavior. Neurosci Biobehav Rev 14:463–472
Blaser RE, Rosemberg DB (2012) Measures of anxiety in Zebrafish (Danio rerio): dissociation of black/white preference and novel tank test. PLoS ONE 7:e36931
Blumstein D, Daniel JC (2007) Quantifying Behavior the JWatcher Way. Sinauer Associates Inc, Sunderland, MA
Boorse GC, Denver RJ (2006) Widespread tissue distribution and diverse functions of corticotropin-releasing factor and related peptides. Gen Comp Endocrinol 146:9–18
Bourin M (2015) Animal models for screening anxiolytic-like drugs: a perspective. Dialogues Clin Neurosci 17:295–303
Campos AC, Fogaca MV, Aguiar DC, Guimaraes FS (2013) Animal models of anxiety disorders and stress. Braz J Psychiat 35:S101–S111
Carr JA, Zhang B, Li WJ, Gao MM, Garcia C, Lustgarten J, Wages M, Smith EE (2013) An intrinsic CRF signaling system within the optic tectum. Gen Comp Endocrinol 188:204–211
Cheng BH, McCarthy JM (2018) Understanding the dark and bright sides of anxiety: a theory of workplace anxiety. J Appl Psychol 103:537–560
Cianca V, Bartolini T, Porfiri M, Macri S (2013) A robotics-based behavioral paradigm to measure anxiety-related responses in Zebrafish. PLoS ONE 8:e69661. https://doi.org/10.1371/journal.pone.0069661
Claas B, Dean J (2006) Prey-capture in the African clawed toad (Xenopus laevis): comparison of turning to visual and lateral line stimuli. J Comp Physiol A 192:1021–1036
Coleman RB, Aguirre K, Spiegel HP, Pecos C, Carr JA, Harris BN (2019) The plus maze and scototaxis test are not valid behavioral assays for anxiety assessment in the South African clawed frog. J Comp Physiol A 205:567–582
Cornwell BR, Mueller SC, Kaplan R, Grillon C, Ernst M (2012) Anxiety, a benefit and detriment to cognition: Behavioral and magnetoencephalographic evidence from a mixed-saccade task. Brain Cognition 78:257–267
Cryan JF, Holmes A (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4:775–790
Cryan JF, Sweeney FF (2011) The age of anxiety: role of animal models of anxiolytic action in drug discovery. Brit J Pharmacol 164:1129–1161
de Abreu MS, Maximino C, Banha F, Anastácio PM, Demin KA, Kalueff AV, Soares MC (2020) Emotional behavior in aquatic organisms? Lessons from crayfish and zebrafish. J Neurosci Res 98:764–779
de Almeida LP, Ramos PL, Pandossio JE, Landeira-Fernandez J, Zangrossi H, Nogueira RL (2006) Prior electrical stimulation of dorsal periaqueductal grey matter or deep layers of the superior colliculus sensitizes rats to anxiety-like behaviors in the elevated T-maze test. Behav Brain Res 170:175–181
Dong W, Lee RH, Xu H, Yang S, Pratt KG, Cao V, Song YK, Nurmikko A, Aizenman CD (2009) Visual avoidance in Xenopus tadpoles is correlated with the maturation of visual responses in the optic tectum. J Neurophys 101:803–815
Duggan PE, Prater C, Carr JA, Harris BN (2016) Predator presence decreases food consumption in juvenile Xenopus laevis. Behav Ecol Sociobiol 70:2005–2015
Forcelli PA, DesJardin JT, West EA, Holmes AL, Elorette C, Wellman LL, Malkova L (2016) Amygdala selectively modulates defensive responses evoked from the superior colliculus in non-human primates. Soc Cogn Affect Neurosci 11:2009–2019
Freeman D, Waller H, Harpur-Lewis RA et al (2015) Urbanicity, persecutory delusions, and clinical intervention: the development of a brief CBT Module for helping patients with persecutory delusions enter social urban environments. Behav Cogn Psychoth 43:42–51
Gray LA, Oreilly JC, Nishikawa KC (1997) Evolution of forelimb movement patterns for prey manipulation in anurans. J Exp Zool 277:417–424
Green SL (2009) The Laboratory Xenopus sp., 1st edn. CRC Press, Boca Raton, FL
Gupta D, Radhakrishnan M, Kurhe Y (2014) Insulin reverses anxiety-like behavior evoked by streptozotocin-induced diabetes in mice. Metab Brain Dis 29:737–746
Hamilton M (1959) The assessment of anxiety states by rating. Brit J Med Psychol 32:50–55
Harris BN (2020) Stress hypothesis overload: 131 hypotheses exploring the role of stress in tradeoffs, transitions, and health. Gen Comp Endocrinol 288:113355. https://doi.org/10.1016/j.ygcen.2019.113355
Harris BN, Carr JA (2016) The role of the hypothalamus-pituitary-adrenal/interrenal axis in mediating predator-avoidance trade-offs. Gen Comp Endocrinol 230:110–142
Huang L, Yuan TF, Tan MJ et al (2017) A retinoraphe projection regulates serotonergic activity and looming-evoked defensive behaviour. Nat Commun 8:14908
Islam R, Prater CM, Harris BN, Carr JA (2019) Neuroendocrine modulation of predator avoidance/prey capture tradeoffs: role of tectal NPY2R receptors. Gen Comp Endocrinol 282:113214. https://doi.org/10.1016/j.ygcen.2019.113214
Javanmard M, Shlik J, Kennedy SH, Vaccarino FJ, Houle S, Bradwejn J (1999) Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol Psychiat 45:872–882
Kandel ER (1983) From metapsychology to molecular-biology - explorations into the nature of anxiety. Am J Psychiat 140:1277–1293
Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K, Price Foundation Collaborative Group (2004) Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiat 161:2215-2221
Keers R, Pedroso I, Breen G, Aitchison KJ, Nolan PM, Cichon S, Nothen MM, Rietschel M, Schalkwyk LC, Fernandes C (2012) Reduced anxiety and depression-like behaviours in the circadian period mutant mouse afterhours. PLoS ONE 7:e38263. https://doi.org/10.1371/journal.pone.0038263
Kelleher SR, Silla AJ, Byrne PG (2018) Animal personality and behavioral syndromes in amphibians: a review of the evidence, experimental approaches, and implications for conservation. Behav Ecol Sociobiol 72:79
Kessler MS, Debilly S, Schoppenthau S, Bielser T, Bruns A, Kunnecke B, von Kienlin M, Wettstein JG, Moreau JL, Risterucci C (2012) fMRI fingerprint of unconditioned fear-like behavior in rats exposed to trimethylthiazoline. Eur Neuropsychopharmacol 22:222–230
Kopij G (1996) Breeding and feeding ecology of the Reed Cormorant, Phalacrocorax africanus, in the Free State. S Afr Acta Ornithol 31:89–99
Kopij G (1998) Diet of Whitebreasted Cormorant Phalacrocorax carbo nestlings in the south-eastern Free State, South Africa. S Afr J Wildl Res 28:100–102
Krohne HW (2001) Stress and coping theories. In: Smelser NJ, Baltes PB (eds) International Encyclopedia of the Social and Behavioral Sciences. Elsevier, Amsterdam, pp 15163–15170
Kysil EV, Meshalkina DA, Frick EE et al (2017) Comparative analyses of Zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish 14:197–208
Lang PJ, Bradley MM, Cuthbert BN (1998) Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol Psychiat 44:1248–1263
Lea AJ, Blumstein DT (2011) Age and sex influence marmot antipredator behavior during periods of heightened risk. Behav Ecol Sociobiol 65:1525–1533
Lischinsky JE, Lin DY (2019) Looming danger: Unraveling the circuitry for predator threats. Trends Neurosci 42:841–842
Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY (2007) The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology 148:5531–5540
Liu YJ, Wang Q, Li B (2011) Neuronal responses to looming objects in the superior colliculus of the cat. Brain Behav Evol 77:193–205
Loveridge A (1953) Zoological results of a fifth expedition to East Africa. IV Amphibians from Nyasaland and Tete. Bull Mus Comp Zool 110:325–406
McFadyen J, Mattingley JB, Garrido MI (2019) An afferent white matter pathway from the pulvinar to the amygdala facilitates fear recognition. Elife 8:e40766. https://doi.org/10.7554/eLife.40766
Merali Z, Levac C, Anisman H (2003a) Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiat 54:552–565
Merali Z, Michaud D, McIntosh J, Kent P, Anisman H (2003b) Differential involvement of amygdaloid CRH system(s) in the salience and valence of the stimuli. Prog Neuro-Psychopharmacol Biol Psychiat 27:1201–1212
Mohammad F, Ho J, Woo JH, Lim CL, Poon DJJ, Lamba B, Claridge-Chang A (2016) Concordance and incongruence in preclinical anxiety models: systematic review and meta-analyses. Neurosci Biobehav Rev 68:504–529
Morris DW, Dupuch A, Moses M, Busniuk K, Otterman H (2019) Differences in behavior help to explain lemming coexistence. J Mammal 100:1211–1220
Muller-Gass A, Porath A, Anisman H, Merali Z (2000) Novelty-induced delay in snack approach: a robust and rapid naturalistic model for anxiety assessment. Soc Neurosci Abstr 30(659):2
Muthuraju S, Talbot T, Brandao ML (2016) Dopamine D-2 receptors regulate unconditioned fear in deep layers of the superior colliculus and dorsal periaqueductal gray. Behav Brain Res 297:116–123
Narayan EJ, Cockrem JF, Hero JM (2013) Sight of a predator induces a corticosterone stress response and generates fear in an amphibian. PLoS ONE 8:e73564. https://doi.org/10.1371/journal.pone.0073564
Perusini JN, Fanselow MS (2015) Neurobehavioral perspectives on the distinction between fear and anxiety. Learn Mem 22:417–425
Pineles SL, Mostoufi SM, Ready CB, Street AE, Griffin MG, Resick PA (2011) trauma reactivity, avoidant coping, and PTSD symptoms: a moderating relationship? J Abnorm Psychol 120:240–246
Prater CM, Garcia C, McGuire LP, Carr JA (2018a) Tectal corticotropin-releasing factor (CRF) neurons respond to fasting and a reactive stressor in the African Clawed Frog, Xenopus laevis. Gen Comp Endocrinol 258:91–98
Prater CM, Harris BN, Carr JA (2018b) Tectal CRFR1 receptors modulate food intake and feeding behavior in the South African clawed frog Xenopus laevis. Horm Behav 105:86–94
Prater CM, Harris BN, Carr JA (2020) Tectal CRFR1 receptor involvement in avoidance and approach behaviors in the South African clawed frog. Xenopus Laevis. Horm Behav 120:104707. https://doi.org/10.1016/j.yhbeh.2020.104707
Rodgers RJ, Cao BJ, Dalvi A, Holmes A (1997) Animal models of anxiety: an ethological perspective. Brazil J Med Biol Res 30:289–304
Samuels BA, Mendez-David I, Faye C, David SA, Pierz KA, Gardier AM, Hen R, David DJ (2016) Serotonin 1A and serotonin 4 receptors: essential mediators of the neurogenic and behavioral actions of antidepressants. Neuroscientist 22:26–45
Sayin U, Sutula TP, Stafstrom CE (2004) Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia 45:1539–1548
Schiwitz NC, Schalk CM, Saenz D (2020) Activity level-predation risk tradeoff in a tadpole guild: Implications for community organization along the hydroperiod gradient. Am Midl Nat 183:223–232
Serova LI, Laukova M, Alaluf LG, Pucillo L, Sabban EL (2014) Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol 24:142–147
Shen MY, Perreault ML, Bambico FR, Jones-Tabah J, Cheung M, Fan T, Nobrega JN, George SR (2015) Rapid anti-depressant and anxiolytic actions following dopamine D1–D2 receptor heteromer inactivation. Eur Neuropsychopharmacol 25:2437–2448
Shi YB, Liang VCT (1994) Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochim Biophys Acta Gene Regul Mech 1217:227–228
Smith KS, Engin E, Meloni EG, Rudolph U (2012) Benzodiazepine-induced anxiolysis and reduction of conditioned fear are mediated by distinct GABA(A) receptor subtypes in mice. Neuropharmacology 63:250–258
Steimer T (2002) The biology of fear- and anxiety-related behaviors. Dialogues Clin Neurosci 4:231–249
Steuwe C, Daniels JK, Frewen PA, Densmore M, Theberge J, Lanius RA (2015) Effect of direct eye contact in women with PTSD related to interpersonal trauma: psychophysiological interaction analysis of connectivity of an innate alarm system. Psychiat Res-Neuroim 232:162–167
Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueffl AV (2014) Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci 37:264–278
Tafet GE, Nemeroff CB (2020) Pharmacological treatment of anxiety disorders: the role of the HPA axis. Front Psychiat 11:443
Takahara T, Kohmatsu Y, Maruyama A, Doi H, Yamanaka H, Yamaoka R (2012) Inducible defense behavior of an anuran tadpole: cue-detection range and cue types used against predator. Behav Ecol 23:863–868
Tomczak M, Tomczak E (2014) The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trend Sport Sci 21:19–25
Videlier M, Bonneaud C, Cornette R, Herrel A (2014) Exploration syndromes in the frog Xenopus (Silurana) tropicalis: correlations with morphology and performance? J Zool 294:206–213
Videlier M, Cornette R, Bonneaud C, Herrel A (2015) Sexual differences in exploration behavior in Xenopus tropicalis? J Exp Biol 218:1733–1739
Wu LQ, Niu YQ, Yang J, Wang SR (2005) Tectal neurons signal impending collision of looming objects in the pigeon. Eur J Neurosci 22:2325–2331
Zhou Z, Liu XM, Chen SP et al (2019) A VTA GABAergic neural circuit mediates visually evoked innate defensive responses. Neuron 103:473–488
Acknowledgements
We thank the Texas Tech University Department of Biological Sciences and the Texas Tech University Association of Biologists for funds to NMM. We also acknowledge Drs. Karina Alvina, Lauren Gollahon, Naima Moustaid-Moussa, and Ernest Smith for assistance with experimental design and Marcos Arciniega and Hailey Luckie for help with behavior analysis. Finally, we thank the anonymous reviewers and editorial team at BES for their valuable contributions. This work was completed in partial fulfillment of the requirements for a doctoral degree in the Department of Biological Sciences at Texas Tech University (NMM).
Funding
Funding was provided by the National Science Foundation IOS #1,656,734, Texas Tech University Department of Biological Sciences, and a Graduate Student Research Support Award provided by Texas Tech University Graduate School.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the design of the study. Material preparation, data collection, and analysis were performed by NMM. Writing and editing was done by NMM with guidance and editing from JAC. Guidance, resources, and supervision on this project was provided by JAC.
Corresponding author
Ethics declarations
Ethics approval
All procedures involving animals were approved by the Texas Tech Animal Care and Use Committee (protocol number 18089–11) and follow Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) guidelines including the Eighth Edition of the Guide for the Care and Use of Laboratory Animals (NRC 2011) and the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, Council of Europe (ETS 123).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by A. Taylor Baugh
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Menon, N.M., Carr, J.A. Anxiety-like behavior and tectal gene expression in a foraging/predator avoidance tradeoff task using adult African clawed frogs Xenopus laevis. Behav Ecol Sociobiol 76, 115 (2022). https://doi.org/10.1007/s00265-022-03219-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03219-0