Abstract
Separately delivered visual and lateral line stimuli elicit similar but not identical orientation and approach by intact, sighted Xenopus. Response frequencies for visual stimuli declined sharply for distant or caudal stimuli while those for lateral line stimuli changed little. Turn angles correlated highly with stimulus angles but were smaller on average, so regression slopes were less than one. Regression slopes were smaller for visual than for lateral line stimuli, but this apparent difference was due to different distributions of stimulus distance interacting with the toad’s rotation center. Errors in final headings, most often under-rotations, did not differ by modality. Frequencies of lunges and arm capture movements were higher for visual stimuli both overall and especially for rostral proximal stimuli. The results demonstrate accurate orientation by sighted Xenopus to visual and lateral line stimuli; they are consistent with expectations based on in-register tectal maps. Orientation to lateral line stimuli is similar to previous results with blinded animals, revealing no heightened acuity in the latter. Modality differences indicate that the lateral line system is better for omnidirectional orientation and approach to distant stimuli whereas the visual system is more attuned to nearby rostral stimuli and more apt to mediate strikes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In adapting their behavior to their surroundings, animals often utilize information from more than one sensory system (e.g., Dusenbury 1992; Stein and Meredith 1993; Coombs and New 2002). The aquatic African clawed toad, Xenopus laevis, can locate and capture prey using its lateral line system (Kramer 1933; Görner 1976; Elepfandt 1982, 1989; Claas and Münz 1996), but physiological evidence (Zittlau et al. 1986) and casual observation as well as the dominant role of vision in prey-capture for many terrestrial amphibians (e.g., Lindquist and Bachmann 1982; Ewert 1997) suggest that visual information can also be used.
Accurate orientation implies that stimulus location is encoded within the central nervous system. The vertebrate midbrain, which participates in many oriented responses including prey-capture, escape, and eye movement, contains topographically ordered maps of the animal’s surroundings based on visual, acoustic, somatosensory (e.g., Stein and Meredith 1993), electrosensory (Heiligenberg 1991), and lateral line (Zittlau et al. 1986) inputs. In Xenopus, visual (e.g., Gaze et al. 1974; Udin and Keating 1981) and lateral line (Zittlau et al. 1986) maps appear to be in register (Zittlau et al. 1986) as tectal maps are in other animals (e.g., Knudsen 1982, 2002; Stein and Meridith 1993; King 1999), so that neighboring visual and non-visual neurons in different layers of the tectum are responsive to similar stimulus directions and multimodal neurons have overlapping receptive fields for visual and non-visual stimuli. The tectum is commonly thought to play a key role in sensorimotor integration, transforming information on target location into appropriate motor commands for many orienting behaviors (e.g., Valentine et al. 2002), although the nature of the transformation may be complex (Grobstein 1988).
In-register multimodal maps may facilitate stimulus detection (Stein and Meredith 1993; King 1999) and do allow common efferent pathways to execute behavior elicited through different modalities (Stein and Meredith 1993). In the barn owl, visual receptive fields of multimodal units in the optic tectum lie within the larger auditory receptive fields near the best direction for auditory stimulation (Knudsen 1982, 2002); visual and acoustic stimuli elicit similar head turns serving to bring the stimulus location to the center of the animal’s gaze (Knudsen et al. 1979, 1984; Knudsen and Knudsen 1989; du Lac and Knudsen 1990). In Xenopus, an accurate turn in response to surface waves also aligns the midline of the animal and the center of its visual field with the stimulus location. Unlike the owl’s behavior, Xenopus’s turning is not a simple rotation around an anatomically defined axis. The advance in the new direction after the turn is quite variable. Prior analyses of lateral line responses in Xenopus have focused on the accuracy of turning, as does the present study.
Previous behavioral studies of the Xenopus lateral line used blinded toads (Görner 1973, 1976; Elepfandt 1984; Görner et al. 1984; Claas et al. 1993; Claas and Münz 1996) to exclude visually mediated escape or orientation responses. Because blinding typically damages or destroys the dense array of lateral line stitches around the eyes, which are at the water surface in the toad’s normal alert posture, orientation of intact toads may actually be better than that demonstrated in blinded toads if these stitches were especially important for sensing surface waves. Alternatively, orientation of intact toads might be poorer if optomotor responses reduce the amplitude of turns mediated by the lateral line or the absence of vision in blinded toads heightens the acuity of the lateral line system. Therefore, orientation of intact toads to lateral line stimuli was examined first for comparison with previous studies.
More important, orientation to visual stimuli has not been studied in Xenopus, although the existence of visual and lateral line maps in the tectum has been confirmed physiologically (Zittlau et al. 1986). The present study compares the turning responses of African clawed toads to visual and lateral line stimuli presented separately near or on the water surface. The expectation was that the visual and the lateral line stimuli should elicit similar turns because they elicit activity at common locations in the tectal map. Parameters compared included response frequency, turning angle, heading error, and prey-capture elements such as lunging or gathering movements with the arms.
Material and methods
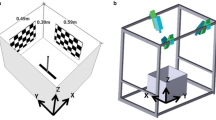
The experimental animals were X. laevis purchased from Xenopus I (Dexter, MI, USA) as small tadpoles or immediate postmetamorphic juveniles. Seven of the nine animals ranged in size from 28 to 32 mm (snout-to-vent length); two larger animals measured 38 mm (o4) and 48 mm (o1). Behavioral testing was carried out in an octagonal, glass-bottomed aquarium (44 cm across with sides 18 cm long and 13 cm high) filled with tap water to a depth of 4 cm (Fig. 1). The aquarium was placed on a glass plate over a circular opening (33 cm diameter) in a wooden table. A 25 W red incandescent lamp (GE 4409) provided light from above, shining onto white paper spanning the top of a 47 cm cubic frame surrounding the arena.
Experimental conditions. a The “large arena” for lateral line stimuli. The pipette assembly for generating an air pulse (arrow) to produce surface waves is raised for clarity. b The “small arena” for visual stimuli and, as illustrated in the right half, for lateral line tests. p: pipette, connected by plastic tube to a pipette bulb (not shown), s: slit at water level to allow surface waves to enter the small arena, sp: sphere of black fabric producing a dark stimulus when touched to the translucent cylinder. c Stimulus and response parameters. st: stimulus location, sd: stimulus distance, α: stimulus angle, β: turn angle, rc: rotation center, βi: ideal turn angle, and he: heading error. On average, the rotation center was between head and vent; the caudal location is exaggerated here for clarity
Several different test enclosures were used within the aquarium. For lateral line testing comparable to previous studies, the enclosure (“large arena”; Fig. 1a) was a submerged plastic ring of 27.5 cm diameter and 3.2 cm height. Gauze was stretched from the ring to the outer aquarium walls to restrict the animal to the test enclosure. Surface waves were produced by air puffs delivered through one of several Pasteur pipettes mounted with their tips 5 cm above the water surface. Each pipette was connected by a hard plastic tube to a 2 ml pipette bulb, which was squeezed quickly and completely by hand to produce an air puff directed onto the water surface. A 24 cm diameter circle cut from overhead transparency film printed with a Julesz noise pattern (Gregory 1966; 1 mm×1 mm pixels) was fitted onto the pipette tips. This film stabilized the pipette array; the noise pattern provided a uniform visual background above the animal and made the surface waves easily visible in video recordings. It also concealed the pipettes and their tips from the toad’s view; we never observed toads to orient toward a pipette in the absence of an air puff or spring at one as they often did toward air bubbles or other visual features. Typical stimuli, measured according to Rudolph (1967) as applied by Zittlau et al. (1986), had mean peak-to-peak amplitudes ranging from 90 μm at 180 mm to 215 μm at 6 cm and 822 μm at 1 cm with standard deviations less than 50 μm at each distance; these amplitudes are similar to those used previously (e.g., Görner et al. 1984; Traub and Elepfandt 1990).
For visual tests, the enclosure (“small arena”; Fig. 1b left side) was a clear Plexiglas cylinder with a diameter of 15.4 cm and a height of 11 cm. It was wrapped with white tissue paper. Manually touching the outside of the enclosure with a round piece of black fabric (diameter ca. 3 mm) fixed at the tip of a probe produced a black-on-white visual stimulus on the inside of the arena. The tissue paper concealed the probe until contact, providing a stimulus with a quick onset and small, well-defined angular extent. (Responses could be readily elicited by moving the probe in the air above the toad, but such stimuli had ill-defined onsets and directions, so they were not systematically employed or analyzed.) Contact between probe and enclosure also switched on a LED to mark stimulus onset. Stimuli were less than 1 cm above the water surface. They persisted for the duration of contact with the cylinder (median 0.9 s; quartiles 0.6–1.3 s). Because toads responded poorly to caudal visual stimuli and often remained close to and facing the enclosure wall after a response, rostral stimuli at large distances and caudal stimuli at short distances were hard to obtain. In an attempt to increase the distance of the toad from rostral visual stimuli and make distributions of distance more like those of lateral line trials, a 6 mm wide Plexiglas ring was placed inside the cylinder and just below the water level in some trials.
The toads’ tendency to stay close to the enclosure wall in the visual tests necessitated tests of lateral line stimuli in the small arena. Two slits were cut around the circumference of the small arena (Fig. 1b right side). They extended from ca. 4 mm below the water surface to ca. 3 mm above it and left two 1 cm wide supports on opposite sides of the arena. Air puffs were produced by two pipettes mounted on a rotating arm; the surface waves originated 1–1.5 cm outside the arena and entered through the slits. A large mesh net on the inside of the slits kept the toad inside the arena.
During each test session, individual stimuli were presented only when the toad was stationary. For responsive toads, stimuli could be repeated at intervals of several seconds to elicit a series of responses. At irregular intervals, the toad was rewarded with a red mosquito larva. Individual sessions lasted up to 20 min a day; testing was repeated on subsequent days until sufficient responses were acquired. Sessions and data analysis ended when the toad ceased responding to repeated stimuli. Stimuli eliciting no response were included in the analysis if the toad did respond to a subsequent stimulus.
Test sessions were recorded on video using a Panasonic CCTV camera (WV-PP330) with an 8× zoom lens (WV-LV62/8) and a Panasonic AG5710 SVHS recorder. The camera was 69 cm below the bottom of the aquarium; the field of view included 10 cm calibration markers aligned with the camera’s vertical and horizontal directions. The video recorder was controlled via a BCD SB100 Time Code Controller, which also added a frame counter to the video image. After testing, videotapes were inspected frame by frame to determine stimulus onset, initial movement of the toad (latency), and the final position after the response. Using an ELSA Erazor III card and Main Actor Version 3 software (Main Concept USA), frames with the stimulus onset and the final position were captured to disk.
From these image files, the stimulus location and the initial and final positions of the toad were digitized using a Delphi program. Stimulus angle and distance were calculated relative to the point on the midline between the arms (Görner et al. 1984; Claas and Münz 1996); the rostral extension of the midline is 0° and clockwise angles are positive (Fig. 1c). Observed turn angle and the ideal turn angle necessary for the toad to exactly face the stimulus were determined relative to the intersection of the midline extensions before and after the response; this intersection is called the rotation center. The heading error (Fig. 1c) was calculated using the rotation center, rather than the thoracic midpoint of the toad before or after the turn, as the vertex for the angle describing the toad’s final deviation from the source direction because it is unaffected by how far the toad swims but does reflect the true heading. If the midline of the toad after the turn intersects the stimulus location, heading error is zero and the head and mouth are facing the stimulus location or swam through it. (Because the axis of the turn is variable and not usually between the arms, the directions of translation of this midpoint, the midpoint between the eyes or the tip of the snout do not accurately reflect the toad’s heading after the turn.) Signs of heading errors were adjusted so that positive and negative values represent over- and under-rotation, respectively. This measure was not appropriate occasionally when, for small stimulus angles, lateral displacement and a small counter-rotation combined to place the rotation center beyond the stimulus location and cause computed heading errors close to 180°, even though the toad accurately faced the stimulus; such values were among the outliers excluded.
Statistical analyses [Fisher exact tests and χ 2 for contingency tables, logistic regressions for binomial dependent variables, simple linear regressions, comparison of two regression lines, general linear regression models with categorical and quantitative factors, t-tests, and multifactor analysis of variance (ANOVA)] were performed with Statgraphics (Version 5, Manugistics Inc.); loess fits were performed using SAS (Version 8e for Windows). Logistic regressions fit the binomial outcome measurements, zero or one, to the function frequency(outcome1) = exp(eta)/(1 + exp(eta)), where eta is a polynomial function of the independent variables; the percentage of explained deviance is an approximation to the R 2 of standard regression and serves as a measure of strength of association. For some tests, stimulus angles were grouped in 30° classes denoted by the middle angle. Extreme outliers more than three standard deviations from the mean or, alternatively, more than three interquartile intervals from the median were excluded for final calculation of some parametric statistics as noted.
Results
Responses of sighted toads to surface wave stimuli in the large enclosure
Response frequency
In motivated toads, surface waves readily elicited turns toward and, usually, approach to the source. Nine toads were tested; 1,841 responses were obtained to 2,573 stimuli. Response frequencies (Fig. 2a) differed according to stimulus angle, stimulus angle squared, and stimulus angle × stimulus distance (P<0.001, P<0.0001, and P<0.008, respectively, for logistic regression coefficients) but the logistic regression explained only 2% of the deviance. Response frequencies were high for all directions (Fig. 2a); rostrolateral stimuli were slightly more effective. This pattern was confirmed by analyzing responses in 30° stimulus classes with an ANOVA. Response frequencies differed with stimulus angle (χ 2=56.3; degrees of freedom (df)=5; P<0.0001, N=2,573); they were highest for frontal lateral stimuli and significantly lower for both rostral and caudal stimuli. Response frequency did not change with stimulus distance, even when tested separately within each 30° class.
Response probability as a function of stimulus angle and distance for lateral line and for visual stimuli. Plots are computed using the equations from a logistic regression: probability=exp(eta)/(1+exp(eta)). a Lateral line stimuli in the large arena (eta= 0.714 + 0.0138 × |angle| − 0.000116 × |angle|2 + 0.0000263 × |angle| × distance, N=2573, adjusted R 2 =2%); omitting the two large toads removed the interaction term. Curves and coefficients for lateral line stimuli in the small arena, where the two large toads were not tested, were similar but distance × angle was no longer a significant factor (eta = 1.40 + 0.0251 × |angle| − 0.000170 × |angle|2, N=931, adjusted R 2 =4.3%). b Visual stimuli in the small arena (eta=3.49 − 0.0618 × distance − 0.000170|angle|2 + 0.000256 × |angle| × distance, N = 2791, adjusted R 2 =18.5%). Parts a and b show data from the same nine toads. All coefficients were significant at P<0.008
Turn angle and accuracy
Turn angle is the focus of most previous analyses. Toads turned toward the incorrect side (“left–right errors”) in 5% of all responses. Rostral stimuli within 15° of the midline were within the general turning accuracy (see below), so left–right errors (2%) for such stimuli were included with correct turns in all analyses. With this adjustment, toads turned correctly in nearly 97% of all responses.
Left–right errors for more lateral or caudal stimuli would represent extreme outliers (cf. Fig. 3d versus 3a–c) and unduly affect parametric statistics, so they were excluded. Most (33 or 57%) occurred for the −165 and +165° stimulus classes. Even the best turns to the incorrect side were under-rotations and only four of the 1,841 turns exceeded 180°.
Dependence of turn angle on stimulus angle for lateral line stimuli in the large arena. Data are shown for the animals with the largest (a o3 N=257; 6 left–right errors-with |stimulus angle| > 15°-excluded) and smallest (b o1 N=201, 12 left–right errors excluded) slopes, as well as for all animals (c N= 1783; 58 left–right errors excluded) and for turns in the wrong direction, “left–right errors” (d N=58). Points with turn angle equal to stimulus angle lie along the diagonal (dashed line)
Plotting turn angle versus stimulus angle is the conventional representation of response accuracy (e.g., Elepfandt 1982; Görner et al. 1984; Claas and Münz 1996). According to this measure, turn angle was highly correlated with stimulus angle (Fig. 3). Linear regressions accounted for over 90% of the variance (R 2 in Table S1). Regression intercepts were not significantly different from zero for five animals and within 5° of zero for the rest. Treating the data as if all stimuli were on the right side (“pseudounilateral data”; i.e., converting stimulus-turn angle pairs for stimuli on the left into their reflection through the origin) can better reveal trends from small to large stimulus angles. Regressions with such pseudounilateral data had slightly smaller slopes and percent variance explained (Table S1); intercepts were not significantly different from zero in six animals and within 10° in the rest. Thus, turn angle is directly proportional to stimulus angle.
However, regression slopes for both the original and the pseudounilateral data were significantly less than one (range 0.65–0.89, mean 0.81: Table S1; all less than 1 at P<0.01 by t-tests); in other words, the toads turned less than the angle to the stimulus, which seems to imply that the toads under-rotated and did not end up facing the stimulus. This point was examined further using errors based on final heading. (Standard deviations of heading errors were just 16–25° for different animals, but there were a few outliers—including small turns with a large rostral center of rotation. Therefore, for parametric statistics, outliers more than three standard deviations from the mean heading error were removed, eliminating between 2 and 5 points per animal in addition to the left–right errors.)
Heading errors (Fig. 4) were close to zero for small stimulus angles in the pseudounilateral data. As stimulus angles increased, heading errors for all animals became significantly more negative, indicating that the animals did not turn far enough to face the stimulus directly (regression slopes between −0.06 and −0.29 degree per degree, significant for each animal at P<0.03; R 2 between 4 and 43%). Relationships were linear in six animals: coefficients for a quadratic term were negative and significant in the other three. For the 165° stimulus class, these heading errors averaged −25° (range of intra-animal averages: −15 to −40°; median −26°). This measure of final heading indicated that all animals ended up slightly better oriented toward the stimulus location than would be indicated by the turn angle versus stimulus angle plots, where expected differences from regressions would be −15 to −61° (mean −36°, median −29°) at 165°. Differences between the two measures were largest for the two largest animals.
Dependence of heading error on stimulus angle for lateral line stimuli in the large arena. Pseudounilateral data are shown for the animals of figure 3. a: o3 N=251; 12 left–right errors and outliers excluded. b: o1 N=198, 16 values excluded. c: all animals, N=1745; 96 values excluded. Turns leaving the toad facing or passing through the stimulus location (midline aligned with the stimulus location) have heading errors of zero (dashed line); under-rotations have negative heading errors
For turns to the incorrect side, turn angle was negatively correlated with stimulus angle (Fig. 3d; Table S1: L-R Err); the absolute value of the slope was similar to that for turns to the correct side. Data for left–right errors by definition lie in quadrants 2 and 4 (Fig. 3d), so a negative correlation might be expected, but the regression slope was significant and similar when points in quadrant 2 were replaced by their reflections in quadrant 4 (“pseudounilateral data”).
Precision of turning to the correct side varied slightly with stimulus angle. Standard deviations of heading errors in the pseudounilateral data (Fig. 5a) increased only about 10° over the 180° stimulus range (significant at P<0.0001 in an ANOVA with stimulus angle in 30° classes), but the relationship was not linear. The minimum occurred at 75°, which formed a homogenous group with (i.e., was not significantly different from) the 45 and 105° classes; the maximum occurred at 165°. Standard deviations of turn angles increased about 15° over the 180° range in a similar pattern (Fig. 5a) except that the 15° class was part of the homogeneous group around the minimum and only means for the two caudal classes were significantly larger. In other words, precision of turning was better for rostrolateral angles.
Dependence of average within-animal standard deviations of heading error and turn angle on |stimulus angle| for lateral line and for visual stimuli. Pseudounilateral data for nine animals. Means and 95% confidence intervals are from ANOVAs with stimulus angle and animal, based on standard deviations computed for each of nine animals and each 30° stimulus angle class. a Lateral line stimuli in the large arena. Angle is a significant factor for each parameter at P<0.0001. b Visual stimuli in the small arena. Angle is significant for each at P<0.002
Effect of stimulus distance
Although one might expect stimulus distance, particularly short distances, to modify turn parameters in order to bring the mouth close to the stimulus site, the data showed only minor effects for the distances tested. When added as a factor to multiple regressions with the pseudounilateral data, stimulus distance significantly influenced turn angle for just the two larger animals and heading error for only one animal.
Neither did stimulus distance affect the rotation center, as tested separately for each 30° stimulus class using a general linear regression model with the independent factors stimulus distance, and animal. (Regressions were limited to stimulus distances between 10 and 170 mm, which included most stimuli, and to rotation centers less than three times the interquartile distance from the median rotation center to eliminate extreme outliers.) Animal was a highly significant factor with the two larger animals in particular having more caudal mean rotation centers.
Other response parameters
In prey-capture, orientation and approach are followed by a lunge (strike) toward the target and/or by gathering or capture movements of the arms which serve to sweep any nearby objects toward the mouth. Gathering movements with the arms occurred in 10% of the responses; their frequency decreased with increasing stimulus angle (Fig. 6a), but less than 2% of the deviance was explained in logistic regressions. Lunges occurred in less than 5% of responses. Their frequency also decreased with stimulus angle and, in addition, with stimulus distance squared (Fig. 6b); 6.0% of the deviance was explained.
Dependence of gathering movements of the arms and lunges on stimulus angle and distance for lateral line stimuli in the large arena. Plots are computed using the equations from a logistic regression: probability=exp(eta)/(1+exp(eta)). Pseudounilateral data are for nine animals. a Arm movements: eta= −1.513 − 0.00773 × |stimulus angle|, adjusted R 2=1.5%, N=1836; b Lunges: eta=−1.54 − 0.00889 × |stimulus angle| − 0.000101 × stimulus distance2, adjusted R 2=6.0%, N=1835, coefficients significant at P<0.001
Responses to visual stimuli
Response frequency
Visual stimuli also elicited turns in motivated toads. The same nine toads produced 1,472 responses to 2,791 stimuli. Response frequencies varied greatly with stimulus angle; responses were frequent for rostrolateral and rostral angles (Fig. 2b) and infrequent for caudal stimuli. Response frequencies also decreased with stimulus distance (Fig. 2b), even for stimulus angles less than 120° for which mean stimulus distances were constant. (Mean stimulus distances increased from about 50 mm for the 105° class and below to 90 mm for the 165° class because stimuli were presented at the arena perimeter and, for many visual trials, toads were close to and roughly facing the wall after a previous response.)
Turn angle and accuracy
Toads turned to the correct side 98% of the time, slightly but significantly more often than for lateral line stimuli (χ 2=7.26, with Yates correction, P<0.007; N=3,314). Again turn angles were directly proportional to stimulus angles (Fig. 7). Linear regressions accounted for over 89% of the variance (Table S1b). Regression intercepts were not significantly different from zero for five animals and within 5° of zero for the rest. Regressions with pseudounilateral data reduced the explained variance but left slopes essentially unchanged (Table S1b); intercepts were not significantly different from zero in eight animals and within 10° in the ninth.
Dependence of turn angle on stimulus angle for visual stimuli. Data are shown for the animals with the flattest (o1: N=120; 1 left–right error excluded) and the steepest (o10: N=223; 2 left–right errors excluded) slopes, as well as for all animals combined (c N=1454; 18 left–right errors excluded). Insets show the regression lines for visual stimuli (solid line) and the corresponding line for lateral line stimuli in the large arena (large dashed lines). Points with turn angle equal to stimulus angle lie along the diagonal (small dashes)
Regression slopes were again less than one (range 0.52–0.74, mean 0.65: Table S1b; all slopes significantly less than 1 at P<0.01). Moreover, the slope for each toad was significantly less than its slope for lateral line stimuli in the large arena (Table S1b: p:LL-v).
Standard deviations of heading errors for different animals ranged from 11 to 23°. Outliers more than three standard deviations from the mean were excluded from regressions, removing between 2 and 10 points per animal.
Heading errors for small stimulus angles were close to zero (seven animals) or positive (two animals: over-rotation by about 6°). With increasing stimulus angle, heading errors became negative (i.e., under-rotation) with regression slopes between −0.02 and −0.27 degree per degree, which were significantly less than zero for all but one animal at P<0.0002; regressions explained between 0.5 and 35% of the variance. For the 105° stimulus direction—the most caudal class with appreciable visual responses from all animals, these heading errors averaged −14° (range of intra-animal averages: −5 to −25°; median −12°). This measure of final heading again indicated that the animals ended up better oriented toward the stimulus location than would be indicated by the amount of under-rotation apparent in regressions of turn angle on stimulus angle, where expected values would be −21 to −55° (mean and median −40°) at 105°. Differences between the two measures were again largest for the two largest animals.
Compared to heading errors for lateral line stimuli in the large arena, those for visual stimuli showed less over-rotation for small stimulus angles (the overall intercept was 4° less, P<0.005) but a flatter slope (0.028 less negative; P=0.04) for angles that readily elicited responses (<140°: see Fig. 8c) before increasing greatly for caudal angles. In other words, accuracy for visual stimuli was better than that for lateral line stimuli over a wide range of rostral and lateral stimulus angles.
Dependence of heading error on |stimulus angle| for visual stimuli. Pseudounilateral data are shown for the animals with the steepest (a o3 N=124; 4 left–right errors and outliers excluded, intercept NS, slope=0.0001, R 2=0.12) and smallest (b o10 N=220; 5 values excluded, intercept NS, slope P=0.0002, R 2=0.059) slopes, as well as for all animals combined (c N= 1417; 55 values excluded). To better account for the non-linearity at large stimulus angles, loess smoothing curves with ±95% confidence intervals are plotted instead of regressions. Turns leaving the toad facing or passing through the stimulus location (midline aligned with the stimulus location) have heading errors of zero (dashed line); under-rotations have negative heading errors
Within-animal standard deviations of heading errors increased linearly by about 8° from the 15° to the 105° stimulus class (Fig. 5b; pseudounilateral data: P<0.0001 in an ANOVA with the factor stimulus angle), and then varied greatly by animal due to low response frequencies. The minimum occurred for 15°; the three rostrolateral classes (15–75°) formed a homogeneous group. Standard deviations of turn angles increased about 11° (10–21° for the 135° class) in a similar pattern. In other words, the relationship of precision to stimulus angle was monotonic, not the asymmetric U-shaped function found for lateral line stimuli. (Continued presence of the visual stimulus when the response began did not decrease the standard error of regression estimates or the number of left–right errors.)
Other response parameters
Both gathering movements and lunges were more common and often occurred together in responses to visual stimuli, particularly for stimuli close to and in front of the toad. Gathering movements occurred in 39% of responses (compared to 10% for lateral line stimuli in the large arena); frequencies decreased with increasing stimulus angle, with increasing stimulus distance, and with decreases in their product (Fig. 9: the interaction term boosts the rates for distant caudal and distal stimuli in general—making them more similar to distal rostral stimuli and to proximal lateral stimuli—and for combinations of intermediate angles and distances in particular—making the peak at the origin more rounded).
Dependence of gathering movements of the arms and lunges on stimulus angle and distance for visual stimuli. Plots are computed using the equations from a logistic regression: probability=exp(eta)/(1+exp(eta)). Pseudounilateral data are for 9 animals and restricted to |stimulus angle|<120° due to the paucity of responses for larger angles. Arm movements: eta= 3.51 − 0.0428* |stimulus angle| − 0.0590*stimulus distance + 0.000443 × |stimulus angle| × stimulus distance, adjusted R 2=12.0%, N=1402, coefficients significant at P<0.002. Lunges: eta= 4.21−0.0546 × |stimulus angle| − 0.0782 × stimulus distance + 0.000410 × |stimulus angle| × stimulus distance, adjusted R 2=19.7%, N=1400, coefficients significant at P<0.025
Lunges occurred in 47% of responses (compared to 5% for lateral line stimuli); 97% of responses to rostrofrontal stimuli included a lunge. The frequency decreased with the same factors that affected gathering movements.
Responses to visual and surface wave stimuli in the small enclosure
In an attempt to make stimulus distances and wall proximity of lateral line trials more like those of visual trials, four of the toads were also tested with surface waves in the small arena (Fig. 1b). This limited maximum stimulus distances to about 15 cm. Surface waves originated 10–15 mm outside the cylinder, so toads could not swim to the stimulus location and some turns could be influenced if toads began near the wall and encountered it while turning. Visual stimuli appeared directly on the cylinder, so their mean stimulus distance remained slightly smaller: differences varied with stimulus angle, ranging from 10 to 57 mm for 15 and 135° classes, respectively. For trials eliciting responses, mean distance of visual stimuli was independent of angle for angles less than 120°, which included most of the visual responses, whereas mean distance of lateral line stimuli increased 31 mm over this range of angles.
Response frequency still decreased less with angle and with distance for lateral line stimuli than for visual stimuli. Logistic regressions for each were similar to those reported above. For surface waves, response frequency did not significantly change with distance; it was highest for rostrolateral stimuli, slightly less for rostral stimuli and lower still for caudal stimuli. For visual stimuli, response frequency was greater than 50% for distances less than 60 mm and angles less than 90°; the logistic regression explained only 25% of the deviance.
Turn angle and accuracy
Slopes of regressions of turn angle on stimulus angle were still smaller for visual stimuli, but differences were smaller than when lateral line data from the large arena were used in the comparison (Table S1c, Fig. 10). The difference in slope remained even when the regressions were restricted to stimulus angles less than 110° or even 70°, where distributions of stimulus distances for the two modalities overlapped considerably (ranges 14–102 mm versus 21–114 mm for visual and lateral line stimuli, respectively) although mean distance remained significantly shorter for visual stimuli (42 mm and standard deviation 15 mm versus 70 mm and standard deviation 23 mm).
Dependence of turn angle on stimulus angle for lateral line and visual stimuli in the small arena. Data are shown for the animals with the smallest (a o10, N=396; 4 left–right errors excluded, R 2=95.5%, difference significant at P=0.003) and the largest (b o3, N=231; 3 left–right errors excluded, R 2=95.9%, difference significant at P=0.0001) differences in slopes. Points with turn angle equal to stimulus angle lie along the diagonal (dashed line)
To test whether proximity to the cylinder affected turns, responses were classified in two ways. First, a dichotomous classification determined whether the toad was close enough to the cylinder that when it turned toward the stimulus it would contact the wall. In regressions of turn angle on stimulus angle, slopes for visual stimuli were significantly smaller than those for lateral line stimuli for each class, but the difference was smaller for turns near a wall (Table S2), primarily because the slope for lateral line stimuli decreased with wall proximity. Similar conclusions resulted from a trichotomous classification according to initial position: toad touching the wall, body within one body length of the wall, or body more than one body length from the wall. For a given stimulus angle, turn angles were greater for lateral line stimuli than for visual stimuli and, for lateral line stimuli, the farther the toad was from the wall the larger the turn.
Despite these differences in turn angle, heading errors in the pseudounilateral data did not differ by stimulus modality in a general linear regression model with stimulus angle and distance as independent factors. Modality also was not a significant factor in an ANOVA with stimulus angle in 30° classes, stimulus distance in 20 mm classes from 20 to 100 mm, and animal as independent factors. Magnitudes of mean heading errors increased with stimulus angle, but only from −1 to −10° at 105°; they also increased with distance, but only from −3° at 30 mm to −14° at 90 mm.
Neither did rotation center differ by stimulus modality in analogous regressions. It was only affected by stimulus angle, but less than 4% of the variance was explained. Wall proximity did shift the rotation center caudally by about 4 mm.
To illustrate the influences of stimulus distance and the rotation center with its large variability, the ideal turn angle (β i, Fig. 1c) was plotted against stimulus angle. Most of these ideal turn angles occupied an area bounded by the diagonal—corresponding to responses with rotation centers near zero and ideal turn angles equal to stimulus angles—and a superimposed sinusoid representing about 15° of under-rotation for 90 and −90° stimuli—corresponding to responses with caudal rotation centers. Subtracting the stimulus angle from the ideal turn angle makes the under-rotation clearer (Fig. 11). For lateral line stimuli in the small arena (Fig. 11a), ideal turn angles equal to the stimulus angle were rare for stimuli within about 20° of 90 or −90°. The distribution for visual stimuli (Fig. 11b) resembled that for lateral line stimuli between −120 and +120° but with a steeper slope; in other words, for lateral stimuli, the under-rotation is larger.
Dependence of ideal turn angle on stimulus angle for lateral line and visual stimuli in the small arena. Points represent the difference between the stimulus angle and the turn angle needed for zero heading error, given the distance and rotation center in each response. Negative values to the right of zero and positive values to the left indicate the required turn is smaller than the stimulus angle. Curves are loess smoothing curves with 95% confidence intervals. The slope in the interval between −100° and +100° is less for lateral line stimuli (a) than for visual stimuli (b) at P<0.012
Plotting the heading error (Fig. 1c) between −100 and +100°, which includes most of the visual responses, showed no difference between lateral line and visual responses in either slope or intercept. A slight under-rotation (slope of −0.08±0.01 degree per degree, equivalent to 8° per 100°, P<0.0001) remained for both modalities.
Dependencies of standard deviations of turn angle and heading error on stimulus angle resembled those of Fig. 5: standard deviations differed by angle (P<0.001; ANOVA) but not by stimulus modality. For turn angle, the interaction between modality and stimulus angle was significant (P=0.006); standard deviations for visual stimuli were smaller for rostral classes but increased more rapidly for caudal stimuli than did those for lateral line stimuli. For heading error, standard deviations for visual stimuli increased monotonically with angle but those for lateral line stimuli increased only for the two caudal groups.
Other response parameters
Both lunges and capture movements of the arms were significantly more frequent for visual than for lateral line stimuli (logistic regression with pseudounilateral data: stimulus modality significant at P<0.001). Functions for logistic regressions resembled those described above. For lateral line stimuli, logistic regressions explained about 10% of the deviance and the decrease with stimulus angle was significant. For visual stimuli, logistic regressions explained 11 and 20% of the deviance for capture movements and lunges, respectively. Both decreased with stimulus angle and were modified—albeit in opposite directions—by the square of stimulus angle; lunges also decreased with distance whereas capture movements increased with the interaction between distance and stimulus angle.
Responses to surface wave stimuli in the large and small enclosures
For the four toads tested with surface wave stimuli in both the small and the large arenas, the total response frequency was significantly higher in the small arena but only by 0.05 (out of 1). Otherwise the pattern in pseudounilateral data was as described for the large arena: response frequency did not significantly change with distance and was lower for rostral stimuli and especially for caudal midline stimuli compared to lateral stimuli. In a second order logistic regression, arena size (P>0.6) and distance (P>0.68) were not significant alone or in any interactions (P>0.16), whereas stimulus angle and stimulus angle^2 were significant (P <0.0008). Less than 5% of the deviance was explained for either arena.
Turn angle was directly proportional to stimulus angle in both arenas but regression slopes were smaller in the small arena (Table S1d). The amount of variance explained was similar.
Heading errors were identical up to about 100°, where under-rotation increased more steeply for the large arena before it did for the small arena.
The mean rotation center was 9 mm more rostral in the large arena (−6 mm versus −15 mm, P<0.0001); in the same ANOVA, it was significantly more rostral for the 15° class, compared to all others and distance was not a factor. In an analogous ANOVA, the mean heading error corresponded to 4° more under-rotation in the small arena (−10° versus −14°, P<0.0001).
Dependencies of standard deviations of turn angle and heading error on stimulus angle resembled those of Fig. 5a: standard deviations differed by angle (P < 0.001; ANOVA) and those in the small arena were about 3° less than those in the large arena for each parameter.
Frequencies of lunges and gathering movements of the arms did not differ significantly according to arena. Both decreased with absolute stimulus angle and gathering movements also decreased with distance, but logistic regressions explained less than 12% of the deviance.
Discussion
Similarity of visual and lateral line turns
Lateral line responses in the sighted animals studied here in the large arena were similar to those reported previously for blinded animals in similar conditions, indicating that damage to stitches around the eyes of blinded animals does not seriously impair accuracy or responsiveness. Turns to the wrong side were rare (3% when small rostral angles are omitted; cf. Elepfandt 1982: 1%; Görner et al. 1984: 1.5%; Claas and Münz 1996: 1%). Turning was both precise and accurate: regressions of turn angle on stimulus angle explained over 90% of the variance and the slopes were near 1. These values are comparable to previous values for animals blinded after metamorphosis (cf. Claas and Münz 1996: average slopes of 0.83, range: 0.8–1.02, R 2 range of 88.4–97.8%); slopes for animals blinded as larvae were lower, averaging 0.58 (Claas 1994). Thus, the visual system and any optomotor responses it might elicit do not oppose and reduce turns commanded by the lateral line system. Absence of interference of vision with lateral line responses was also noted in another lateral line specialist, surface-feeding fish (Mogdans et al. 2002). Neither does there appear to be heightened lateral line acuity in blinded Xenopus, such as occurs for the acoustic systems of ferrets—blinded as infants or adults, cats, and humans (King and Parsons 1999). Unlike blinded toads, which often remain submerged during testing (e.g., Görner et al. 1984), sighted animals invariably come to the surface once surface wave stimuli begin and thus increase their sensitivity for both lateral line (Elepfandt and Wiedemer 1987) and visual stimuli. As in blinded animals (Görner et al. 1984), standard deviations of turn angle increase by a little more than 10° with increasing stimulus angle; here, precision, like responsiveness, was best for rostrolateral stimulus angles.
The apparent effect of modality on turn angle, as revealed in initial regressions of turn angle versus stimulus angle and supported by comparisons in the small arena and for responses initiated at similar distances from the arena wall, was unexpected, because it would suggest that activation of a particular tectal location leads to slightly different motor behaviors according to whether the input is from lateral line or visual systems. Alternatively, response differences might indicate map disparities, as discussed by Stein and Meredith (1993) and evident in barn owls manipulated during development to dissociate visual and acoustic receptive fields (Knudsen and Knudsen 1989; Knudsen et al. 1984).
Surprisingly, these differences in measured turn angle did not translate into differences in orientation accuracy; final headings were equally well oriented for both modalities. Differences in rotation center, one possible explanation, were not significant, which further supports the similarity of turns mediated by visual and lateral line systems. However, the rotation center, besides varying greatly from trial to trial, usually differed from our reference point for measuring stimulus angle, the midpoint between the arms. This difference together with different distributions of stimulus distances for visual and lateral line stimuli explained why turn angles were smaller for visual responses. In other words, for rotation centers caudal to the reference point, proximal stimuli, which were over-represented for visual responses, required less of a turn to face the stimulus than did more distant stimuli, which were over-represented for surface wave responses. This explanation also fits the lateral line responses classified according to wall proximity: when animals were near the wall, stimuli were closer and the difference between stimulus angle and turn angle could be larger—and the regression slope smaller—for the same final heading.
Completely equalizing stimulus distances in visual and lateral line responses was difficult for several reasons. First, visual stimuli had to be at the margin of the arena in order to conceal the probe’s approach and produce well-defined stimulus onsets and directions. Second, to study turning responses, the toads must be unrestrained, so their behavioral preferences influenced distributions of stimuli eliciting responses. Finally, because toads responded well to distant and to caudal lateral line stimuli, their positions within the small arena and hence the combinations of stimulus angle and distance were more varied than for visual tests where the toads tended to remain close to and facing the arena wall after they responded to a stimulus.
The location of the rotation center reflects the motor pattern and mass of the animal, so it is not surprising that it was not identical in all animals. In most trials it was caudal to our reference point and closer to the center of mass. Although studying a range of sizes was not the aim of the present study, the data indicate that the rotation center, as defined here, can be outside the body in individual trials and on average is caudal to the arms by a third to a half the length of the animal.
The present results underline the importance of considering animal size, rotation center, and stimulus distance when comparing turn angles among studies, even though distance alone does not appear to affect turn accuracy. Görner et al. (1984) stated that regression slopes for their young and old animals (3.5 cm versus 5.5 cm snout to vent lengths) did not differ, although the published values fit the trend reported here. Remarking on the even greater under-rotation reported for caudal stimulation of still larger adult animals compared to juveniles (Elepfandt 1982), they speculated that accuracy might decrease with size. Elepfandt (1982) suggested motivation differences as a possible cause. Our results suggest that this apparent inaccuracy may partly reflect the definitions of stimulus and turn angles. The same may apply to reported reductions in measured turning angle for shallow water (Elepfandt 1982); toads may adopt different postures, leading to different rotation centers.
Thus, the behavioral data on accuracy of turning as measured by heading error and actual versus ideal turn angle are consistent with physiological findings of unimodal and bimodal (visual and lateral line) tectal units (Lowe 1986) with overlapping receptive fields arranged in a topological map (Zittlau et al. 1986). Similarly, normal barn owls (Knudsen 1982, 2002) turn accurately to either visual or acoustic stimuli that activate multimodal tectal units with visual receptive fields embedded in the auditory receptive fields. More generally, because either lateral line or visual system can mediate turning, these systems interact in a redundant or possibly synergistic manner (Braun et al. 2002). Lesion experiments (B. Claas et al., in preparation) also support a functional role for the tectal map in surface wave orientation by Xenopus.
How to interpret turns to the wrong side is unclear. Such errors were more common for lateral line stimuli than for visual stimuli, possibly because the visual map is a direct topographic map from the receptor surface whereas the lateral line map is a computational map. The shorter duration of the lateral line stimulus may have further increased left–right errors.
Curiously, turn amplitude for left–right errors, although variable, was correlated with stimulus angle for lateral line stimuli in the large arena; in other words, turn amplitude on average is correct even though the direction is not. This suggests some independence in their specification, a feature also noted in lesioned animals by Elepfandt (1982). Temporally separate specification of motor pattern amplitude and direction has also been observed in human motor control (Favilla et al. 1989). A correlation would not be expected for several alternative explanations, although these may explain some left–right errors. First, some incorrect lateral line turns may have been oriented to diffuse reflected waves, although it has been claimed that Xenopus distinguishes original wave and reflection (P. Görner, personal communication). Latencies of left–right errors were about 200 ms longer on average, but distributions overlapped considerably and 200 ms is too short for reflections to arrive. Second, a lateral line stimulus may release a response oriented toward an otherwise subthreshold visual stimulus (“synergy”: Braun et al. 2002) in the surroundings or to a compromise direction between these two, such as occasionally occurs for spatially separate visual and auditory stimuli (Stein and Meredith 1993). Such interference may include residual activity from previous stimuli; toads making a gross error sometimes appeared to turn toward the previous stimulus site or swim straight ahead. Finally, a few spontaneous turns occurring within the expected response window (Claas and Münz 1996) may have been mistaken for responses.
Typical responses involved an initial turn followed by a variable advance in the new direction, so analyzing the turning accuracy separately from distance is reasonable. Turns took several forms—including an initial backward push for lateral and caudal stimuli, so the axis of rotation was not linked to a fixed anatomical position. Thus, an anatomically fixed motor frame of reference for the turn is not apparent; human pointing illustrates that it need not be the same as a sensory frame of reference.
The error measure used here, one based on the midline axis and the rotation center, best reflects heading and expresses whether the toad can capture a target at the stimulus location by swimming straightforward. Alternative measures, such as the angle to the stimulus location relative to the new position of midpoint between the arms or the direction of translation of our reference point or the head did not accurately represent the toad’s final heading.
The accuracy of turning when the variable rotation center is considered suggests that stimulus location, and hence tectal output, specifies a combination of turn angle and rotation center. The data provide little support for adaptation of rotation center to stimulus parameters; little variance was explained by either stimulus angle or distance. The toads appear to coordinate turn angle and rotation center from trial to trial so as to face the stimulus. On-going investigations of the motor behavior may reveal how this synergy is achieved. Wall proximity did induce slightly more caudal rotation centers, as one would expect for the toad to avoid the wall during turning.
Differences between visual and lateral line responses
Some qualitative differences indicate that visual and lateral line systems contribute unequally to different behavioral responses (“fractionalization”: Braun et al. 2002). Lunges were more frequent for visual stimuli than for lateral line stimuli; this was true even when similar stimulus distances were compared. Capture elements in lateral line responses were restricted to gathering movements with the arms—indicating the toad expects to find prey—or occasional strikes—often at nearby visual features such as air bubbles or parts of the enclosure—in place of or following a turn.
Strikes were most frequent for rostroproximal visual stimuli, as they are in other Ranids (e.g., Ingle 1976; Ewert 1984) where this strike region includes—but is not solely determined by—the binocular visual field (Ingle 1976). The binocular field in the tectum is also the area from which snaps are readily elicited by tectal simulation in Bufo bufo (Schürg-Pfeiffer et al. 1993). In Xenopus, the visual field of each lateral, upward pointed eye is large, including all but a small contralateral crescent of the dorsal hemisphere, so the binocular field is extensive (Udin 1990). Published data on retinotectal maps are usually related to the axis of the eye rather than the animal’s field of view at the water surface, but the binocular field, estimated from data of Gaze et al. (1970) and Udin (1990), appears to be ±20° from the midline at the rostral water surface, ±45° at an elevation of 30°, and 360° at an elevation of 40°. Elevation of most visual stimuli would be less than about 30°, which would occur for a 1 cm height and 2 cm distance. So the area of high lunge frequency appears to extend beyond the binocular field. The visual stimuli used here often were long enough that they would be present after the turn brought them into the rostral field of view, but the majority of turns or lunges are single, ballistic movements, as evidenced by the absence of an effect of presence of the visual stimulus on turn precision or left–right errors.
Related to these behavioral differences were differences in responsiveness for different stimulus directions. Although response frequencies for lateral line stimuli were slightly higher for rostrolateral stimuli, the overall effect of stimulus direction and distance was small. Qualitatively, the arrangement of the lateral lines and individual stitches would not suggest a strong directional bias in sensitivity. Görner (1973), using a rod dipped near the margin of the animal, also found maximum response frequencies for rostrolateral stimuli. In contrast, response frequencies for visual stimuli decreased with distance and direction, even though the visual field covers the entire 360°. Retinal magnification factor or ganglion cell densities measured in some amphibians decrease with distance from the optical axis (e.g., Rana: Jacobson 1962; Grüsser-Cornehls 1984; measurements for Xenopus have not been published), so both visual sensitivity and acuity may decrease for more caudal areas. Due to the toad’s predilection to remain at rest close to the margin of the enclosure, caudal visual stimuli were necessarily farther from the eyes and therefore possibly less easily detectable. Preference should not be affected by this change if the size constancy found in toads (reviewed in Ingle 1976; Ewert 1984) applies to Xenopus. The 5 mm stimuli used here subtended about 14° at 20 cm and 3° at 90 cm; they resemble the 10 mm stimuli subtending 4° at 11 cm that are optimal for slightly larger common toads, Bufo bufo (Ewert 1984).
Unlike some predatory fish (New 2002), where vision appears to control long-range orientation but lateral line inputs can control strikes at short range, Xenopus appears to rely more on the lateral line for long-range orientation like turning and approach. The relatively small eyes of Xenopus indicate that it is less dependent on vision than Esox, Bufo , or Rana. Gathering movements presumably indicate an expectation that prey may be nearby. If the lateral line turn leads to a rostral and proximal visual stimulus, strike and gathering movements follow. Differential behavioral responses depending on stimulus parameters have also been noted in terrestrial toads, which snap at near stimuli and hop to approach more distal stimuli (Grüsser and Grüsser-Cornehls 1976; Ewert 1984). The efficacy of rostral visual stimuli is also evident in the preference of leopard frogs for frontal over lateral stimuli (Stull and Gruberg 1998). Separation of function within subsystems of one sense has been found the lateral line system of fish (e.g., Montgomery et al. 2002).
In-register maps are thought to facilitate detection of multimodal stimuli originating from the same location (Stein and Meredith 1993; Braun et al. 2002); conversely, spatially separated stimuli may depress detection. The slight decrease in lateral line responsiveness to rostral stimuli might reflect slight inhibition or absence of visual facilitation in locations where many natural lateral line stimuli would include a salient visual component (B. Claas et al., in preparation). Stimulus amplitudes were not adjusted here to optimally test intermodal facilitation or inhibition (Stein and Meredith 1993). Tests of combined stimuli are in progress.
In conclusion, responses to visual and lateral line stimuli, despite initial superficial differences, are fundamentally similar with respect to turning and orientation, which is consistent with the control of turning via a common, multimodal map in the tectum (Grobstein 1988). Differences in other aspects of the response need not imply different functions of the tectal map: for either modality, the tectum could control orientation while other areas modulate activity and engage different levels of the prey-capture behavior, as forebrain and pretectal areas appear to do in toads (Ewert 1984; Schürg-Pfeiffer et al. 1993). The differences do suggest that lateral line and vision play slightly different roles: in Xenopus, the lateral line better serves initial orientation and approach to possible prey appearing from any direction, whereas vision better serves attack and capture of nearby prey, particularly in front of the animal.
References
Braun CB, Coombs S, Fay RR (2002) What is the nature of multisensory interaction between octavolateralis subsystems? Brain Behav Evol 59:162–176
Claas B (1994) Removal of eyes in early larval stages alters the responses of the clawed toad, Xenopus laevis, to surface waves. Physiol Behav 56:423–428
Claas B, Münz H (1996) Analysis of surface wave direction by the lateral line system of Xenopus: source localization before and after inactivation of different parts of the lateral line. J Comp Physiol A 178:253–268
Claas B, Münz H, Görner P (1993) Reaction to surface waves by Xenopus laevis Daudin: are sensory systems other than the lateral line involved? J Comp Physiol A 172:759–765
Coombs S, New JG (2002) Preface: multimodal sensory guidance of complex behaviors. Brain Behav Evol 59:159–161
Dusenbury DB (1992) Sensory ecology: how animals acquire and respond to information. WH Freeman, New York
Elepfandt A (1982) Accuracy of taxis response to water-waves in the clawed toad (Xenopus laevis Daudin) with intact or with lesioned lateral line system. J Comp Physiol 148:535–545
Elepfandt A (1984) The role of ventral lateral line organs in water-wave localization in the clawed toad (Xenopus laevis). J Comp Physiol 154:773–780
Elepfandt A (1989) Wave analysis by amphibians. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line: neurobiology and evolution. Springer, Berlin Heidelberg New York, pp. 527–541
Elepfandt A, Wiedemer L (1987) Lateral-line responses to water-surface waves in the clawed frog, Xenopus laevis. J Comp Physiol A 160:667–682
Ewert J-P (1984) Tectal mechanisms that underlie prey-catching and avoidance behaviors in toads. In: Vanegas H (ed) The comparative neurology of the optic tectum. Plenum, New York, pp. 247–416
Ewert J-P (1997) Neural correlates of key stimulus and releasing mechanism: a case study and two concepts. Trends Neurosci 20:332–339
Favilla M, Henning W, Ghez C (1989) Trajectory control in targeted force impulses. VI. Independent specification of response amplitude and direction. Exp Brain Res 75:280–294
Gaze RM, Keating MJ, Szekely G, Beazley L (1970) Binocular interaction in the formation of specific intertectal neuronal connections. Proc R Soc Lond B Biol Sci 175:107–147
Gaze RM, Keating MJ, Chung SH (1974) The evolution of the retinotectal map during development in Xenopus. Proc R Soc Lond B Biol Sci 185:301–330
Görner P (1973) The importance of the lateral line system for the perception of surface waves in the claw toad, Xenopus laevis, Daudin. Experientia 29:295–296
Görner P (1976) Source localization with labyrinth and lateral line in the clawed toad (Xenopus laevis). In: Schuijf A, Hawkins AD (eds) Sound reception in fish. Elsevier, Amsterdam, pp. 171–184
Görner P, Moller P, Weber W (1984) Lateral-line input and stimulus localization in the African clawed toad Xenopus sp. J Exp Biol 108:315–328
Gregory RL (1966) Auge und Gehirn: Zur Psychophysiologie des Sehens. Kindler Verlag, München
Grobstein P (1988) Between retinotectal projection and directed movement: topography of a sensorimotor interface. Brain Behav Evol 31:34–48
Grüsser-Cornehls U (1984) The neurophysiology of the amphibian optic tectum. In: Vanegas H (ed) Comparative neurology of the optic tectum. Plenum, New York, pp. 211–245
Grüsser O-J, Grüsser-Cornehls U (1976) Neurophysiology of the anuran visual system. In: Llinas R, Precht W (eds) Frog neurobiology: a handbook. Springer, Berlin Heidelberg New York, pp. 297–385
Heiligenberg W (1991) Neural nets in electric fish. MIT, Cambridge
Ingle DJ (1976) Behavioral correlates of central visual function in anurans. In: Llinas R, Precht W (eds) Frog neurobiology: a handbook. Springer, Berlin Heidelberg New York, pp. 435–451
Jacobson M (1962) The representation of the retina on the optic tectum of the frog. Correlation between retinotectal magnification factor and retinal ganglion cell count. Q J Exp Physiol 47:170–178
King AJ (1999) Sensory experience and the formation of a computational map of auditory space in the brain. Bioessays 21:900–911
King AJ, Parsons CH (1999) Improved auditory spatial acuity in visually deprived ferrets. Eur J Neurosci 11:3945–3956
Knudsen EI (1982) Auditory and visual maps of space in the optic tectum of the owl. J Neurosci 2:1177–1194
Knudsen EI (2002) Instructed learning in the auditory localization pathway of the barn owl. Nature (London) 417:322–328
Knudsen EI, Knudsen PF (1989) Visuomotor adaptation to displacing prisms by adult and baby barn owls. J Neurosci 9:3297–3305
Knudsen EI, Blasdel GG, Konishi M (1979) Sound localization by the barn owl measured with the search coil technique. J Comp Physiol 133:1–11
Knudsen EI, Esterly SD, Knudsen PF (1984) Monaural occlusion alters sound localization during a sensitive period in the barn owl. J Neurosci 4:1001–1011
Kramer G (1933) Untersuchungen uber die Sinnesleistungen und das Orientierungsverhalten von Xenopus laevis Daud. Zool Jb Physiol 52:629–676
du Lac S, Knudsen EI (1990) Neural maps of head movement vector and speed in the optic tectum of the barn owl. J Neurophysiol 63:131–146
Lindquist SB, Bachmann MD (1982) The role of visual and olfactory cues in the prey catching behavior of the tiger salamander Ambystoma tigrinum. Copeia 1982:81–90
Lowe DA (1986) Organization of lateral line and auditory areas in the midbrain of Xenopus laevis. J Comp Neurol 245:498–513
Mogdans J, Barenbrock J, Bleckmann H (2002) Sighted topminnows, Aplocheilus lineatus, use the lateral line for surface wave discrimination. Copeia 2002:190–194
Montgomery JC, Macdonald F, Baker CF, Carton AG (2002) Hydrodynamic contributions to multimodal guidance of prey capture behavior in fish. Brain Behav Evol 59:190–198
New JG (2002) Multimodal integration in the feeding behaviors of predatory teleost fish. Brain Behav Evol 59:177–189
Rudolph P (1967) Zum Ortungsverhalten von Gyrinus substraticus Steph (Taumelkäfer). Z Vergl Physiol 50:341–361
Schürg-Pfeiffer E, Spreckelsen C, Ewert J-P (1993) Temporal discharge patterns of tectal and medullary neurons chronically recorded during snapping toward prey in toads Bufo bufo spinosus. J Comp Physiol A 173:363–376
Stein BE, Meredith MA (1993) The merging of the senses. MIT, Cambridge
Stull AK, Gruberg ER (1998) Prey selection in the leopard frog: choosing in biased and unbiased situations. Brain Behav Evol 52:37–45
Traub B, Elepfandt A (1990) Sensory neglect in a frog: evidence of early evolution of attentional processes in vertebrates. Brain Res 520:105–107
Udin SB (1990) Plasticity in the ipsilateral visuotectal projection persists after lesions of one nucleus isthmi in Xenopus. Exp Brain Res 79:338–344
Udin SB, Keating MJ (1981) Plasticity in a central nervous pathway in Xenopus: anatomical changes in the isthmo-tectal projection after larval eye rotation. J Comp Neurol 203:575–594
Valentine DE, Sinha SR, Moss CF (2002) Orienting responses and vocalizations produced by microstimulation in the superior colliculus of the echolocating bat, Eptesicus fuscus. J Comp Physiol A 188:89–108
Zittlau KE, Claas B, Münz H (1986) Directional sensitivity of lateral line units in the clawed toad Xenopus laevis Daudin. J Comp Physiol A 158:469–477
Acknowledgment
Volker Duerr (University of Bielefeld) generously provided a Delphi program that was modified for the video frame analysis. These experiments were performed in accord with the NAS Guide for the Care and Use of Laboratory Animals and approved by the CSU IACUC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Claas, B., Dean, J. Prey-capture in the African clawed toad (Xenopus laevis): comparison of turning to visual and lateral line stimuli. J Comp Physiol A 192, 1021–1036 (2006). https://doi.org/10.1007/s00359-006-0137-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-006-0137-2