Abstract
Sexual signals are gained and lost over evolutionary time. While signal gain has obvious fitness benefits, signal loss should present significant costs due to decreased mating opportunities. Just as female mating preferences can promote evolutionary gain and elaboration of sexual signals, they may also maintain signal loss. We investigated how two components of female mate choice are involved in rapid sexual signal loss in the Pacific field cricket (Teleogryllus oceanicus), in which many males have lost the ability to sing. Males that can sing (“normal-wings”) and obligately silent males (“flatwings”) coexist in Hawaiian populations. While we know that females prefer not to mate with flatwings, we tested whether females discriminate against flatwing males before copulation due to the lack of a song or something inherent about their wing morphology. We combined this assessment with a test of post-copulatory preference by presenting females with either a normal-wing or flatwing male in the presence or absence of a courtship song stimulus. Females took significantly longer to mount males in the absence of a courtship song regardless of male wing morph. This is the first evidence that females discriminate against the absence of a song during mate choice, not male wing morph. However, females retained spermatophores for equally long regardless of male wing morph and whether they heard a courtship song, suggesting no post-copulatory barriers in the absence of a song. Pre- and post-copulatory sexual selections may not operate synchronously in this system, which may help explain the success of the silent morph in wild populations.
Significance statement
Signal loss is ubiquitous in nature, despite the associated decrease in mating opportunities. We assessed how the interaction of pre- and post-copulatory preferences may accommodate signal loss. Song attracts mates in Pacific field crickets, but some males have lost the ability to sing in response to an acoustically orienting parasitoid. We measured how long females took to mount males (pre-copulatory choice) and the length of sperm transfer, i.e., spermatophore retention time (post-copulatory choice), when presented with normal and silent males in the presence and absence of a song stimulus. Females took longer to mate in the absence of a courtship song but kept spermatophores for full sperm transfer, regardless of song presence or male type. Pre-copulatory choice in this system acts against silent males, while post-copulatory choice may not. Asynchrony between these two episodes of sexual selection may allow signal loss to persist in spite of the challenges it presents to mating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual signals are gained and lost over evolutionary time, depending on the strength and form of selection acting on a population (Wiens 2001). Often required for mate attraction (Darwin 1871; Andersson 1994), sexual signals have important implications for reproductive success and species divergence (Houde and Endler 1990; Andersson 1994; Boughman 2001; Panhuis et al. 2001; Safran et al. 2013; Barrera-Guzmán et al. 2018). Obvious fitness benefits are associated with signal gain or elaboration, as sexual signals can increase mating opportunities and hence reproductive success (Kirkpatrick and Ryan 1991). In contrast, the loss of a sexual signal should present significant challenges for finding and securing mates. Nevertheless, examples of signal loss are widespread across taxa (Wiens 2001), demonstrating that such losses are commonly facilitated and maintained over evolutionary time. Though signal gain and elaboration are well studied, signal loss is poorly understood, partially because phylogenetic studies have only recently demonstrated a taxonomically widespread loss of sexual traits (Wiens 2001).
Female preference, a known driver for the rise and elaboration of sexually selected traits (Andersson 1994; Jones and Ratterman 2009), may also facilitate sexual signal loss (Weigel et al. 2015). Studies in birds and fish (Basolo 1998; Morris 1998; Morris 2005; Omland 2006) demonstrate that signal loss may occur when there is a prior or co-occurring reduction of female preferences for a male sexual signal (Wiens 2001). Female mate choice is also a multi-step process where females can exercise mating preferences before and after mating, known as pre- and post-copulatory sexual selections, respectively. Episodes of pre- and post-copulatory selections are found especially in polyandrous systems, where females mate with multiple partners, driving selection on male signals in both competitions for mates and post-mating fertilization (Rowe and Arnqvist 2002, reviewed in Kvarnemo and Simmons 2013, Dougherty and Shuker 2016). Female preferences may change at different stages of the mating process; therefore, pre- and post-copulatory sexual selection may reinforce one another (Bangham et al. 2002; Pilastro et al. 2004; Hosken et al. 2008; Sbilordo and Martin 2014; Devigili et al. 2015; McDonald et al. 2017), be asynchronous, or act antagonistically (Warner et al. 1995; Danielsson 2001; Preston et al. 2001; Schneider and Lesmono 2009; Rowe et al. 2010). Incongruence between these two episodes of selection on the same or linked traits may allow for an overall reduction in preference, and therefore weaken selection favoring male sexual signals. Though pre- and post-copulatory sexual selections are clearly linked, few studies have looked at how they interact to drive the gain, elaboration, or loss of sexual signals (Hunt et al. 2009; Rose et al. 2013; Evans and Garcia-Gonzalez 2016).
We use the unique case of rapid sexual signal loss in the Pacific field cricket (Teleogryllus oceanicus) to assess how pre- and post-copulatory mating preferences contribute to sexual signal loss. Like in several field cricket species, mating in T. oceanicus consists of males producing a long-range calling song by rubbing together specialized wing structures and females responding phonotactically by approaching the male (Alexander 1961). Once the female is in close range, the male produces a courtship song, after which the female mounts and accepts his spermatophore. The spermatophore, a small protein capsule containing a package of sperm, attaches to the female’s abdomen and drains sperm into the spermatheca to be stored until fertilization (Alexander 1961, Balakrishnan and Pollack 1996, Alexander and Otte 1967). Females can remove spermatophores at any point in the process and limit the amount of sperm transfer, which increases with time (in Gylllodes supplicans: Sakaluk 1984, in Gryllus bimaculatus: Simmons 1986, in Teleogryllus oceanicus: Simmons et al. 2003). After transfer, females allow differential access of sperm to storage, influencing fertilization by cryptic choice (Tuni et al. 2013; Simmons et al. 2014). Female T. oceanicus mate multiple times in the wild (Tanner et al. 2019cin press), consequently exerting strong post-copulatory sexual selection (Simmons and Beveridge 2010).
In the Hawaiian Islands, where T. oceanicus was introduced, males face significant predation pressure from the acoustically orienting parasitoid fly, Ormia ochracea, which uses male calling song to find its hosts (Zuk et al. 1995; Zuk et al. 1993). Over a period of just a few years, a novel wing mutation spreads, apparently in response to this predation pressure, eliminating the “file and scraper” morphology on the wings necessary to produce a song and rendering its bearers obligately mute (Zuk et al. 2006). Males with this wing mutation (“flatwings”) and males without it (“normal-wings”) coexist in different proportions in three Hawaiian populations (Zuk et al. 2018). Flatwing males are hidden from the parasitoid but cannot attract mates due to the lack of sexual signal (Zuk et al. 2006); similarly, females prefer not to mate with flatwings (Tinghitella and Zuk 2009).
Flatwings are able to bypass their inability to produce a calling song by acting as satellites to normal-wing males. As satellites, flatwings settle in close proximity to singing males and attempt to intercept females attracted to the calling song (Zuk et al. 2006). While both normal- and flatwings engage in satellite behavior, flatwing males are obligate, not facultative satellites. Perhaps because of this necessity, flatwings are just as phonotactic as females, localizing and showing preference for attractive calling songs (Olzer and Zuk 2018). By adopting this alternative mating strategy, flatwings navigate the first barrier to mating—lack of a calling song. It is unclear, however, how flatwings mate without producing a courtship song.
Even in the absence of a courtship song, females of different populations are willing to mate with flatwings in lab conditions (Tinghitella and Zuk 2009). Flatwings coexist with normal-wings in stable proportions across the Hawaiian Islands (Zuk et al. 2018), implying that silent males do achieve matings in the wild. Females reared in song-less environments (characteristic of populations with a high density of flatwings) demonstrate a relaxed preference for calling song quality, implying plasticity in mate choice (Bailey and Zuk 2008; Bailey and Zuk 2012). Female behavioral plasticity appears important for flatwings to navigate their inability to produce a courtship song.
However, previous work on female response to flatwings has assessed only pre- but not post-copulatory selection, demonstrating that females avoid mating with flatwing males (Tinghitella and Zuk 2009). As previously established, the relationship between pre- and post-copulatory preferences can either strengthen or weaken selection on a trait, and examining the interaction between these two modes of selection allows us to understand how signal loss persists in this system. We assessed if females discriminate against silent males on the basis of wing morph or the absence of a courtship song, and whether spermatophore retention time differs in response to male wing morph or absence of a courtship song. If pre- and post-copulatory selections act uniformly in the same direction and favor either the normal-wing morph or the presence of a courtship song, it should create a braking force against signal loss. If they act asynchronously in different directions, selection against the silent male morph may be weakened. Following metrics established with other cricket species, we measured female latency to mount as a metric for pre-copulatory choice and spermatophore retention time as one component of post-copulatory choice. Latency to mount is inversely proportional to female preference (in Teleogryllus commodus: Shackleton et al. 2005, in Teleogryllus oceanicus: Rebar et al. 2011), and spermatophore retention time is proportional to amount of sperm transferred (in Gryllodes supplicans: Sakaluk 1984, in Teleogryllus oceanicus: Simmons et al. 2003); females can remove spermatophores prematurely and limit sperm transfer (in Acheta domesticus: Mautz and Sakaluk 2008, in Teleogryllus oceanicus: Rebar et al. 2011). We conducted mating trials where female crickets were presented with either normal-wing or flatwing males in the presence or absence of a courtship song, allowing us to evaluate the relative importance of wing morph and song during multiple episodes of female mate choice.
Methods
Study system
We used T. oceanicus from a laboratory colony originating from Kauai, first collected in 2004 after the establishment of the flatwing morph. This colony is replenished annually with eggs from field-caught Kauai females to reduce potential for inbreeding. We reared the crickets in Caron Insect Growth Chambers at 75% humidity, 25 °C and on a 12:12 photo-reversed light:dark cycle. Prior to final molt, we placed juvenile males and females in plastic containers (35.9 × 20 × 12.4 cm) with no more than 10–12 same-sex individuals to control for mating experience. Crickets had egg carton for shelter, Teklad rabbit chow for food, and water ad libitum in damp cotton pads. Upon final molt (eclosion), we housed crickets in individual 118-mL plastic cups with shelter, food, and water. At this time, we surgically muted normal-wing males by removing the scraper used to produce a song. Flatwings received the same treatment to control for handling effects. Adult crickets are considered sexually mature 6 days after final molt (Bailey and Zuk 2008), so we used individuals 6–12 days post-eclosion.
Synthetic courtship song construction
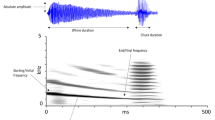
Courtship song in T. oceanicus consists of a long, amplitude-modulated set of pulses called a chirp, followed by a long trill of several repeated pulses (Zuk et al. 2008, Rebar et al. 2009, Balakrishnan and Pollack 1996). We measured relevant temporal components of lab-recorded courtship songs (ten songs from ten males) using Raven Lite (Version 2.0, Cornell Laboratory of Ornithology, Ithaca, NY, USA). Then, we constructed a synthetic courtship song using averaged values of these temporal parameters (Table 1) using a custom script and graphical user interface (GUI; Tanner et al. 2019b). We made the courtship song at a sampling rate of 44.1 kHz and a bit depth of 16. All pulses in the chirp had a frequency modulation sweep of 800 Hz. We then concatenated the song to make a 5-min stimulus (the duration of a mating trial) using Audacity® (Version 2.2.2, Audacity Team).
Mating trials
Since virgin females are often more responsive to males than non-virgin females (Tanner et al. 2019a), we mated all females once to a random male from the Kauai colony at least 6 days post-eclosion and 24 h before their mating trial. We left the spermatophore attached for 30 min, at which point we removed it with forceps to control for the amount of sperm each female received.
We conducted mating trials in an anechoic chamber at 22–25 °C under red lighting. “Song” trials involved playing the standardized courtship song, while “No Song” trials were conducted in silence. We randomized the order of all trials before data collection. During trials, we placed a randomly paired male and female cricket in a plastic container (12 cm × 17 cm) with a mesh-covered opening at one side to allow for better sound propagation. We placed a paper towel on the bottom of the plastic container and replaced it after each trial to prevent overlap in chemical cues. We also placed a speaker (Sony SRS-M30 Active Speaker System) at the mesh end of the plastic container and broadcast the synthetic courtship song at 68–71 dB (measured 10 cm from the container) during song trials. We confirmed the sound level before trials with a digital sound level meter (Graigar Technology 8922 RS232 AZ). In “Song” trials, we started the song stimulus as soon as we placed the male and female together inside of the mating arena. We then timed latency to mount, measured as the time from the beginning of song stimulus to the point at which the female mounts the male. The time females take to mount a male is inversely proportional to preference, so we used latency to mount as a metric for pre-copulatory choice (Shackleton et al. 2005; Rebar et al. 2011). After the female mounted the male, we switched off the courtship song. For “No Song” trials, we also started measuring latency to mount from the time when the male and female were placed into the container together, but did not broadcast the song stimulus. After spermatophore transfer, we placed the female into a 118-mL cup and left her in a dark and quiet room to measure the duration of time that the spermatophore remains attached to the female. The amount of time that a spermatophore is attached to the female abdomen is proportional to how much sperm is transferred (Simmons et al. 2003). While most of the sperm is generally transferred in approximately 40 min (Simmons et al. 2003), females can prematurely terminate sperm transfer by removing the spermatophore before this point (Rebar et al. 2011). Across different species of crickets, males that transfer a greater amount of sperm, though not necessarily through spermatophore retention, sire a higher proportion of offspring (Sakaluk 1986; Simmons 1987; Sakaluk and Eggert 1996; Calos and Sakaluk 1998; Eggert et al. 2003; García-González and Simmons 2005; Bussière et al. 2006; Mautz and Sakaluk 2008), making spermatophore retention time a suitable metric for post-copulatory choice in many cases. We checked every 5 min to see if the female still had the spermatophore and removed the spermatophore at 40 min, at which point most sperm empties into the female’s spermatheca (Simmons et al. 2003).
We note that we removed spermatophores at a standardized time instead of measuring natural variation in removal times. However, 40 min is a reasonable time frame for standardized spermatophore retention in this species. Simmons et al. (2003) shows that the majority (~ 72%) of sperm in a spermatophore had already transferred by 40 min. The likelihood of females selecting to differentially remove spermatophores much later than when discriminating between two types of males is low. Since the removal at 40 min was standardized across treatments and likely does not limit more than a small amount of remaining sperm from transferring into the spermatheca, we believe that this method still allowed us to examine spermatophore retention time as a post-copulatory metric. We preferred this option to allow females to keep spermatophores indiscriminately, as females can sometimes retain empty spermatophore sacs after sperm transfer has ended. Therefore, measuring natural removal times may not be the best way to measure post-copulatory choice.
A trial was considered successful if the female kept the spermatophore until her transfer to a cup. A trial was unsuccessful if the female did not mount the male in 5 min or if the female mounted the male but there was no spermatophore transfer. In 91 of the 209 trials (43%), females failed to mount and the trial was terminated. Three spermatophores dislodged during transfer to the cup after mating. We included these data in the final analyses, though they did not change any results. We allowed each male and female up to 3 mating attempts before excluding them from the experiment, but did not repeat any mating combinations (Table 2).
Data analysis
We conducted all statistical analyses on RStudio (2015, RStudio Inc., Boston, MA).
Likelihood to mount
We tested the effects of song stimulus and wing morph on the probability that a female mounted a male with a mixed-effects generalized linear model with binomial distribution. The model held the male and female identifiers as random effects since individuals had multiple opportunities for a successful trial. We also held the wing morph of the male from the pre-trial mating as a random effect.
Latency to mount
We examined the effects of song stimulus and male wing morph on female latency to mount with a linear regression (n = 19 flatwing (FW) No Song, n = 23 FW Song, n = 30 normal-wing (NW) No Song, n = 32 NW Song, n = 105 in total). We initially included male and female age, number of tries for successful mating (for both males and females), and male wing morph in pre-trial matings as random effects. In the final model, we removed male age, male number of tries, and pre-mating male wing morph due to non-significance (male age F = 1.13, p = 0.29; number of male attempts before successful mating F = 2.79, p = 0.097; pre-trial mating male wing morph F = 3.47, p = 0.07).
Spermatophore retention
We examined the independent effects of song stimulus and wing morph on spermatophore retention time with two Mann-Whitney U tests (n = 42 FW, n = 63 NW, n = 56 Song, n = 49 No Song, n = 105 in total per independent effect). Because our response variable (spermatophore retention time) was non-normally distributed (Shapiro-Wilk goodness of fit, p < 0.001), we used the Mann-Whitney U, a non-parametric test of medians. We also tested for an interaction effect with a mixed-effects generalized linear model with binomial distribution. In this model, we held the male wing morph of the pre-trial mating and the number of tries before successful matings (males and females) as random effects. To run the binomial model, we converted our spermatophore retention data to binary variables: whether or not females kept spermatophores for the full 40 min (enough time for most sperm to transfer). Since most females kept spermatophores for 40 min, this time served as a natural breaking point in the data.
Separating the two response variables
It can be an error to conclude that a significant difference in latency to mount and a non-significant difference in spermatophore retention time mean that the traits are independent. Lastly, we used the residuals from the latency to mount linear model as a random effect in the spermatophore retention mixed-effects linear model to control for the effects of latency to mount on retention times.
Results
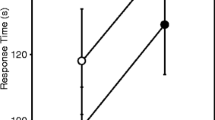
Song stimulus (mixed-effects generalized linear model, Wald chi-square = 0.272, p = 0.602, df = 208) and male wing morph (mixed-effects generalized linear model, Wald chi-square = 0.088, p = 0.735, df = 208) had no independent effects on female probability of mounting. The two variables also showed no interaction on probability of mounting (Wald chi-square = 0.006, p = 0.936, df = 208). Females took significantly longer to mount males in the absence of a courtship song, regardless of male wing morph (linear regression, F1,104 = 13.91, p = 0.0003, Fig. 1). Male wing morph did not have an effect on female latency to mount (linear regression, F1,104 = 0.74, p = 0.289). Song stimulus and male wing morph showed no interaction on latency to mount (linear regression, F1,104 = 0.285, p = 0.392). Spermatophore retention times did not differ based on the presence or absence of a courtship song (Mann-Whitney U non-parametric test of medians, w = 1574, p = 0.12, df = 104) or male wing morph (Mann-Whitney U non-parametric test of medians, w = 1178, p = 0.254, df = 104). Song stimulus and male wing morph showed no interaction on the probability of females retaining spermatophores for 40 min (mixed-effects generalized linear model, Wald chi-square = 0.658, p = 0.417, df = 104). Residuals from the latency to mount model had no effect on spermatophore retention time.
Discussion
Here, we provide the first empirical evidence that in T. oceanicus, 1) females discriminate against absence of a song, not wing morph per se, prior to mating and 2) females allow spermatophores to remain attached for full transfer without regard to song stimulus or male morph. Females were quicker (Fig. 1), though not more likely, to mount males when perceiving a courtship song and demonstrated no difference in likelihood or latency to mount when presented with flatwing or normal-wing males. Therefore, females appear to discriminate in some ways against flatwing males for their inability to produce a song, not because of other factors associated with their wing morphology. Furthermore, since females took significantly longer to mount in the absence of a song (Fig. 1), we demonstrate that females exert strong pre-copulatory selection against silent males. The results of a power analysis showed that we had a sufficiently large sample size to detect this effect (G*Power, type of test: F test for linear regression, type of power analysis: post hoc, power = 0.8).
Females also held spermatophores for approximately 40 min regardless of the presence or absence of a song stimulus or male wing morph. There is a possibility that females discriminate in spermatophore retention times, but we were unable to detect the difference without larger sample sizes. However, the results of a power analysis showed that our sample size was high enough to detect the female effect on spermatophore retention times, if one existed (G*Power, type of test: F test for linear regression, type of power analysis: post hoc, power = 0.99). As 40 min is an enough time to allow most of the sperm to drain into the spermatheca (Simmons et al. 2003), females appear to indiscriminately allow transfer of flatwing and normal-wing sperm from spermatophores. We demonstrate that females do not exert post-copulatory selection against silent males via prematurely removing spermatophores and terminating sperm transfer.
Though flatwings are shielded from the parasitoid fly and therefore successful in survival, we show that females are faster to mate with singing males over silent ones, potentially reducing flatwing reproductive success. Despite this obvious pre-copulatory challenge to mating, flatwing males have maintained a stable presence in two Hawaiian populations (approximately 90% on Kauai, 50% on Oahu, < 1% on the big island of Hawaii; Zuk et al. 2018). Flatwings can successfully bypass female preference for singing males by acting as satellites to singing males (Zuk et al. 2006; Olzer and Zuk 2018). Though both normal- and flatwing males can act as satellites, flatwings are demonstrably more likely to engage in satellite behavior (Olzer and Zuk 2018). Flatwings also face low risk of parasitization from this alternative mating strategy and are almost completely protected from the fly, which should give them a substantial survival advantage (Zuk et al. 2006). When raised in song-free environments in the lab, flatwing males demonstrate substantial social plasticity, walking around more than normal-wing males and presumably increasing chances of encountering receptive females (Balenger and Zuk 2015). Similarly, females raised in song-free environments characteristic of a high flatwing density demonstrate relaxed requirements for attractive songs and are more accepting of silent males (Tinghitella and Zuk 2009; Bailey and Zuk 2008; Bailey and Zuk 2012). Success with alternative mating tactics and female behavioral plasticity both appear to act to help flatwings overcome pre-copulatory barriers to mating. Our results show that once flatwings bypass these pre-copulatory challenges, their reproductive success is at least not limited by premature spermatophore removal.
Although we demonstrate that females keep spermatophores attached for full sperm transfer regardless of male wing morph, this does not necessarily translate to equal proportion of offspring between the two morphs. Females in T. oceanicus can control via cryptic choice how much sperm is allocated to storage in the spermatheca, regardless of how long spermatophores are left attached (Tuni et al. 2013; Simmons et al. 2014). While males across several cricket species experience higher paternity rates with higher amounts of sperm transfer (Sakaluk 1986; Sakaluk and Eggert 1996; Calos and Sakaluk 1998; Eggert et al. 2003; García-González and Simmons 2005; Bussière et al. 2006; Mautz and Sakaluk 2008), more recent evidence suggests that in T. oceanicus, spermatophore retention time does not have a significant effect on paternity, only in the amount of sperm transferred (Simmons et al. 2003). Once females store differential amounts of sperm by cryptic choice, in some species, sperm mixes indiscriminately in storage and male paternity is almost entirely determined by how much sperm is represented in the spermatheca (in Gryllus bimaculatus: Simmons 1987, in Gryllodes sigillatus: Sakaluk and Eggert 1996, in Teleogryllus oceanicus: García-González and Simmons 2005). We would need to directly evaluate sperm allocation in mated females, as well as flatwing paternity success, to claim that females exert no post-copulatory choice against flatwings altogether. However, the lack of differences in spermatophore retention indicates some form of post-mating advantage, or lack of disadvantage for singing males. Spermatophore retention time is an established post-copulatory metric in several cricket species, including, still, T. oceanicus. Though retaining a spermatophore for full sperm transfer does not translate to fertilization rates, females still have the option to prematurely terminate sperm transfer itself and even further limit chances of fertilization. Therefore, our results still indicate a lack of post-copulatory selection in a strong metric.
Overall, we find that females take longer to mount in the absence of a song (Fig. 1) and do not discriminate in spermatophore removal between morphs or song presence. One can take the former as evidence that silent males face some pre-copulatory challenges and the latter as evidence for limited post-copulatory selection against silent males. Hence, we suggest that a mismatch between pre- and post-copulatory sexual selections may help maintain signal loss in this system by facilitating persistence of the flatwing morph. Sexual selection research often focuses on female preference as a driver for the gain and elaboration, but not the loss, of sexually selected traits. Though there is a substantial evidence for pre- and post-copulatory sexual selections acting as directional agents in trait evolution (reviewed in Birkhead and Pizzari 2002), few studies have evaluated their specific roles in signal loss. In T. oceanicus, pre- and post-copulatory selections appear to act incongruently, with females preferring song before mating, but neither preferring nor disfavoring song after. This uncovers a piece of the puzzle explaining the success of the flatwing morph and how sexual signal loss has persisted in T. oceanicus Hawaiian populations but also serves as an explanation for how pre- and post-copulatory selections can interact to prolong signal loss. Though the two forces do not act antagonistically (i.e., females take less time to mount but remove spermatophores faster in the presence of song, or vice versa), female preference is not consistent, and post-copulatory selection still does not reinforce the preference demonstrated in pre-copulatory choice. Our results highlight the importance of examining how different episodes of female preference facilitate the taxonomically widespread loss of sexual traits.
Data availability
The raw data for this study can be found in the Dryad digital data repository:
Kota MV, Uquhart EM, Zuk M. Data from: Spermatophore Retention May Accommodate Sexual Signal Loss in Pacific Field Crickets. Dryad Digital Repository. https://datadryad.org/stash/share/OLWZYmZ1a0jJsO1ReT8i7-5O_4bC9Yi9EQjzHo7hhAc.
References
Alexander RD (1961) Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behav. 17:130–223
Alexander RD, Otte D (1967) The Evolution of Genitalia and Mating Behavior in Crickets (Gryllidae) and other Orthoptera. Univ. Mich. Mus. Zool. Misc. Pub. 133:1–62
Andersson M (1994) Sexual selection. Princeton, New Jersey, United States
Bailey NW, Zuk M (2008) Acoustic experience shapes female mate choice in field crickets. Proc R Soc B 275:2645–2650
Bailey NW, Zuk M (2012) Socially flexible female choice differs among populations of the Pacific field cricket: geographical variation in the interaction coefficient psi (ψ). Proc R Soc B 279:3589–3596
Balakrishnan R, Pollack GS (1996) Recognition of courtship song in the field cricket, Teleogryllus oceanicus. Anim Behav 51:353–366
Balenger SL, Zuk M (2015) Roaming Romeos: male crickets evolving in silence show increased locomotor behaviors. Anim Behav 101:213–219
Bangham J, Chapman T, Partridge L (2002) Effects of body size, accessory gland and testis size on pre- and postcopulatory success in Drosophila melanogaster. Anim Behav 64:915–921
Barrera-Guzmán AO, Aleixo A, Shawkey MD, Weir JT (2018) Hybrid speciation leads to novel male secondary sexual ornamentation of an Amazonian bird. Proc Natl Acad Sci U S A 115:218–225
Basolo AL (1998) Evolutionary change in a receiver bias: a comparison of female preference functions. Proc R Soc B 265:2223–2228
Birkhead TR, Pizzari T (2002) Postcopulatory sexual selection. Nat Rev Genet 3:262–273
Boughman JW (2001) Divergent sexual selection enhances reproductive isolation in sticklebacks. Nat. 411:944–948
Bussière LF, Hunt J, Jennions MD, Brooks R (2006) Sexual conflict and cryptic female choice in the black field cricket, Teleogryllus commodus. Evolution 60:792–800
Calos JB, Sakaluk SK (1998) Paternity of offspring in multiply-mated female crickets: the effect of nuptial food gifts and the advantage of mating first. Proc R Soc B 265:2191–2195
Danielsson I (2001) Antagonistic pre- and post-copulatory sexual selection on male body size in a water strider (Gerris lacustris). Proc R Soc B 268:77–81
Darwin C (1871) The descent of man: and selection in relation to sex, London, England
Devigili A, Evans JP, Di Nisio A, Pilastro A (2015) Multivariate selection drives concordant patterns of pre- and postcopulatory sexual selection in a livebearing fish. Nat Commun 6:8291
Dougherty LR, Shuker DM (2016) Variation in pre- and post-copulatory sexual selection on male genital size in two species of lygaeid bug. Behav Ecol Sociobiol 70:625–637
Eggert AK, Reinhardt K, Sakaluk SK (2003) Linear models for assessing mechanisms of sperm competition: the trouble with transformations. Evolution 57:173–176
Evans JP, Garcia-Gonzalez F (2016) The total opportunity for sexual selection and the integration of pre- and post-mating episodes of sexual selection in a complex world. J Evol Biol 29:2338–2361
García-González F, Simmons LW (2005) Sperm viability matters in insect sperm competition. Curr Biol 15:271–275
Hosken DJ, Taylor ML, Hoyle K, Higgins S, Wedell N (2008) Attractive males have greater success in sperm competition. Curr Biol 18:R553–R554
Houde AE, Endler JA (1990) Correlated evolution of female mating preferences and male color patterns in the guppy Poecilia reticulata. Science 248:1405–1408
Hunt J, Breuker CJ, Sadowski JA, Moore AJ (2009) Male-male competition, female mate choice and their interaction: determining total sexual selection. J Evol Biol 22:13–26
Jones AG, Ratterman NL (2009) Mate choice and sexual selection: what have we learned since Darwin? Proc Natl Acad Sci U S A 106:10001–10008
Kirkpatrick M, Ryan MJ (1991) The evolution of mating preferences and the paradox of the lek. Nat. 350:33–38
Kvarnemo C, Simmons LW (2013) Polyandry as a mediator of sexual selection before and after mating. Philos Tr Soc B 3681–16
Mautz BS, Sakaluk SK (2008) The effects of age and previous mating experience on pre- and post-copulatory mate choice in female house crickets (Acheta domesticus L.). J. Insect Behav 21:203–212
McDonald GC, Spurgin LG, Fairfield EA, Richardson DS, Pizzari T (2017) Pre- and postcopulatory sexual selection favor aggressive, young males in polyandrous groups of red junglefowl. Evolution 71:1653–1669
Morris MR (1998) Female preference for trait symmetry in addition to trait size in swordtail fish. Proc R Soc B 265:907–911
Morris MR (2005) Further examination of female preference for vertical bars in swordtails: preference for “no bars” in a species without bars. J Fish Biol 53:56–63
Olzer R, Zuk M (2018) Obligate, but not facultative, satellite males prefer the same male sexual signal characteristics as females. Anim Behav 144:37–43
Omland KE (2006) Examining two standard assumptions of ancestral reconstructions: repeated loss of dichromatism in dabbling ducks (Anatini). Evolution 51:1636–1646
Panhuis T, Butlin R, Zuk M, Tregenza T (2001) Sexual selection and speciation. Trends Ecol Evol 16:364–371
Pilastro A, Simonato M, Bisazza A, Evans JP (2004) Cryptic female preference for colorful males in guppies. Evolution 58:665–669
Preston BT, Stevenson IR, Pemberton JM, Wilson K (2001) Dominant rams lose out by sperm depletion. Nat. 409:681–682
Rebar D, Bailey NW, Zuk M (2009) Courtship song’s role during female mate choice in the field cricket Teleogryllus oceanicus. Behav Ecol 20:1307–1314
Rebar D, Zuk M, Bailey NW (2011) Mating experience in field crickets modifies pre- and postcopulatory female choice in parallel. Behav Ecol 22:303–309
Rose E, Paczolt KA, Jones AG (2013) The contributions of premating and postmating selection episodes to total selection in sex-role-reversed gulf pipefish. Am Nat 182:410–420
Rowe L, Arnqvist G (2002) Sexually antagonistic coevolution in a mating system: combining experimental and comparative approaches to address evolutionary processes. Evolution 56:754–767
Rowe M, Swaddle JP, Pruett-Jones S, Webster MS (2010) Plumage coloration, ejaculate quality and reproductive phenotype in the red-backed fairy-wren. Anim Behav 79:1239–1246
Safran RJ, Scordato ESC, Symes LB, Rodríguez RL, Mendelson TC (2013) Contributions of natural and sexual selection to the evolution of premating reproductive isolation: a research agenda. Trends Ecol Evol 28:643–650
Sakaluk SK (1984) Male crickets feed females to ensure complete sperm transfer. Science 4636:609–610
Sakaluk SK (1986) Sperm competition and the evolution of nuptial feeding behavior in the cricket, Gryllodes supplicans. Evolution 40:584–593
Sakaluk SK, Eggert AK (1996) Female control of sperm transfer and intraspecific variation in sperm precedence: antecedents to the evolution of a courtship food gift. Evolution 50:694–703
Sbilordo SH, Martin OY (2014) Pre- and postcopulatory sexual selection act in concert to determine male reproductive success in Tribolium castaneum. Biol J Linn Soc 112:67–75
Schneider JM, Lesmono K (2009) Courtship raises male fertilization success through post-mating sexual selection in a spider. Proc R Soc B 276:3105–3111
Shackleton MA, Jennions MD, Hunt J (2005) Fighting success and attractiveness as predictors of male mating success in the black field cricket, Teleogryllus commodus: the effectiveness of no-choice tests. Behav Ecol Sociobiol 58:1–8
Simmons LW (1986) Inter-male competition and mating success in the field cricket, Gryllus bimaculuts (De Geer). Anim Behav 34:567–579
Simmons LW (1987) Sperm competition as a mechanism of female choice in the field cricket, Gryllus bimaculatus. Behav Ecol Sociobiol 21:197–202
Simmons LW, Beveridge M (2010) The strength of postcopulatory sexual selection within natural populations of field crickets. Behav Ecol 21:1179–1185
Simmons LW, Wernham J, Garcia-Gonzalez F, Kamien D (2003) Variation in paternity in the field cricket Teleogryllus oceanicus. Behav Ecol 14:539–545
Simmons LW, Lovegrove M, Almbro M (2014) Female effects, but no intrinsic male effects on paternity outcome in crickets. J Evol Biol 27:1644–1649
Tanner JC, Garbe LM, Marlene Zuk M (2019a) When virginity matters: age and mating status affect female responsiveness in crickets. Animal Behaviour 147:83–90
Tanner JC, Justison J, Bee MA. (2019b) SynSing: Open-Source MATLAB Code for Generating Synthetic Signals in Studies of Animal Acoustic Communication. Bioacoustics 29:1-22
Tanner JC, Swanger E, Zuk M (2019c) Sexual signal loss in field crickets maintained despite strong sexual selection favoring singing males. Evolution 73:1482–1489
Tinghitella RM, Zuk M (2009) Asymmetric mating preferences accommodated the rapid evolutionary loss of a sexual signal. Evolution 63:2087–2098
Tuni C, Beveridge M, Simmons LW (2013) Female crickets assess relatedness during mate guarding and bias storage of sperm toward unrelated males. J Evol Biol 26:1261–1268
Warner RR, Shapiro DY, Marcanato A, Petersen CW (1995) Sexual conflict: males with highest mating success convey the lowest fertilization benefits to females. Proc R Soc B 262:135–139
Weigel EG, Testa ND, Peer A, Garnett SC (2015) Context matters: sexual signaling loss in digital organisms. Ecol Evol 5:3725–3736
Wiens JJ (2001) Widespread loss of sexually selected traits: how the peacock lost its spots. Trends Ecol Evol 16:517–523
Zuk M, Simmons LW, Cupp L (1993) Calling characteristics of parasitized and unparasitized populations of the field cricket Teleogryllus oceanicus. Behav Ecol Sociobiol 33:339–343
Zuk M, Simmons LW, Rotenberry JT (1995) Acoustically-orienting parasitoids in calling and silent males of the field cricket Teleogryllus oceanicus. Ecol Entomol 20:380–383
Zuk M, Rotenberry JT, Tinghitella RM (2006) Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol Lett 2:521–524
Zuk M, Bailey NW, Gray B, Rotenberry JT (2018) Sexual signal loss: the link between behaviour and rapid evolutionary dynamics in a field cricket. J Anim Ecol 87:623–633
Zuk M, Rebar D, Scott SP (2008) Courtship song is more variable than calling song in the field cricket Teleogryllus oceanicus. Animal Behaviour 76(3):1065–1071
Acknowledgments
We thank Kirstine Grab, Adam Hartman, Jake Hjort, Justa Heinen-Kay, Rachel Nichols, Grace Richmond, Kristin Robinson, Erin Schwister, Daina Strub, Rachel Olzer, and Jessie Tanner for assistance with cricket rearing, experimental logistics, and critical feedback.
Funding
We would like to thank our funding sources, the National Science Foundation (IOS-1261575) and the Daniel and Margaret Carper Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We conducted this research in the absence of any competing commercial or financial interests.
Ethical approval
While invertebrates are not subject to IACUC regulation, we adhered to ABS/ASAB guidelines for the ethical treatment of animals in behavioral research.
Additional information
Communicated by K. Shaw
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kota, M.V., Urquhart, E.M. & Zuk, M. Spermatophore retention may accommodate sexual signal loss in Pacific field crickets. Behav Ecol Sociobiol 74, 95 (2020). https://doi.org/10.1007/s00265-020-02850-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02850-z