Abstract
Although females’ mating preferences are influenced by male characteristics, there are a number of factors intrinsic to females and unrelated to male phenotype that can modulate female choice. We assessed the effects of age and mating experience on mechanisms of pre- and post-copulatory mate choice in female house crickets, Acheta domesticus L., by randomly assigning males to females, but independently varying the age and number of previous matings of females at the time of experimental matings. Latency to mating, a measure of a female’s pre-copulatory preference, was influenced by female age at the time of mating, with older females mating sooner than younger females. The reduced selectivity of older females appears consistent with life-history theory, which predicts that the reproductive value of females should decline with age. The length of time that females retained the spermatophore after mating, a measure of a female’s post-copulatory mating preference, was not influenced by female age at the time of mating, the number of previous matings, or any interaction between the two main effects. Contrary to previous reports, male mass had no effect on either the latency to mating or female retention of the spermatophore in A. domesticus. We conclude that female age and mating experience can moderate female selectivity, but that their impact varies according to the mechanism by which females favor particular sires.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female mate choice is a pervasive force shaping the evolution of male sexual signals (Andersson 1994). Although considerable effort has been directed at identifying the male traits upon which mating preferences are based, less attention has been paid to factors unrelated to male phenotype that influence the expression of females’ preferences. Such factors can be partitioned into those extrinsic to the female (i.e., environmental conditions) and those intrinsic to the female (i.e., age, experience or genetic makeup). Extrinsic factors that have been shown to influence female mate choice include the risk of predation (Csada and Neudorf 1995; Hedrick and Dill 1993), ambient light (Gamble et al. 2003), and seasonal changes (Borg et al. 2006). Extrinsic factors that increase the costs of mate choice, such as an increased risk of predation, are likely to favor females that accept lower quality males (Csada and Neudorf 1995; Hedrick and Dill 1993), and thereby increase variation in the expression of female mating preferences.
Intrinsic factors that influence how females choose their mates include female condition (Burley and Foster 2006; Hunt et al. 2005; Luttbeg et al. 2001), reproductive state (Lynch et al. 2005), hormonal state (Lynch et al. 2006), and female age and mating history (Forslund and Part 1995; Prosser et al. 1997; Moore and Moore 2001; Martin and Hosken 2002; Mack et al. 2003). Female age and mating history represent a special case because these factors often are positively correlated and may interact in their effect on female mating preferences. As females age, their reproductive effort is expected to decline and selection is expected to be weak (Futuyma 1998), such that females are expected to become less selective of prospective mates as they get older. However, any age-related threshold to mating is likely to be modulated by the number times a female has mated previously. Assuming that the benefits of mating to females increase at a diminishing rate and that the costs of mating remain constant, females may benefit by adopting a higher threshold to mating as the number of previous matings increases. Hence, the effect of previous mating experience may counteract the decreased selectivity of older females. Although some attempts have been made to dissect the interaction between age and mating experience (Prosser et al. 1997; Kodric-Brown and Nicoletto 2001; Moore and Moore 2001; Martin and Hosken 2002), no study has succeeded in completely disentangling the effects of these two variables.
The house cricket, Acheta domesticus L., is well suited to investigating the effects of age and mating experience on both pre- and post-copulatory mechanisms of female choice. In crickets, females visit individual males sequentially at males’ calling sites (reviewed in Zuk and Simmons 1997). Female A. domesticus exhibit pre-copulatory mating preferences for larger males (Crankshaw 1979; Gray 1997; Kiflawi and Gray 2000) and those males producing songs with particular acoustical properties (Crankshaw 1979). Pre-copulatory choice is expressed not only through the differential attraction of females to calling males, but also through the length of time it takes a female to mount a courting male. Latency to mating has been shown to be a reliable indicator of male mating success in another cricket species, Teleogryllus commodus (Shackleton et al. 2005). Post-copulatory choice in crickets is mediated through the length of time that the female retains the externally-attached spermatophore before removing it after copulation (Sakaluk 1984; Simmons 1986; Bussière et al. 2006). The spermatophore of male house crickets, which consists of a simple sperm-filled ampulla that remains attached outside of the female’s genital opening after mating, requires approximately 55 min to be emptied of sperm (Sakaluk 2000). Females often remove the spermatophore before complete sperm transfer has occurred, either by reaching back with their mandibles to consume it or by scraping it off against the substrate (Sakaluk 1984, 2000). Premature removal of the spermatophore has a significant influence on the paternity of a female’s offspring, with those males transferring a relatively greater number of sperm siring a disproportionate share of the offspring (Sakaluk and Eggert 1996).
Here we assess the effects of age and mating experience on the mechanisms of pre- and post-copulatory choice in female house crickets by randomly assigning males to females, but independently varying the age and number of previous matings of females at the time of experimental matings. Because our focus was on intrinsic factors that influence female selectivity and not the characteristics of males per se, we predicted that latency to mating and female spermatophore removal behavior would vary in accordance with the age and previous mating experience of the female irrespective of the male with whom she had been assigned to mate.
Methods
Crickets were acquired as late-instar nymphs from Fluker Farms (Baton Rouge, LA) and held separately by sex in 55-l plastic storage containers. The colonies were provisioned with water in 40-ml tissue culture flasks plugged with cotton dental rolls, Fluker’s® cricket feed, and egg cartons to increase the rearing surface area and provide shelter. Adults were removed from stock colonies on the day of their eclosion and held in 4.3-l plastic shoe boxes (34.3 × 20.3 × 10.2 cm), provisioned as above, and maintained at 32°C on a 16:8 light/dark cycle in an environmentally-controlled chamber.
To determine the effects of age and mating experience on the mechanisms mediating pre- and post-copulatory female choice, a two way factorial design was employed in which both female age and prior mating experience were varied simultaneously. Three age classes were established in which females were 10 (N = 55), 13 (N = 59), and 16 (N = 61) days of age, respectively, at the time of experimental mating trials. Although the longevity of A. domesticus under natural conditions is unknown, in other species, the average age of crickets collected from the field is about 12–16 days (Zuk 1987; Zuk and Simmons 1997; Sakaluk et al. 2002). Within each age class, females were placed on one of two mating schedules in which females mated three (age 10: N = 31; age 13: N = 29; age 16: N = 35) or four times (age 10: N = 24; age 13: N = 30; age 16: N = 26) prior to experimental matings. While a difference of one mating may not appear to be an appreciable difference, it represents approximately a 33% increase in the total volume of ejaculate received, and previous work has revealed that significant fitness benefits to females may accrue with just one additional mating (Sakaluk and Cade 1980; Simmons 1988; Wagner et al. 2001). While we had considered including a treatment involving a higher number of matings, pilot studies showed that many females would be unlikely to complete their mating schedule by the prescribed age, such that the females in such a treatment would have been largely self-selected.
The non-experimental matings were scheduled so that within each age-by-mating subgroup, females completed their last mating 24 h before the experimental mating. The net result of this protocol is that at the time of experimental matings, females were of varying age and mating status, but all females had mated 24 h previously. This standardized for any effects that time since last mating might have had on female mating behavior during experimental trials. After receiving their first non-experimental mating, females were held individually in 710-ml plastic containers (9 × 9 × 8.4 cm) and provisioned with food and water ad libitum, egg carton, and moistened peat moss as an oviposition substrate and an additional source of water. After each non-experimental mating, females were confined to a narrow tube for 60 min and then spermatophores were removed with forceps before being placed back into their individuals containers. This procedure was adopted to prevent premature spermatophore removal and to standardize the amount of ejaculate received from each mating.
All matings were staged in Plexiglas mating chambers (7.7 × 10.6 × 3.4 cm) and observed under red-light illumination. Females were weighed to the nearest 0.1 mg on day 5 post-eclosion, just prior to being placed with a male for their first non-experimental mating. Males were weighed just prior to experimental matings. Each mating pair was observed for one hour or until copulation occurred.
For experimental matings, we recorded the latency to mating as the time from when the male initiated courtship, a stereotypic behavior easily recognized (Alexander and Otte 1967), to when the female mounted the male. For successful matings, we recorded the duration of spermatophore attachment, measured as the time from when the female dismounted the male to when the female removed the spermatophore, to a maximum of 60 min. Males were removed immediately after mating because harassment of the female by the male can influence the timing of spermatophore removal, at least in some species (Loher and Rence 1978; Bussière et al. 2006). However, previous studies of A. domesticus have shown no effect of post-copulatory mate guarding on spermatophore retention by females (Khalifa 1950; Sakaluk and Cade 1980).
Females that failed to mate with a courting male or failed to remove a spermatophore within 60 min, the period required for the compete evacuation of the spermatophore (Sakaluk 2000), were included in the analysis as censored observations (Allison 1995). During all mating trials, if a male did not court a female within 10 min of being introduced into the mating chamber, he was replaced with a new male. This was done to ensure that the failure of a female to mate was not due to the lack of male sexual readiness; females will only mate with males that produce courtship song (Nelson and Nolen 1997).
If pre-copulatory female choice is influenced by age or mating status, we predicted that latency to mate would increase with the number of previous matings and decrease with an increase in the female’s age. If post-copulatory female choice is influenced by age or mating status, we predicted that the time at which females remove the spermatophore would decrease with an increase in both the previous number of matings and female age.
Statistical Analysis
Data were analyzed using PROC LIFETEST in SAS (SAS Institute 2004). This non-parametric procedure allows the inclusion of censored data stemming from trials in which the females did not mate during the allotted 60-min observation period or, in the case of those females that mated, failed to remove the spermatophore within 60 min of mating. Data were analyzed across age and mating group to test for main effects and across each age by mating sub-group category to test for an interaction between the two main effects. Wilcoxon χ 2 values were used to evaluate significance because this test gives more weight to earlier failure times (Allison 1995) and is less sensitive to later-censored values. Female and male mass were included as covariates in the analysis.
Results
Latency to Mating
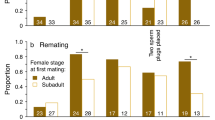
Age had a significant effect on female latency to mating (Wilcoxon χ 2 = 6.29, p = 0.0431), but there was no effect of previous mating experience (Wilcoxon χ 2 = 0.0145, p = 0.90) and no significant interaction between age and mating treatment (Wilcoxon χ 2 = 6.53, p = 0.26) (Fig. 1). Pairwise comparisons between age groups were made using PROC LIFEREG in SAS. Sixteen-day-old females mated sooner than both 10-day-old females (Wald’s χ 2 = 11.9123, p = 0.0360) and 13-day-old females (Wald’s χ 2 = 11.9123, p = 0.0360). However, there was no significant difference in latency to mating between 10- and 13-day-old females (Wald’s χ 2 = 0.008, p = 0.99). Neither female mass, nor male mass, had any effect on latency to mate across age group, mating treatment, and age-by-mating subgroup (Wilcoxon χ 2, all p > 0.2).
Spermatophore Retention
There were no significant effects of age (Wilcoxon χ 2 = 2.64, p = 0.27) or mating treatment (Wilcoxon χ 2 = 0.288, p = 0.5918) on the duration of spermatophore attachment, nor was there any interaction between the two main effects (Wilcoxon χ 2 = 3.59, p = 0.61) (Fig. 2). Female and male mass covariates had no effect on spermatophore retention across age group, mating treatment, and age-by-mating subgroup (Wilcoxon χ 2, all p > 0.8).
Discussion
Latency to mating in female house crickets was influenced by female age at the time of mating, with older females mating sooner than younger females. This response was independent of female size, male size, and mating experience. Because the residual reproductive value of females is expected to decline with age and because selection is weak on older females (Futuyma 1998), the reduced selectivity of older females appears consistent with life-history theory. It is also consistent with previous work showing that older female house crickets are less discriminating than younger females in their phonotactic responses to male calling song, with a higher proportion of younger females orienting to the calls of attractive males (Gray 1999). However, in ground crickets (Allonemobius socius), overall phonotactic responsiveness of females declines with age suggesting that female auditory sensitivity may be diminished as a consequence of senescence (Olvido and Wagner 2004).
Contrary to expectation, the previous number of matings had no effect on a female’s latency to mating. It may be that the difference in the number of previous matings (three versus four) was not sufficient to promote a detectable difference in female receptivity even if, as previous studies have shown, one additional mating can result in discernable fitness benefits to females (Sakaluk and Cade 1980; Simmons 1988; Wagner et al. 2001). Indeed, previous work with Acheta domesticus revealed a marked difference in the remating propensity of once-mated and multiply mated females, with multiply mated females taking significantly longer to remate (Fleischman and Sakaluk 2004). In other cricket species, phonotactic responses of females to conspecific song is diminished with increased sexual experience (Lickman et al. 1998; Loher et al. 1993). Thus, a decrease in female receptivity with increased mating experience probably accrues, but becomes apparent only when the difference in mating experience is greater than was established in the present study.
The length of time that females retained the spermatophore was not influenced by female age at the time of mating, the number of previous matings, or any interaction between the two main effects. Because spermatophore removal by female crickets can greatly influence the paternity of offspring (Sakaluk 1984; Simmons 1986; Bussière et al. 2006), it can function as a particularly effective mechanism of post-copulatory female choice. Previous studies have shown that such preferences can favor larger or more dominant males (Sakaluk 1985; Simmons 1986; Bateman et al. 2001), males offering larger food gifts (Sakaluk 1984; Fedorka and Mousseau 2002), and micropterous males (Sakaluk 1997). Our results suggests that unlike pre-copulatory mechanisms mediating female choice, such as female phonotaxis or latency to mating, post-copulatory mating preferences appear to be largely immune to intrinsic female influences such as age and prior mating experience.
Contrary to previous reports implicating male body size as an important target of female mate choice in other studies of crickets, male mass had no effect on either the latency to female mating or female retention of the spermatophore in A. domesticus. However, most of the previous studies showing a female preference for larger males documented this on the basis of the increased phonotactic attraction of females to the calling song of larger males (Crankshaw 1979; Gray 1997; Kiflawi and Gray 2000). In the present study, however, females were randomly assigned males and could only opt to mount, or not mount, a courting male. It may be that while females attend to the acoustic parameters of male calling song, which functions to attract females over a long distance, they are indifferent to male body size once they have located a calling male and do not discriminate among males of different body size on the basis of the close-range courtship song that males produce upon physically contacting a female. The absence of an effect of male body size on female spermatophore removal is a bit more surprising, as some studies have shown that females tend to retain the spermatophores of larger males longer than those of smaller males (Simmons 1986; Bateman et al. 2001; but see Bussière et al. 2006).
Plasticity in the mechanisms underlying female choice, as demonstrated here, reveals that there are intrinsic factors unrelated to male attributes that may have a direct influence on male mating success. Variation in female age and prior mating experience may promote a continuum of female selectivity that moderates the expression of female choice. Ultimately, this variation may lead to fluctuations in the intensity of sexual selection driving the evolution of male sexual signals and other characteristics (Jennions and Petrie 1997). In addition, fluctuating selection arising from temporal variation in female mating preferences may also contribute to the maintenance of genetic variation in male secondary sexual characteristics (Sasaki and Ellner 1997; Reinhold 1999).
References
Alexander RD, Otte D (1967) The evolution of genitalia and mating behavior in crickets (Gryllidae) and other Orthoptera. Misc Publ Mus Zool Univ Mich 133:1–59
Allison D (1995) Survival analysis using the SAS system: a practical guide. SAS Institute, Cary, NC
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Bateman PW, Gilson LN, Ferguson JWH (2001) Male size and sequential mate preference in the cricket Gryllus bimaculatus. Anim Behav 61:631–637
Borg A, Forsgren E, Amundsen T (2006) Seasonal change in female choice for male size in the two-spotted goby. Anim Behav 72:763–771
Burley NT, Foster VS (2006) Variation in female choice of mates: condition influences selectivity. Anim Behav 72:713–719
Bussière LF, Hunt J, Jennions MD, Brooks R (2006) Sexual conflict and cryptic female choice in the black field cricket, Teleogryllus commodus. Evolution 60:792–800
Crankshaw OS (1979) Female choice in relation to calling and courtship songs in Acheta domesticus. Anim Behav 27:1274–1275
Csada RD, Neudorf DL (1995) Effects of predation risk on mate choice in female Acheta domesticus crickets. Ecol Entomol 20:393–395
Fedorka KM, Mousseau TA (2002) Material and genetic benefits of female multiple mating and polyandry. Anim Behav 64:361–367
Fleischman RR, Sakaluk SK (2004) Sexual conflict over remating in house crickets: no evidence of an anti-aphrodisiac in males’ ejaculates. Behaviour 141:633–646
Forslund P, Part T (1995) Age and reproduction in birds: hypotheses and tests. Trends Ecol Evol 10:374–378
Futuyma DJ (1998) Evolutionary biology. Sinauer, Sunderland
Gamble S, Lindholm AK, Endler JA, Brooks R (2003) Environmental variation and the maintenance of polymorphism: the effect of ambient light spectrum on mating behaviour and sexual selection in guppies. Ecol Lett 6:463–472
Gray DA (1997) Female house crickets, Acheta domesticus, prefer the chirps of large males. Anim Behav 54:1553–1562
Gray DA (1999) Intrinsic factors affecting female choice in house crickets: time cost, female age, nutritional condition, body size, and size-relative reproductive investment. J Insect Behav 12:691–700
Hedrick AV, Dill LM (1993) Mate choice by female crickets is influenced by predation risk. Anim Behav 46:193–196
Hunt J, Brooks R, Jennions MD (2005) Female mate choice as a condition-dependent life-history strategy. Am Nat 166:79–92
Jennions MD, Petrie M (1997) Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev 72:283–327
Khalifa A (1950) Sexual behaviour in Gryllus domesticus L. Behaviour 2:264–274
Kiflawi M, Gray DA (2000) Size-dependent response to conspecific mating calls by male crickets. Proc R Soc Lond B 267:2157–2161
Kodric-Brown A, Nicoletto PF (2001) Age and experience affect female choice in the guppy (Poecilia reticulata). Am Nat 157:316–323
Lickman K, Murray AM, Cade WH (1998) Effect of mating on female phonotactic response in Gryllus integer (Orthoptera: Gryllidae). Can J Zool 76:1263–1268
Loher W, Rence B (1978) The mating behavior of Teleogryllus commodus (Walker) and its central and peripheral control. Z Tierpsychol 46:222–259
Loher W, Weber T, Huber F (1993) The effect of mating on phonotactic behaviour in Gryllus bimaculatus (De Geer). Physiol Entomol 18:57–66
Luttbeg B, Towner MC, Wandesforde-Smith A, Mangel M, Foster SA (2001) State-dependent mate-assessment and mate-selection behavior in female three-spine sticklebacks (Gasterosteus aculeatus, Gasterosteiformes: Gasterosteidae). Ethology 107:545–558
Lynch KS, Rand AS, Ryan MJ, Wilczynski W (2005) Plasticity in female mate choice associated with changing reproductive states. Anim Behav 69:689–699
Lynch KS, Crews D, Ryan MJ, Wilczynski W (2006) Hormonal state influences aspects of female mate choice in the Tungara frog (Physalaemus pustulosus). Horm Behav 49:450–457
Mack PD, Priest NK, Promislow DEL (2003) Female age and sperm competition: last-male precedence declines as female age increases. Proc R Soc Lond B 270:159–165
Martin OY, Hosken DJ (2002) Strategic ejaculation in the common dung fly Sepsis cynipsea. Anim Behav 63:541–546
Moore PJ, Moore AJ (2001) Reproductive aging and mating: the ticking of the biological clock in female cockroaches. Proc Natl Acad Sci U S A 98:9171–9176
Nelson CM, Nolen TG (1997) Courtship song, male agonistic encounters, and female mate choice in the house cricket, Acheta domesticus (Orthoptera: Gryllidae). J Insect Behav 10:557–570
Olvido AE, Wagner WE (2004) Signal components, acoustic preference functions and sexual selection in a cricket. Biol J Linn Soc 83:461–472
Prosser MR, Murray AM, Cade WH (1997) The influence of female age on phonotaxis during single and multiple song presentations in the field cricket, Gryllus integer (Orthoptera: Gryllidae). J Insect Behav 10:437–449
Reinhold K (1999) Evolutionary genetics of sex-limited traits under fluctuating selection. J Evol Biol 12:897–902
Sakaluk SK (1984) Male crickets feed females to ensure complete sperm transfer. Science 223:609–610
Sakaluk SK (1985) Spermatophore size and its role in the reproductive behaviour of the cricket, Gryllodes supplicans (Orthoperta: Gryllidae). Can J Zool 63:1652–1656
Sakaluk SK (1997) Cryptic female choice predicated on wing dimorphism in decorated crickets. Behav Ecol 8:326–331
Sakaluk SK (2000) Sensory exploitation as an evolutionary origin to nuptial food gifts in insects. Proc R Soc Lond B 267:339–343
Sakaluk SK, Cade WH (1980) Female mating frequency and progeny production in singly and doubly mated house and field crickets. Can J Zool 58:404–411
Sakaluk SK, Eggert AK (1996) Female control of sperm transfer and intraspecific variation in sperm precedence: antecedents to the evolution of a courtship food gift. Evolution 50:694–703
Sakaluk SK, Schaus JM, Eggert A-K, Snedden WA, Brady PL (2002) Polyandry and fitness of offspring reared under varying nutritional stress in decorated crickets. Evolution 56:1999–2007
SAS Institute (2004) SAS® online doc version 9.1.3. SAS Institute, Cary NC
Sasaki A, Ellner S (1997) Quantitative genetic variance maintained by fluctuating selection with overlapping generations: variance components and covariances. Evolution 51:682–696
Shackleton MA, Jennions MD, Hunt J (2005) Fighting success and attractiveness as predictors of male mating success in the black field cricket, Teleogryllus commodus: the effectiveness of no-choice tests. Behav Ecol Sociobiol 58:1–8
Simmons LW (1986) Female choice in the field cricket Gryllus bimaculatus (De Geer). Anim Behav 34:1463–1470
Simmons LW (1988) The contribution of multiple mating and spermatophore consumption to the lifetime reproductive success of female field crickets (Gryllus bimaculatus). Ecol Entomol 13:57–69
Wagner WE Jr, Kelly RJ, Tucker KR, Harper CJ (2001) Females receive a lifespan benefit from male ejaculates in a field cricket. Evolution 55:994–1001
Zuk M (1987) Age determination of adult field crickets: methodology and field applications. Can J Zool 65:1564–1566
Zuk M, Simmons LW (1997) Reproductive strategies of the crickets (Orthoptera: Gryllidae). In: Choe JC, Crespi BJ (eds) Mating systems in insects and arachnids. Cambridge University Press, Cambridge, pp 89–109
Acknowledgements
We thank Tracie Ivy and Carie Weddle for their assistance in cricket maintenance. We are grateful for the helpful suggestions from Diane Byers and Rachel Bowden on the design of this experiment. This research was funded by grants from the National Science Foundation grant to SKS and grants from the Beta Lambda Chapter of the Phi Sigma Biological Honors Society to BSM. Support was also provided to BSM through a National Science Foundation GK-12 grant to Cynthia Moore.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mautz, B.S., Sakaluk, S.K. The Effects of Age and Previous Mating Experience on Pre- and Post-copulatory Mate Choice in Female House Crickets (Acheta domesticus L.). J Insect Behav 21, 203–212 (2008). https://doi.org/10.1007/s10905-008-9120-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-008-9120-9