Abstract
The expensive brain hypothesis predicts that the lowest stable level of energy input sets the upper limit to a species’ brain size. This prediction receives comparative support from the effects of experienced seasonality (including hibernation) and diet quality on mammalian brain size. Here, we test another prediction, which concerns the temporal stability of energy inputs. Allomaternal care in mammals can be provided by breeding males or other helpers (usually earlier offspring). Male care should be stable and reliable since otherwise no breeding would occur. Care by others, in contrast, should fluctuate, as the availability of helpers often varies. One would therefore predict, other things being equal, that the presence of male care in addition to maternal care should show positive correlated evolution with brain size, whereas care by others would not. However, because females can readily respond through litter size adjustments to variable amounts of energy inputs, helper inputs may be used to increase fertility. A detailed comparative analysis of a large sample of mammals (N = 478 species) showed that male help is correlated with the evolution of larger brains, whereas alloparental help is correlated with higher fertility, but only in species where male care is also present (as in cooperative breeders). Humans evolved an unusual form of multi-family cooperative breeding, which involves stable and reliable care by both fathers and alloparents. This combination helps to explain why humans differ from the other apes in having both an extremely large brain and a relatively high reproductive output.
Significance statement
Allomaternal care provides breeding females with energy, directly or indirectly, and so would be expected to affect fertility and/or brain size. Which path evolution actually took remains controversial, partly because previous studies did not separate between care provided by the breeding male (paternal care) and care by non-breeding helpers (alloparental care). We distinguish between them because we expect that selection only favours increased brain size if the increase in energy available to the female is predictable and constant. Using a sample of 478 mammals, we show that paternal care, which is both reliable and stable, shows correlated evolution with brain size, whereas alloparental care, which fluctuates with varying availability of helpers, is correlated with higher fertility. Thus, constraints on brain size, imposed by its high-energy costs, may predict brain size better than the fitness benefits of improved cognitive abilities per se.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain size for a given body size varies appreciably among mammalian species (e.g. Striedter 2005) and relates to intellectual or cognitive performance (Deaner et al. 2007; Reader et al. 2011). These enhanced cognitive abilities engender general behavioural flexibility (Fernandes et al. 2014; Borrego and Gaines 2016; Burkart et al. 2016), and therefore provide numerous benefits in both the social (e.g. Byrne and Whiten 1988; Barrett and Henzi 2005; Emery et al. 2007; Dunbar and Shultz 2017) and ecological domain (e.g. Parker and Gibson 1977; Sol 2009; van Woerden et al. 2012; Benson-Amram et al. 2016; Heldstab et al. 2016b; Navarrete et al. 2016; DeCasien et al. 2017; Powell et al. 2017). However, the benefit of enhanced cognitive abilities is counterbalanced by the energetic demands of larger and hence metabolically more expensive brains. Brain tissue is among the most metabolically expensive tissues in the body to maintain and grow (Holliday 1986; Rolfe and Brown 1997; Niven and Laughlin 2008; Bauernfeind et al. 2014; Kuzawa et al. 2014) and benefits from a supply of energy at all times (Mink et al. 1981; Lukas and Campbell 2000). Thus, one would predict that species can only evolve relatively larger brains than their ancestors if they can constantly sustain the high energetic costs of brain development and maintenance, and if these costs do not outweigh the fitness benefits of superior cognitive abilities.
The expensive brain hypothesis (Isler and van Schaik 2009a) postulates that the increased costs of an evolutionary brain enlargement can be paid for by two complementary mechanisms, for which comparative studies of mammals, birds, amphibians and fish have provided extensive support (Clutton-Brock and Harvey 1980; Fish and Lockwood 2003; Isler and van Schaik 2006b, 2009a, 2009b; van Woerden et al. 2010, 2012, 2014; Barton and Capellini 2011; Navarrete et al. 2011; Kotrschal et al. 2013; Weisbecker et al. 2015; Heldstab et al. 2016a, 2018b; Luo et al. 2017; Genoud et al. 2018; Yu et al. 2018). The first, trade-off pathway is a redirection of energy allocated to other body functions such as locomotion (Isler and van Schaik 2006b; Navarrete et al. 2011; Heldstab et al. 2016a), rate of development (Isler and van Schaik 2009a; Barton and Capellini 2011; Yu et al. 2018) and fertility (Isler and van Schaik 2009a, 2009b; Kotrschal et al. 2013). The second pathway of providing the increased energy needs of larger brains consists of a stable net increase in energy input. This has been supported in mammals in general (particularly so in Eutheria, but not in Metatheria) where the basal metabolic rate (BMR), a proxy of the net energy input, is positively correlated with brain size (Isler and van Schaik 2006a; Genoud et al. 2018). The increased energy input could be achieved by improved diet quality (Clutton-Brock and Harvey 1980; Fish and Lockwood 2003), or by avoiding periods of starvation in both mammals (van Woerden et al. 2010, 2012, 2014; Weisbecker et al. 2015) and frogs (Luo et al. 2017). Hibernators, whose intake varies most dramatically (e.g. Lovegrove et al. 2014), are also found to have smaller brains (Veitschegger 2017; Heldstab et al. 2018b).
The broad support for the expensive brain hypothesis suggests that organisms have a brain as large as they can sustain energetically. The expensive brain hypothesis should therefore also predict effects of energy inputs during brain growth due to allomaternal care by the breeding male (paternal care) or non-breeding helpers (alloparental care). Brains benefit from high and stable energetic input especially during the growth and differentiation phase (Holliday 1986; Bauernfeind et al. 2014; Kuzawa et al. 2014) because developing brains cannot be starved temporarily without permanent cognitive damage (Lukas and Campbell 2000). This energetic constraint on brain development is expected to be relieved by energy subsidies to breeding females and immatures, allowing the evolution of bigger brains in species with allomaternal care, including humans (Burkart et al. 2009; Hrdy 2009; van Schaik and Burkart 2011; Isler and van Schaik 2012). However, empirical evidence for this prediction is mixed. A large comparative study across more than 400 mammals found support for this hypothesis. Isler and van Schaik (2012) found a positive correlation between brain size and the amount of allomaternal care. Likewise, cooperatively breeding mammals and most altricial birds do not show the steep decline in rmax (maximum rate of population increase) with brain size found among independent breeders, suggesting that allomaternal care enables species to increase their brain size without compromising their demographic viability (Isler and van Schaik 2009b). In contrast, two other studies, one in the parvorder Corvida (Iwaniuk and Arnold 2004) and one in cichlid fishes (Reddon et al. 2016) found no relationship between brain size and cooperative breeding.
Some of the ambiguity in results of previous studies may have arisen because most studies did not or only partially separate care according to the identity of the carer, although benefits and costs, and therefore the reliability of allomaternal care may differ between the male breeder and alloparents. For instance, Isler and van Schaik (2009b, 2012) did not distinguish between the contribution by fathers or non-parents to offspring care and hence the positive correlation between brain size and allomaternal care could be driven by paternal care, by alloparental care or both. Similarly, in the cichlid fish study, Reddon et al. (2016) compared cooperatively and independently breeding species but the independently breeding species comprised species with biparental and uniparental care (usually maternal care). And finally, Iwaniuk and Arnold (2004) solely tested for a difference in brain size between independently and cooperatively breeding species but did not test for a difference in encephalisation between species with uniparental and species with biparental care. We therefore reassess the relationship between brain size and allomaternal care by separating the effect of allomaternal care provided by males (paternal care) from that provided by other group members (alloparental care).
The goal of this paper is to re-evaluate the effects of energy inputs on brain size in light of the expensive brain hypothesis. As mentioned above, organisms cannot opportunistically respond with brain enlargement to highly fluctuating increases in energy supply. Thus, selection should only favour increased brain size if the increase in energy available to the female is predictable and constant. We therefore predict the following pattern. In species with male care (in addition to the care of the breeding female) or cooperative breeding (in which the breeding male also always participates in caring), the care provided by the breeding male is reliably present, being a stable trait of such species. Indeed, even if the male reduces his contribution in response to the activity of helpers (Price 1992; Rothe et al. 1993; Santos et al. 1997; Bales et al. 2000), there is nonetheless a stable amount of extra energy available to the breeding female. Thus, the expensive brain hypothesis predicts that natural selection will favour females responding to the reliable presence of male care by producing larger-brained offspring.
In contrast, the care provided by helpers is much more variable depending on various factors such as the age of the parents, group composition (number of helpers available) and variation in environmental conditions. Thus, new breeding pairs usually have no helpers or only young inexperienced helpers who tend to provide less help than experienced older caregivers (Tardif et al. 1992; Heinsohn and Cockburn 1994; Woxvold et al. 2006; Rymer and Pillay 2014). Moreover, whereas the help provided by the breeding male is unaffected by his body condition or food abundance, other non-breeding group members generally adjust their helping efforts in relation to their body condition and according to food availability (Harrington et al. 1983; Creel and Creel 2002; Nichols et al. 2012; Marshall et al. 2016). Finally, subordinates can also start to breed themselves, in which case their help to the dominant female could end abruptly or be minimal to begin with (Clutton-Brock et al. 2002; Young et al. 2005; Brouwer et al. 2011; Zöttl et al. 2013). In sum, energy inputs due to alloparental help should be less reliable and therefore less likely to allow for a selective change in the energy allocation to the growing brain during development. Instead, we suggest that these more fluctuating energy inputs are allocated to reproduction. It is well known that females respond to increased food availability with increased fertility (Tyler 1987; Wauters and Lens 1995; van Noordwijk and van Schaik 1999; Heesen et al. 2013; Arlet et al. 2015). The positive effect of the amount of alloparental care on fertility is well established in birds (Russell and Rowley 1988; Mumme 1992; Komdeur 1994; Klauke et al. 2013; Dixit et al. 2017) and mammals (Moehlman 1979; Fairbanks 1990; Solomon 1991; Koenig 1995; Garber and Leigh 1997; Mitani and Watts 1997; Ross and MacLarnon 2000; Russell et al. 2003).
In this paper, we therefore test this modified prediction of the expensive brain hypothesis (Fig. 1): if the level of steady energy inputs relates to a species’ brain size, the presence and amount of paternal care by the breeding male (in addition to maternal care), being reliable and steady, should be correlated with increased brain size, whereas the presence and amount of allomaternal care by non-breeding helpers (alloparental care) should not be and rather be correlated with increased fertility. We test these predictions in a large sample of 478 mammalian species using a new approach that clearly distinguishes care provided by the breeding male (paternal care) from care by non-breeding helpers (alloparental care).
Predicted pattern of correlated evolution between different sources of allomaternal care and brain size or fertility. We expect paternal care, which is predictable in amount and time, to be associated with large relative brain size, but alloparental care, which is less predictable, to be related to higher fertility rates
Material and methods
Brain size, body mass and fertility
Data on brain size and body mass were mainly retrieved from Isler and van Schaik (2012) and supplemented with data from published compilations (Isler et al. 2008; Isler and van Schaik 2009b; Heldstab et al. 2016a; Matějů et al. 2016). Where available, we used female values to reduce error due to sexual dimorphism. Annual fertility of an average female was defined as the product of the number of offspring per litter times the number of litters per year as in Isler and van Schaik (2009a). Data on the number of offspring per litter and litters per year were taken from Myers et al. (2006); Jones et al. (2009); Rowe and Myers (2011); Santos et al. (2015); and West and Capellini (2016), building on the large datasets compiled by Isler and van Schaik (2009a, 2012).
Allomaternal care behaviours
To quantify the continuous amount of allomaternal care, we complemented the database of Isler and van Schaik (2012) and Heldstab et al. (2017) which includes the frequency of occurrence of the following care behaviours: provisioning (separating provisioning aimed at offspring from provisioning aimed at the mother), carrying, protection, and a variable that comprises other energetically influential care behaviours such as huddling, communal nesting and pup retrieval. As scores for provisioning the mother were restricted to the order Carnivora in the dataset of Isler and van Schaik (2012), we added additional data on this behaviour from West and Capellini (2016) for other orders. Communal nursing (allonursing) was excluded in this study because lactating mothers have not been shown to derive any energetic benefits from it (Baldovino and Di Bitetti 2008; MacLeod et al. 2015; Heldstab et al. 2017). To distinguish the effects of allomaternal care provided by males (paternal care) from that provided by other group members (alloparental care), we summed up the frequency of occurrence of all allomaternal care behaviours separately for the father and other group members, as in Heldstab et al. (2017).
To investigate whether the results reported in this study are robust with respect to different coding schemes of allomaternal care, we additionally conducted all analyses by using a binary classification of paternal and alloparental care with 1 indicating the presence and 0 the absence of the trait. We scored the presence of paternal care or alloparental care if the frequency of occurrence of care was higher than 5%. We additionally only scored the presence of paternal care if it comprised more than just defence of territory or protection against predators or infanticide. Such paternal care is probably not energetically significant, and such species are not generally categorised as having male care in studies employing a binary coding scheme (e.g. Woodroffe and Vincent 1994; Lukas and Clutton-Brock 2013; West and Capellini 2016).
In total, data on allomaternal care behaviours and brain size and/or fertility were available for 478 mammals across all major orders. Bats and cetaceans were excluded from our study because reliable data on allomaternal care of both cetaceans and bats are notoriously difficult to obtain. The full dataset is available in the Supplementary Material (ESM 1). Figure 2 shows the distribution of the various care categories in the dataset.
Covariates
Living in social groups (gregariousness), diet quality, diurnality and substrate use have been shown to correlate with brain size in mammals (e.g. Harvey et al. 1980; Gittleman 1986; Bernard and Nurton 1993; Pérez-Barbería and Gordon 2005; Kirk 2006; Dunbar and Shultz 2007; DeCasien et al. 2017; Powell et al. 2017). Although it is less clear how these variables should be related to allomaternal care or fertility, our large sample size allowed us to include these potentially confounding variables into our analyses. Data for these covariates were collated from the literature (data were from Russell 1974; Gittleman 1989; Myers et al. 2006; Jones et al. 2009; van Woerden et al. 2010; Rowe and Myers 2011; Kuznetsova et al. 2013; Lukas and Clutton-Brock 2013; Wilman et al. 2014; Heldstab et al. 2016a; DeCasien et al. 2017). Gregariousness was classified as follows: solitary (or mother with infants) (0); usually solitary, but occasionally seen in pairs or groups, or facultative group denning (0.5); pairs (with infants) (1); usually in pairs, but gregarious at times or in part of the geographic range (1.5) and permanently gregarious (the group comprises more adults than just the parents) (2). To control for diet quality, species were divided into five categories based on their main diet: aquatic faunivore or piscivore (1), frugivore/folivore or granivore (2), frugivore/faunivore or omnivore (3), herbivore or folivore (4) and carnivore, faunivore or insectivore (5). A binary coding was used for activity period, with (1) for nocturnal, cathemeral or crepuscular species and (2) for diurnal species. For substrate use, each species was assigned to one of four substrate use categories: aquatic or semi-aquatic (1), fossorial or semi-fossorial (2), terrestrial or semi-arboreal (3) and arboreal (4).
Statistical analyses
All statistical analyses were done in JMP™ 13.0 (SAS Institute Inc. 1989–2016) and in R3.4.1 (R Core Team 2017). Fertility, brain size and body mass values were loge transformed before analysis to reduce the skew of their distribution. Because the phylogenetic signal lambda (λ) was always close to 1, the use of methods to control for phylogenetic non-independence was required (Pagel 1999). We therefore built phylogenetic generalised least-squares regressions (PGLS) models (Freckleton et al. 2002) using the ‘caper’ package (Orme 2013) in R. The phylogeny was based on an updated version (Fritz et al. 2009) of the mammalian supertree (Bininda-Emonds et al. 2007) and is given in Fig. S1 (Supplementary Material ESM 2). We used PGLS models with either brain size or fertility as dependent variables, and paternal or alloparental care, female body mass and all possible confounding variables (gregariousness, diet quality, diurnality and substrate use) as independent variables.
We also tested for an additive effect of care by including paternal care, alloparental care and combined care (paternal and alloparental care) as independent variables into the same model. In most species that exhibit alloparental care, paternal care is also observed (see Fig. 2), potentially resulting in collinearity problems in this particular statistical analysis. To assess potential multicollinearity between paternal, alloparental and combined care in the additive model, we generated variance inflation factors (VIF) (Quinn and Keough 2002; Dormann et al. 2013) using non-phylogenetic generalised linear models and the function ‘vif’ (‘car’ package: (Fox and Weisberg 2011)) in R. VIFs quantify how much the variance of an estimated model parameter is increased because of multicollinearity between predictors. The VIF for alloparental care and combined care was higher than 4, which indicates a problematic amount of covariance among predictors (Rogerson 2001; Hair et al. 2006). To solve this, we categorised species into having either paternal care only (no alloparental care), alloparental care only (no paternal care) or combined care (paternal and alloparental care). After this, the VIFs of all independent variables were less than 2, which indicates an acceptable amount of covariance among predictors (Supplementary Material ESM 3, Table S1). To choose the best fitting from a set of models, we used AIC values (Akaike Information Criterion Akaike 1974).
We additionally also performed multi-model averaging as in Heldstab et al. (2017, 2018a) with brain size or fertility as dependent variables, and paternal care only, alloparental care only, combined care, female body mass and all possible confounding variables (gregariousness, diet quality, diurnality and substrate use) as independent variables to test whether our results are robust with respect to different statistical approaches.
We performed information-theoretic model selection based on AICc across all possible models built with the independent variables mentioned above. As the AICc did not clearly distinguish the most highly ranked models, we accounted for uncertainty by using multi-model averaging (Grueber et al. 2011) in the candidate model set, which included all models with ∆AICc < 3. ∆AICc is the difference in AICc between the focal model and the AICc of the best-fitting model in the candidate model set. Estimates of each parameter were averaged across the candidate models, with means weighted by the Akaike weight of a given model. The relative importance of a predictor was obtained by summing the Akaike’s weights of the models in the candidate model set including the focal predictor (Symonds and Moussalli 2011). The method to perform model averaging with the PGLS function in the ‘caper’ package (Orme 2013) is described by Garamszegi and Mundry (2014) and the corresponding material is available at http://www.mpcm-evolution.org.
A phylogenetically informed comparative approach to analyse the combined mammalian dataset as described above was preferred over analysing different clades or orders separately. First, the larger sample size of a combined dataset allows to test models with several covariates. Second, in a phylogenetic analysis with lambda close to 1, grade shifts between clades are represented in the values of one node, and thus are neither neglected nor exert overdue influence. Third, care behaviours are evolutionary relatively stable within clades, while ecological influences on brain size evolution likely show more variation in the tip nodes of the phylogenetic tree. We therefore expect that patterns are much weaker within clades or may even be obscured completely.
Results
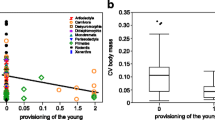
The results confirmed our two main predictions. In a comprehensive sample of more than 400 mammals, paternal care, the more reliable sort of allomaternal care, shows positive correlated evolution with relative brain size (Table 1, Fig. 3a). Alloparental care had an additive effect on brain size, but only if it was associated with reliable paternal care (combined care) (Table 1). Furthermore, paternal care always had a stronger effect on brain size than alloparental or combined care (Table 1). In contrast, alloparental care, which is more variable in amount and time, showed a significant or nearly significant positive relationship with fertility (Table 2, Fig. 3b). This positive correlation between fertility and alloparental care is mainly driven by the positive effect of alloparental care on fertility in species with combined care (paternal and alloparental care); indeed, species with alloparental care only did not show increased fertility (Table 2). Increases in brain size or fertility rate seem to be largely independent selective responses as paternal care was correlated with brain size but not fertility, and alloparental care only had an additive effect on brain size when paternal care was already present (Tables 1 and 2). Our results are robust with respect to different coding schemes of allomaternal care (binary or continuous) (Supplementary Material ESM 3, Tables S2, S3). We also found no difference in the results when we controlled for various possible confounding variables (gregariousness, diet, diurnality and substrate use) (Supplementary Material ESM 3, Tables S4-S7), suggesting that these findings are not spurious by-products of other correlations. Our results are also strikingly similar for different statistical approaches (simple model selection vs multi-model averaging) (Supplementary Material ESM 3, Tables S8-S15).
Relative brain size is positively correlated with the amount of paternal care (a). Fertility (corrected for body mass) shows a positive relationship with alloparental care (b). Details of phylogenetic models are shown in Tables 1 and 2. Species values are listed in the Supplementary Material (ESM 1). For a graphical representation of the same correlations but with suborder mean values, see Fig. S2 (Supplementary Material ESM 4)
Discussion
Reproduction is energetically very expensive, especially in taxa with fast life histories (e.g. Zenuto et al. 2002; McNab 2006; Speakman 2008). The expensive brain hypothesis therefore postulates that one major pathway toward the evolution of relatively larger brains is reduced allocation to reproduction (Isler and van Schaik 2009a). One way for females to achieve such a reduction in reproductive costs is by distributing these costs over other individuals such as the breeding male or non-breeding group members. However, studies investigating the relationship between brain size and allomaternal care have so far produced inconclusive results. We suspected that this happened because they did not separate between care provided by the breeding male (paternal care) and care by non-breeding helpers (alloparental care). By separating these two care types, we found that allomaternal help by the breeding male was correlated with the presence of larger brains, but not with fertility. Allomaternal help by others was correlated with the presence of higher fertility and only correlated with larger brains in species with combined care (where breeding males also help). Allomaternal help by others in the absence of male care had no influence on either fertility or brain size.
Allomaternal care and brain size
In accordance with the expensive brain hypothesis, we found that an additional influx of energy in the form of predictable paternal care toward the mother and the offspring is associated with an evolutionary increase in brain size, whereas care provided by non-breeding group members, which is more variable in amount and time, only had an influence on brain size if reliable paternal care was also present. Indeed, abundant studies show that the identity of the caretaker, be it the breeding male or other helpers, influences the predictability of care received by the breeding female and her offspring. For instance, in banded mongoose (Mungos mungo) (Nichols et al. 2012; Marshall et al. 2016), adult males (potential fathers) maintained or even increased their investment in care as food supply decreased, whereas non-breeding group members helped less when food was scarce. Similarly, in wolves (Canis lupus) and African wild dogs (Lycaon pictus), prey availability affected the ability or willingness of non-breeding pack members to care for the pups whereas the help of the breeding male remained mostly constant (Malcolm and Marten 1982; Harrington et al. 1983). In regions where prey was scarce, wolf pairs produced more surviving pups than did larger packs with additional potential helpers (Harrington et al. 1983), showing that additional potential helpers can even hinder pup survival. Indeed, in wolves and wild dogs, helpers are sometimes fed at the den by other individuals or raid caches near the den, thus intercepting food potentially available for the pups (Haber 1977; Malcolm and Marten 1982; Murie 2011). Furthermore, young helpers in wild dogs, such as yearlings, failed to regurgitate during periods of food scarcity and thus stopped helping (Malcolm and Marten 1982; Creel and Creel 2002). Together, these studies suggest that the amount of alloparental help and the probability of its expression depend on prevailing environmental conditions influencing food availability (see also Macdonald and Moehlman 1982).
Besides ecological conditions, the ability of subordinate helpers to reproduce independently is also expected to influence an individual’s investment in alloparental care. In cooperatively breeding meerkats (Suricata suricatta) (Clutton-Brock et al. 2002), cichlid fish (Neolamprologus pulcher) (Zöttl et al. 2013) and paper wasps (Polistes dominulus) (Tibbetts 2007), dominant individuals stay around and help to raise offspring, whereas subordinates reduce investment in helping behaviours such as babysitting, pup feeding, digging, cooperative foraging or predator defence shortly before dispersing. This resembles patterns found in some other social vertebrates such as prairie voles (Microtus ochrogaster) (Lonstein and De Vries 2000, 2001), Californian mice (Peromyscus californicus) (Gubernick and Laskin 1994) and Damaraland mole-rats (Fukomys damarensis) (Zöttl et al. 2018), which reduce the amount of alloparental care with increasing chances of independent reproduction. Thus, alloparental help is unpredictable and variable in amount and timing as helpers in various species adjust their caring effort depending on both food availability and future reproduction. Our result that the presence and frequency of paternal care (in addition to maternal care) were much more strongly correlated with brain size than alloparental care (in addition to maternal care) supports the idea that selection favours larger brains whenever females experience a predictable and constant increase in energy.
An additional reason why alloparental care was less important for brain size in our study might also be that the quality of paternal and alloparental care differs. In a huge variety of mammal and bird species, breeding males contribute more to offspring care than alloparents. Examples include African wild dogs (Lycaon pictus) (Malcolm and Marten 1982), banded mongooses (Mungos mungo) (Gilchrist and Russell 2007), striped mice (Rhabdomys pumilio) (Schubert et al. 2009), golden lion tamarins (Leontopithecus rosalia) (Siani 2009), chestnut-crowned babblers (Pomatostomus ruficeps) (Browning et al. 2006), long-tailed tits (Aegithalos caudatus) (MacColl and Hatchwell 2003), white-fronted bee-eaters (Merops bullockoides) (Emlen and Wrege 1991), apostlebirds (Struthidea cinerea) (Woxvold et al. 2006) and laughing kookaburras (Dacelo novaeguineae) (Legge 2000) (but see as counterexamples Bennett and Faulkes 2000; Clutton-Brock et al. 2004). This is likely the case because alloparents, in contrast to breeding males, are often young and unexperienced (Lancaster 1971; Hrdy 1976; Roberts et al. 1998) and did not improve infant survival (Harrington et al. 1983; Jaquish et al. 1997). In agreement with our study, all these results suggest that paternal care is more important for females than the help of others. Finally, a recent comparative study (Heldstab et al. 2017) showed that reproducing females in species with any sort of allomaternal care can afford to reduce reliance on fat reserves, but also that care provided by the breeding male was more important than the help of other non-breeding group members.
The results of our study are also consistent with those of earlier comparative studies. Studies in birds and non-primate taxa found that brain size is associated with an increase in paternal care (West 2014) or biparental care and pair-bonding (Shultz and Dunbar 2007, 2010). The authors of these studies argue that pair-bonding species are more encephalised due to the higher degree of coordination and cooperation that is necessary to maintain stable pair-bonded relationships. Here, we provide an additional or alternative explanation for larger brains in monogamous species: predictably available paternal care, which is particularly common among species with socially monogamous mating systems (Lukas and Clutton-Brock 2013), allows for a constant extra energy input during brain growth, making the evolution of larger brains possible.
As discussed by Isler and van Schaik (2012), our explanation does not require a special explanation for primates, as the extra energy may be used to increase fertility instead of enlarging brain size (see below). Obviously, it is possible to coordinate close pair bonds and cooperative care even with relatively small brains. Finally, our results also explain why the study of corvids (Iwaniuk and Arnold 2004) and cichlid fishes (Reddon et al. 2016) failed to find any effect on brain size: they compared species with male care with those with male care and care by helpers.
In conclusion, our results are consistent with the expensive brain hypothesis, and therefore strongly suggest that brains tend to be as large as the species can afford it energetically. Thus, constraints on brain size, imposed by its energetic costs, may predict brain size better than the fitness benefits of improved cognitive abilities per se.
Allomaternal care and fertility
If allomaternal care is variable and unsteady in amount and timing and therefore does not allow a constant high energy supply for brain growth, we expect that selection will favour mothers who invest the consequent load reduction into the production of a higher number of offspring, rather than in larger-brained offspring. As expected, we found that alloparental care resulted in higher fertility rates, but only in species with combined care. Previous studies in mammals support this finding, showing that females of cooperative breeders commonly have larger litters and shorter interbirth intervals than non-cooperative social species (Garber and Leigh 1997; Mitani and Watts 1997; Moehlman and Hofer 1997; Ross and MacLarnon 2000; Lukas and Clutton-Brock 2012; West and Capellini 2016).
We found that species with alloparental care where breeding males do not help (alloparental care only) show no increase in fertility. One explanation for this finding is that in these species, alloparental care is less systematic than in species with combined care, such as cooperative breeders. For instance, the frequencies of occurrence of alloparental care behaviours in species where males provide no care are relatively low. For instance, we see only 1% alloparental carrying in black-and-white ruffed lemurs (Varecia variegata), 8.4% in Venezuelan red howlers (Alouatta seniculus), 10.3% in patas monkeys (Erythrocebus patas), 5% alloparental provisioning in red slender lorises (Loris tardigradus), spectral tarsiers (Tarsius tarsier) or 10% babysitting in ring-tailed lemurs (Lemur catta). Furthermore, some attempts to provide alloparental help have been shown to be detrimental to mothers and infants (Hrdy 1976; Silk 1980; Malcolm and Marten 1982; Sommer 1989; Maestripieri 1994). In our dataset, this was also found in various species with alloparental care only, namely guerezas (Colobus guereza) (Wooldridge 1969), Lowe’s monkeys (Cercopithecus lowei) (Bourlière 1970), vervet monkeys (Chlorocebus aethiops) (Gartlan 1969) and patas monkeys (Erythrocebus patas) (Zucker and Kaplan 1981).
In our study, paternal care (in addition to maternal care) showed no correlation with fertility. There is some controversy in the literature as to whether paternal care is related to increased fertility in mammals. The comparative studies that find higher fertility (larger litters: (Lukas and Clutton-Brock 2013) or reduced birth intervals (West and Capellini 2016)) did not distinguish between male care only and male care plus alloparental care (cooperative breeding), making it impossible to disentangle their separate effects. Stockley and Hobson (2016) made this distinction, and separately analysed species with paternal care only (without cooperative breeding). However, they used a very conservative definition of cooperative breeding which led, among many others, to the classification of some callitrichids, e.g. Saguinus labiatus and Cebuella pygmaea, as non-cooperative breeders. In this sample, and only for provisioning but not for other helping behaviours, they found higher fertility in species with paternal care only. In conclusion, in our study, we found that fertility was increased in species with alloparental care but mainly due to species with combined care showing higher fertility rates. However, future studies should examine in more detail whether the positive effect of paternal care on fertility found in previous studies is solely driven by species with combined care.
Allomaternal care and human evolution
Humans have the largest relative brain size across the whole animal kingdom (Jerison 1973; Striedter 2005). The costs of sustaining such a large brain are extremely high as humans spend about 20–25% of their resting metabolism on the brain (neonates even up to 60%) (Mink et al. 1981). Among hunter-gatherers, we also see intensive paternal care, mainly in the form of provisioning (male contribution to subsistence) (Hewlett 1993; Marlowe 1999, 2000; Quinlan 2007; Dyble et al. 2016).
Humans also stand out among apes by having a relatively high reproductive output. The main underlying difference is our system of cooperative care for infants and mothers (Hrdy 2005; Burkart et al. 2009; Burkart and van Schaik 2010). Allomaternal care among human foragers is provided by both reproductive men and non-reproductive group members of both sexes and of kin and non-kin, comprising help in the form of food provisioning, carrying, protecting and babysitting the infants (Hawkes et al. 1998; Hill and Hurtado 2009; Hrdy 2009; Dyble et al. 2016; Jaeggi et al. 2016). Humans therefore resemble other mammalian species with combined care (where the male and other non-breeding group members help) in this respect by having higher reproductive rates compared to other species, including other apes, with bi-parental care or maternal care only. However, in contrast to other mammals, where the amount of alloparental care is highly variable depending on various factors such as the age of the parents, group composition and variation in environmental conditions, alloparental care provided by humans is very stable and reliable, at least among foragers. Universal notions of fairness, equity and social punishment, e.g. through excluding uncooperative individuals from future help, sustain cooperation among foragers, including alloparental care (e.g. Hill et al. 1993; Gurven 2004). As a result, mothers are even provided with more food than they actually need to cover the maximum additional costs of gestation or lactation (Kaplan et al. 2000; Butte and King 2005; Sellen 2007). We therefore suggest that the unusual stability of energy input due to alloparental care allowed humans not only to increase their reproductive output but also to evolve even larger brains than other mammalian species with combined care resulting in the rapid and unparalleled brain expansion during hominin evolution (cf. Burkart et al. 2009; Burkart and van Schaik 2010; van Schaik and Burkart 2010).
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723. https://doi.org/10.1109/TAC.1974.1100705
Arlet ME, Isbell LA, Kaasik A, Molleman F, Chancellor RL, Chapman CA, Mänd R, Carey JR (2015) Determinants of reproductive performance among female gray-cheeked mangabeys (Lophocebus albigena) in Kibale National Park, Uganda. Int J Primatol 36:55–73. https://doi.org/10.1109/TAC.1974.1100705
Baldovino MC, Di Bitetti MS (2008) Allonursing in tufted capuchin monkeys (Cebus nigritus): milk or pacifier? Folia Primatol 79:79–92. https://doi.org/10.1159/000108780
Bales K, Dietz J, Baker A, Miller K, Tardif SD (2000) Effects of allocare-givers on fitness of infants and parents in callitrichid primates. Folia Primatol 71:27–38. https://doi.org/10.1159/000021728
Barrett L, Henzi P (2005) The social nature of primate cognition. Proc R Soc Lond B 272:1865–1875. https://doi.org/10.1098/rspb.2005.3200
Barton RA, Capellini I (2011) Maternal investment, life histories, and the costs of brain growth in mammals. P Natl Acad Sci USA 108:6169–6174. https://doi.org/10.1073/pnas.1019140108
Bauernfeind AL, Barks SK, Duka T, Grossman LI, Hof PR, Sherwood CC (2014) Aerobic glycolysis in the primate brain: reconsidering the implications for growth and maintenance. Brain Struct Funct 219:1149–1167. https://doi.org/10.1007/s00429-013-0662-z
Bennett NC, Faulkes CG (2000) African mole-rats: ecology and eusociality. Cambridge University Press, Cambridge
Benson-Amram S, Dantzer B, Stricker G, Swanson EM, Holekamp KE (2016) Brain size predicts problem-solving ability in mammalian carnivores. P Natl Acad Sci USA 113:2532–2537. https://doi.org/10.1073/pnas.1505913113
Bernard RTF, Nurton J (1993) Ecological correlates of relative brain size in some South-African rodents. S Afr J Zool 28:95–98. https://doi.org/10.1080/02541858.1993.11448300
Bininda-Emonds OR, Cardillo M, Jones KE, MacPhee RD, Beck RM, Grenyer R, Price SA, Vos RA, Gittleman JL, Purvis A (2007) The delayed rise of present-day mammals. Nature 446:507–512. https://doi.org/10.1038/nature05634
Borrego N, Gaines M (2016) Social carnivores outperform asocial carnivores on an innovative problem. Anim Behav 114:21–26. https://doi.org/10.1016/j.anbehav.2016.01.013
Bourlière F (1970) Ecology and behaviour of Lowe’s guenon (Cercopithecus campbelli lowei) in the Ivory Coast. In: Napier JR, Napier PH (eds) Old World monkeys: evolution, systematics and behaviour. Academic Press, New York, pp 297–350
Brouwer L, van de Pol M, Atema E, Cockburn A (2011) Strategic promiscuity helps avoid inbreeding at multiple levels in a cooperative breeder where both sexes are philopatric. Mol Ecol 20:4796–4807. https://doi.org/10.1111/j.1365-294X.2011.05325.x
Browning RC, Baker EA, Herron JA, Kram R (2006) Effects of obesity and sex on the energetic cost and preferred speed of walking. J Appl Physiol 100:390–398. https://doi.org/10.1152/japplphysiol.00767.2005
Burkart JM, van Schaik CP (2010) Cognitive consequences of cooperative breeding in primates? Anim Cogn 13:1–19. https://doi.org/10.1007/s10071-009-0263-7
Burkart JM, Hrdy SB, van Schaik CP (2009) Cooperative breeding and human cognitive evolution. Evol Anthropol 18:175–186. https://doi.org/10.1002/evan.20222
Burkart JM, Schubiger MN, van Schaik CP (2016) The evolution of general intelligence. Behav Brain Sci 40:1–65. https://doi.org/10.1017/S0140525X16000959
Butte NF, King JC (2005) Energy requirements during pregnancy and lactation. Public Health Nutr 8:1010–1027. https://doi.org/10.1079/PHN2005793
Byrne RW, Whiten A (1988) Machiavellian intelligence. Social expertise and the evolution of intellect in monkeys, apes, and humans. Clarendon Press, Oxford
Clutton-Brock T, Harvey PH (1980) Primates, brains and ecology. J Zool 190:309–323. https://doi.org/10.1111/j.1469-7998.1980.tb01430.x
Clutton-Brock T, Russell A, Sharpe L, Young A, Balmforth Z, McIlrath G (2002) Evolution and development of sex differences in cooperative behavior in meerkats. Science 297:253–256. https://doi.org/10.1126/science.1071412
Clutton-Brock T, Russell A, Sharpe L (2004) Behavioural tactics of breeders in cooperative meerkats. Anim Behav 68:1029–1040. https://doi.org/10.1016/j.anbehav.2003.10.024
Creel S, Creel NM (2002) The African wild dog: behavior, ecology, and conservation. Princeton University Press, Princeton
Deaner RO, Isler K, Burkart J, van Schaik C (2007) Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav Evol 70:115–124. https://doi.org/10.1159/000102973
DeCasien AR, Williams SA, Higham JP (2017) Primate brain size is predicted by diet but not sociality. Nat Ecol Evol 1:112. https://doi.org/10.1038/s41559-017-0112
Dixit T, English S, Lukas D (2017) The relationship between egg size and helper number in cooperative breeders: a meta-analysis across species. PeerJ 5:e4028. https://doi.org/10.7717/peerj.4028
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Dunbar RIM, Shultz S (2007) Evolution in the social brain. Science 317:1344–1347. https://doi.org/10.1126/science.1145463
Dunbar RIM, Shultz S (2017) Why are there so many explanations for primate brain evolution? Philos Trans R Soc B 372:20160244. https://doi.org/10.1098/rstb.2016.0244
Dyble M, Thompson J, Smith D, Salali GD, Chaudhary N, Page AE, Vinicuis L, Mace R, Migliano AB (2016) Networks of food sharing reveal the functional significance of multilevel sociality in two hunter-gatherer groups. Curr Biol 26:2017–2021. https://doi.org/10.1016/j.cub.2016.05.064
Emery NJ, Seed AM, von Bayern AM, Clayton NS (2007) Cognitive adaptations of social bonding in birds. Philos Trans R Soc B 362:489–505. https://doi.org/10.1098/rstb.2006.1991
Emlen ST, Wrege PH (1991) Breeding biology of white-fronted bee-eaters at Nakuru: the influence of helpers on breeder fitness. J Anim Ecol 60:309–326. https://doi.org/10.2307/5462
Fairbanks LA (1990) Reciprocal benefits of allomothering for female vervet monkeys. Anim Behav 40:553–562. https://doi.org/10.1016/S0003-3472(05)80536-6
Fernandes HBF, Woodley MA, Nijenhuis JT (2014) Differences in cognitive abilities among primates are concentrated on G: phenotypic and phylogenetic comparisons with two meta-analytical databases. Intelligence 46:311–322. https://doi.org/10.1016/j.intell.2014.07.007
Fish JL, Lockwood CA (2003) Dietary constraints on encephalization in primates. Am J Phys Anthropol 120:171–181. https://doi.org/10.1002/ajpa.10136
Fox J, Weisberg S (2011) An {R} companion to applied regression, vol 2. Sage, Thousand Oaks
Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160:712–726. https://doi.org/10.1086/343873
Fritz SA, Bininda-Emonds ORP, Purvis A (2009) Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics. Ecol Lett 12:538–549. https://doi.org/10.1111/j.1461-0248.2009.01307.x
Garamszegi LZ, Mundry R (2014) Multimodel-inference in comparative analyses. In: Garamszegi LZ (ed) Modern phylogenetic comparative methods and their application in evolutionary biology. Springer, Berlin, pp 305–331
Garber PA, Leigh SR (1997) Ontogenetic variation in small-bodied new world primates: implications for patterns of reproduction and infant care. Folia Primatol 68:1–22. https://doi.org/10.1159/000157226
Gartlan J (1969) Sexual and maternal behavior of the vervet monkey, Cercopithecus aethiops. J Reprod Fertil 6:137–150
Genoud M, Isler K, Martin RD (2018) Comparative analyses of basal rate of metabolism in mammals: data selection does matter. Biol Rev 93:404–438. https://doi.org/10.1111/brv.12350
Gilchrist JS, Russell AF (2007) Who cares? Individual contributions to pup care by breeders vs non-breeders in the cooperatively breeding banded mongoose (Mungos mungo). Behav Ecol Sociobiol 61:1053–1060. https://doi.org/10.1007/s00265-006-0338-2
Gittleman JL (1986) Carnivore brain size, behavioral ecology, and phylogeny. J Mammal 67:23–36. https://doi.org/10.2307/1380998
Gittleman JL (1989) Carnivore group living: comparative trends. In: Gittleman JL (ed) Carnivore behavior, ecology, and evolution. Springer, Berlin, pp 183–207
Grueber C, Nakagawa S, Laws R, Jamieson I (2011) Multimodel inference in ecology and evolution: challenges and solutions. J Evol Biol 24:699–711. https://doi.org/10.1111/j.1420-9101.2010.02210.x
Gubernick DJ, Laskin B (1994) Mechanisms influencing sibling care in the monogamous biparental California mouse, Peromyscus californicus. Anim Behav 48:1235–1237. https://doi.org/10.1006/anbe.1994.1356
Gurven M (2004) To give and to give not: the behavioral ecology of human food transfers. Behav Brain Sci 27:543–559. https://doi.org/10.1017/S0140525X04000123
Haber GC (1977) Socio-ecological dynamics of wolves and prey in a subarctic ecosystem. PhD dissertation, University of British Columbia
Hair J, Black W, Babin B, Anderson R, Tatham R (2006) Multivariate data analysis. Pearson Prentice Hall, Upper Saddle River
Harrington FH, Mech LD, Fritts SH (1983) Pack size and wolf pup survival: their relationship under varying ecological conditions. Behav Ecol Sociobiol 13:19–26. https://doi.org/10.1007/BF00295072
Harvey PH, Clutton-Brock T, Mace GM (1980) Brain size and ecology in small mammals and primates. P Natl Acad Sci USA 77:4387–4389. https://doi.org/10.1073/pnas.77.7.4387
Hawkes K, O’Connell JF, Jones NB, Alvarez H, Charnov EL (1998) Grandmothering, menopause, and the evolution of human life histories. P Natl Acad Sci USA 95:1336–1339. https://doi.org/10.1073/pnas.95.3.1336
Heesen M, Rogahn S, Ostner J, Schülke O (2013) Food abundance affects energy intake and reproduction in frugivorous female Assamese macaques. Behav Ecol Sociobiol 67:1053–1066. https://doi.org/10.1007/s00265-013-1530-9
Heinsohn R, Cockburn A (1994) Helping is costly to young birds in cooperatively breeding white-winged choughs. Proc R Soc Lond B 256:293–298. https://doi.org/10.1098/rspb.1994.0083
Heldstab SA, van Schaik CP, Isler K (2016a) Being fat and smart: a comparative analysis of the fat-brain trade-off in mammals. J Hum Evol 100:25–34. https://doi.org/10.1016/j.jhevol.2016.09.001
Heldstab SA, Kosonen ZK, Koski S, Burkart JM, van Schaik CP, Isler K (2016b) Manipulation complexity in primates coevolved with brain size and terrestriality. Sci Rep 6:24528. https://doi.org/10.1038/srep24528
Heldstab SA, van Schaik CP, Isler K (2017) Getting fat or getting help? How female mammals cope with energetic constraints on reproduction. Front Zool 14:29. https://doi.org/10.1186/s12983-017-0214-0
Heldstab SA, Müller DWH, Graber SM, Bingaman Lackey L, Rensch E, Hatt J-M, Zerbe P, Clauss M (2018a) Geographical origin, delayed implantation and induced ovulation explain reproductive seasonality in carnivores. J Biol Rhythms 33:402–419. https://doi.org/10.1177/0748730418773620
Heldstab SA, Isler K, van Schaik CP (2018b) Hibernation constrains brain size evolution in mammals. J Evol Biol 31:1582–1588. https://doi.org/10.1111/jeb.13353
Hewlett BS (1993) Intimate fathers: the nature and context of Aka Pygmy paternal infant care. University of Michigan Press, Ann Arbor
Hill K, Hurtado AM (2009) Cooperative breeding in South American hunter–gatherers. Proc R Soc Lond B 276:3863–3870. https://doi.org/10.1098/rspb.2009.1061
Hill K, Kaplan H, Hawkes K (1993) On why male foragers hunt and share food. University of Chicago Press, Chicago
Holliday MA (1986) Body composition and energy needs during growth. In: Falkner F, Tanner JM (eds) Postnatal growth neurobiology, vol 2. Springer, New York, pp 101–117
Hrdy SB (1976) Care and exploitation of nonhuman primate infants by conspecifics other than the mother. Adv Study Behav 6:101–158. https://doi.org/10.1016/S0065-3454(08)60083-2
Hrdy SB (2005) Evolutionary context of human development: the cooperative breeding model. In: Carter CS, Anhert L, Grossmann KE, Hrdy SB, Lamb ME, Porges SW, Sachser N (eds) Attachment and bonding: a new synthesis. MIT Press, Cambridge, pp 9–32
Hrdy SB (2009) Mothers and others: the evolutionary origins of mutual understanding. Harvard University Press, Cambridge
Isler K, van Schaik CP (2006a) Metabolic costs of brain size evolution. Biol Lett 2:557–560. https://doi.org/10.1098/rsbl.2006.0538
Isler K, van Schaik C (2006b) Costs of encephalization: the energy trade-off hypothesis tested on birds. J Hum Evol 51:228–243. https://doi.org/10.1016/j.jhevol.2006.03.006
Isler K, van Schaik CP (2009a) The expensive brain: a framework for explaining evolutionary changes in brain size. J Hum Evol 57:392–400. https://doi.org/10.1016/j.jhevol.2009.04.009
Isler K, van Schaik CP (2009b) Why are there so few smart mammals (but so many smart birds)? Biol Lett 5:125–129. https://doi.org/10.1098/rsbl.2008.0469
Isler K, van Schaik CP (2012) Allomaternal care, life history and brain size evolution in mammals. J Hum Evol 63:52–63. https://doi.org/10.1016/j.jhevol.2012.03.009
Isler K, Kirk EC, Miller JM, Albrecht GA, Gelvin BR, Martin RD (2008) Endocranial volumes of primate species: scaling analyses using a comprehensive and reliable data set. J Hum Evol 55:967–978. https://doi.org/10.1016/j.jhevol.2008.08.004
Iwaniuk AN, Arnold KE (2004) Is cooperative breeding associated with bigger brains? A comparative test in the Corvida (Passeriformes). Ethology 110:203–220. https://doi.org/10.1111/j.1439-0310.2003.00957.x
Jaeggi AV, Hooper PL, Beheim BA, Kaplan H, Gurven M (2016) Reciprocal exchange patterned by market forces helps explain cooperation in a small-scale society. Curr Biol 26:2180–2187. https://doi.org/10.1016/j.cub.2016.06.019
Jaquish C, Tardif S, Cheverud J (1997) Interactions between infant growth and survival: evidence for selection on age-specific body weight in captive common marmosets (Callithrix jacchus). Am J Primatol 42:269–280. https://doi.org/10.1002/(SICI)1098-2345(1997)42:4<269::AID-AJP2>3.0.CO;2-V
Jerison HJ (1973) Evolution of the brain and intelligence. Academic Press, New York
Jones KE, Bielby J, Cardillo M, Fritz SA, O’Dell J, Orme CDL, Safi K, Sechrest W, Boakes EH, Carbone C, Connolly C, Cutts MJ, Foster JK, Grenyer R, Habib M, Plaster CA, Price SA, Rigby EA, Rist J, Teacher A, Bininda-Emonds ORP, Gittleman JL, Mace GM, Purvis A (2009) PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90:2648–2648. https://doi.org/10.1890/08-1494.1
Kaplan H, Hill K, Lancaster J, Hurtado AM (2000) A theory of human life history evolution: diet, intelligence, and longevity. Evol Anthropol 9:156–185. https://doi.org/10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7
Kirk EC (2006) Visual influences on primate encephalization. J Hum Evol 51:76–90. https://doi.org/10.1016/j.jhevol.2006.01.005
Klauke N, Segelbacher G, Schaefer H (2013) Reproductive success depends on the quality of helpers in the endangered, cooperative El Oro parakeet (Pyrrhura orcesi). Mol Ecol 22:2011–2027. https://doi.org/10.1111/mec.12219
Koenig A (1995) Group size, composition, and reproductive success in wild common marmosets (Callithrix jacchus). Am J Primatol 35:311–317. https://doi.org/10.1002/ajp.1350350407
Komdeur J (1994) Experimental evidence for helping and hindering by previous offspring in the cooperative-breeding Seychelles warbler Acrocephalus sechellensis. Behav Ecol Sociobiol 34:175–186. https://doi.org/10.1007/BF00167742
Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N (2013) Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr Biol 23:168–171. https://doi.org/10.1016/j.cub.2012.11.058
Kuzawa CW, Chugani HT, Grossman LI, Lipovich L, Muzik O, Hof PR, Wildman DE, Sherwood CC, Leonard WR, Lange N (2014) Metabolic costs and evolutionary implications of human brain development. P Natl Acad Sci USA 111:13010–13015. https://doi.org/10.1073/pnas.1323099111
Kuznetsova TA, Kam M, Khokhlova IS, Kostina NV, Dobrovolskaya TG, Umarov MM, Degen AA, Shenbrot GI, Krasnov BR (2013) Desert gerbils affect bacterial composition of soil. Microb Ecol 66:940–949. https://doi.org/10.1007/s00248-013-0263-7
Lancaster JB (1971) Play-mothering: the relations between juvenile females and young infants among free-ranging vervet monkeys (Cercopithecus aethiops). Folia Primatol 15:161–182. https://doi.org/10.1159/000155377
Legge S (2000) Helper contributions in the cooperatively breeding laughing kookaburra: feeding young is no laughing matter. Anim Behav 59:1009–1018. https://doi.org/10.1006/anbe.2000.1382
Lonstein JS, De Vries GJ (2000) Influence of gonadal hormones on the development of parental behavior in adult virgin prairie voles (Microtus ochrogaster). Behav Brain Res 114:79–87. https://doi.org/10.1016/S0166-4328(00)00192-3
Lonstein JS, De Vries GJ (2001) Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster). J Comp Physiol 115:53–61. https://doi.org/10.1037//0735-7036.115.1.53
Lovegrove BG, Lobban KD, Levesque DL (2014) Mammal survival at the Cretaceous–Palaeogene boundary: metabolic homeostasis in prolonged tropical hibernation in tenrecs. Proc R Soc Lond B 281:20141304. https://doi.org/10.1098/rspb.2014.1304
Lukas WD, Campbell BC (2000) Evolutionary and ecological aspects of early brain malnutrition in humans. Hum Nat 11:1–26. https://doi.org/10.1007/s12110-000-1000-8
Lukas D, Clutton-Brock T (2012) Life histories and the evolution of cooperative breeding in mammals. Proc R Soc Lond B 279:4065–4070. https://doi.org/10.1098/rspb.2012.1433
Lukas D, Clutton-Brock TH (2013) The evolution of social monogamy in mammals. Science 341:526–530. https://doi.org/10.1126/science.1238677
Luo Y, Zhong MJ, Huang Y, Li F, Liao WB, Kotrschal A (2017) Seasonality and brain size are negatively associated in frogs: evidence for the expensive brain framework. Sci Rep 7:16629. https://doi.org/10.1038/s41598-017-16921-1
MacColl AD, Hatchwell BJ (2003) Sharing of caring: nestling provisioning behaviour of long-tailed tit, Aegithalos caudatus, parents and helpers. Anim Behav 66:955–964. https://doi.org/10.1006/anbe.2003.2268
Macdonald DW, Moehlman PD (1982) Cooperation, altruism, and restraint in the reproduction of carnivores. In: Bateson PPGKP (ed) Perspectives in ethology, vol 5. Plenum Press, New York, pp 433–467
MacLeod KJ, McGhee KE, Clutton-Brock TH (2015) No apparent benefits of allonursing for recipient offspring and mothers in the cooperatively breeding meerkat. J Anim Ecol 84:1050–1058. https://doi.org/10.1111/1365-2656.12343
Maestripieri D (1994) Social structure, infant handling, and mothering styles in group-living Old World monkeys. Int J Primatol 15:531–553. https://doi.org/10.1007/BF02735970
Malcolm JR, Marten K (1982) Natural selection and the communal rearing of pups in African wild dogs (Lycaon pictus). Behav Ecol Sociobiol 10:1–13. https://doi.org/10.1007/BF00296390
Marlowe F (1999) Male care and mating effort among Hadza foragers. Behav Ecol Sociobiol 46:57–64. https://doi.org/10.1007/s002650050592
Marlowe F (2000) Paternal investment and the human mating system. Behav Process 51:45–61. https://doi.org/10.1016/S0376-6357(00)00118-2
Marshall HH, Sanderson JL, Mwanghuya F, Businge R, Kyabulima S, Hares MC, Inzani E, Kalema-Zikusoka G, Mwesige K, Thompson FJ (2016) Variable ecological conditions promote male helping by changing banded mongoose group composition. Behav Ecol 27:978–987. https://doi.org/10.1093/beheco/arw006
Matějů J, Kratochvíl L, Pavelková Z, Řičánková VP, Vohralík V, Němec P (2016) Absolute, not relative brain size correlates with sociality in ground squirrels. Proc R Soc Lond B 283:20152725. https://doi.org/10.1098/rspb.2015.2725
McNab BK (2006) The energetics of reproduction in endotherms and its implication for their conservation. Integr Comp Biol 46:1159–1168. https://doi.org/10.1093/icb/icl016
Mink JW, Blumenschine RJ, Adams DB (1981) Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Phys 241:R203–R212. https://doi.org/10.1152/ajpregu.1981.241.3.R203
Mitani JC, Watts D (1997) The evolution of non-maternal caretaking among anthropoid primates: do helpers help? Behav Ecol Sociobiol 40:213–220. https://doi.org/10.1007/s002650050335
Moehlman PD (1979) Jackal helpers and pup survival. Nature 277:382–383. https://doi.org/10.1038/277382a0
Moehlman PD, Hofer H (1997) Cooperative breeding, reproductive suppression, and body mass in canids. In: Solomon NG, French JA (eds) Cooperative breeding in mammals. Cambridge University Press, Cambridge, pp 76–127
Mumme RL (1992) Do helpers increase reproductive success? Behav Ecol Sociobiol 31:319–328. https://doi.org/10.1007/BF00177772
Murie A (2011) The wolves of Mount McKinley. University of Washington Press, Seattle
Myers P, Espinosa R, Parr C, Jones T, Hammond G, Dewey T (2006) The animal diversity web, http://animaldiversity.ummz.umich.edu/
Navarrete A, van Schaik CP, Isler K (2011) Energetics and the evolution of human brain size. Nature 480:91–94. https://doi.org/10.1038/nature10629
Navarrete AF, Reader SM, Street SE, Whalen A, Laland KN (2016) The coevolution of innovation and technical intelligence in primates. Philos Trans R Soc B 371:20150186. https://doi.org/10.1098/rstb.2015.0186
Nichols HJ, Amos W, Bell MB, Mwanguhya F, Kyabulima S, Cant MA (2012) Food availability shapes patterns of helping effort in a cooperative mongoose. Anim Behav 83:1377–1385. https://doi.org/10.1016/j.anbehav.2012.03.005
Niven JE, Laughlin SB (2008) Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol 211:1792–1804. https://doi.org/10.1242/jeb.017574
Orme D (2013) The caper package: comparative analysis of phylogenetics and evolution in R. R package version 0.5.2, http://CRAN.R-project.org/package=caper
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884. https://doi.org/10.1038/44766
Parker ST, Gibson KR (1977) Object manipulation, tool use and sensorimotor intelligence as feeding adaptations in Cebus monkeys and great apes. J Hum Evol 6:623–641. https://doi.org/10.1016/S0047-2484(77)80135-8
Pérez-Barbería FJ, Gordon IJ (2005) Gregariousness increases brain size in ungulates. Oecologia 145:41–52. https://doi.org/10.1007/s00442-005-0067-7
Powell LE, Isler K, Barton RA (2017) Re-evaluating the link between brain size and behavioural ecology in primates. Proc R Soc Lond B 284:20171765. https://doi.org/10.1098/rspb.2017.1765
Price EC (1992) The benefits of helpers: effects of group and litter size on infant care in tamarins (Saguinus oedipus). Am J Primatol 26:179–190. https://doi.org/10.1002/ajp.1350260304
Quinlan RJ (2007) Human parental effort and environmental risk. Proc R Soc Lond B 274:121–125. https://doi.org/10.1098/rspb.2006.3690
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R Core Team (2017) R: a language and environment for statistical computing. version 3.4.1. R Foundation for Statistical Computing, Vienna, http://www.R-project.org/
Reader SM, Hager Y, Laland KN (2011) The evolution of primate general and cultural intelligence. Philos Trans R Soc B 366:1017–1027. https://doi.org/10.1098/rstb.2010.0342
Reddon AR, O’Connor CM, Ligocki IY, Hellmann JK, Marsh-Rollo SE, Hamilton IM, Balshine S (2016) No evidence for larger brains in cooperatively breeding cichlid fishes. Can J Zool 94:373–378. https://doi.org/10.1139/cjz-2015-0118
Roberts RL, Miller AK, Taymans SE, Carter CS (1998) Role of social and endocrine factors in alloparental behavior of prairie voles (Microtus ochrogaster). Can J Zool 76:1862–1868. https://doi.org/10.1139/z98-156
Rogerson P (2001) Statistical methods for geography, Sage
Rolfe DFS, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77:731–758. https://doi.org/10.1152/physrev.1997.77.3.731
Ross C, MacLarnon A (2000) The evolution of non-maternal care in anthropoid primates: a test of the hypotheses. Folia Primatol 71:93–113. https://doi.org/10.1159/000021733
Rothe H, Darms K, Koenig A, Radespiel U, Juenemann B (1993) Long-term study of infant-carrying behavior in captive common marmosets (Callithrix jacchus): effect of nonreproductive helpers on the parents’ carrying performance. Int J Primatol 14:79–93. https://doi.org/10.1007/BF02196504
Rowe N, Myers M (2011) All the world’s primates. Primate Conservation Inc., Charlestown http://www.alltheworldsprimates.org
Russell EM (1974) Recent ecological studies on Australian marsupials. Austr Mammal 1:189–211. https://doi.org/10.1007/978-94-017-6295-3_20
Russell E, Rowley I (1988) Helper contributions to reproductive success in the splendid fairy-wren (Malurus splendens). Behav Ecol Sociobiol 22:131–140. https://doi.org/10.1007/BF00303548
Russell A, Brotherton P, McIlrath G, Sharpe L, Clutton-Brock T (2003) Breeding success in cooperative meerkats: effects of helper number and maternal state. Behav Ecol 14:486–492. https://doi.org/10.1093/beheco/arg022
Rymer TL, Pillay N (2014) Alloparental care in the African striped mouse Rhabdomys pumilio is age-dependent and influences the development of paternal care. Ethology 120:11–20. https://doi.org/10.1111/eth.12175
Santos CV, French JA, Otta E (1997) Infant carrying behavior in callitrichid primates: Callithrix and Leontopithecus. Int J Primatol 18:889–907. https://doi.org/10.1023/A:1026340028851
Santos MJ, Thorne JH, Moritz C (2015) Synchronicity in elevation range shifts among small mammals and vegetation over the last century is stronger for omnivores. Ecography 38:556–568. https://doi.org/10.1111/ecog.00931
SAS Institute Inc (1989–2016) JMP version 13.0. SAS Institute Inc., Cary
Schubert M, Pillay N, Schradin C (2009) Parental and alloparental care in a polygynous mammal. J Mammal 90:724–731. https://doi.org/10.1644/08-MAMM-A-175R1.1
Sellen DW (2007) Evolution of infant and young child feeding: implications for contemporary public health. Annu Rev Nutr 27:123–148. https://doi.org/10.1146/annurev.nutr.25.050304.092557
Shultz S, Dunbar RIM (2007) The evolution of the social brain: anthropoid primates contrast with other vertebrates. Proc R Soc Lond B 274:2429–2436. https://doi.org/10.1098/rspb.2007.0693
Shultz S, Dunbar RIM (2010) Social bonds in birds are associated with brain size and contingent on the correlated evolution of life-history and increased parental investment. Biol J Linn Soc 100:111–123. https://doi.org/10.1111/j.1095-8312.2010.01427.x
Siani JM (2009) Costs and benefits of cooperative infant care in wild golden lion tamarins (Leontopithecus rosalia). PhD dissertation, University of Maryland, College Park
Silk JB (1980) Kidnapping and female competition among captive bonnet macaques. Primates 21:100–110. https://doi.org/10.1007/BF02383827
Sol D (2009) The cognitive-buffer hypothesis for the evolution of large brains. In: Dukas R, Ratcliffe JM (eds) Cognitive ecology II. Chicago University Press, Chicago, pp 111–134
Solomon NG (1991) Current indirect fitness benefits associated with philopatry in juvenile prairie voles. Behav Ecol Sociobiol 29:277–282. https://doi.org/10.1007/BF00163985
Sommer V (1989) Infant mistreatment in langur monkeys: sociobiology from the wrong end. In: Rasa A, Vogel C, Voland E (eds) The sociobiology of sexual and reproductive strategies. Chapman Hall, New York, pp 100–127
Speakman JR (2008) The physiological costs of reproduction in small mammals. Philos Trans R Soc B 363:375–398. https://doi.org/10.1098/rstb.2007.2145
Stockley P, Hobson L (2016) Paternal care and litter size coevolution in mammals. Proc R Soc Lond B 283:20160140. https://doi.org/10.1098/rspb.2016.0140
Striedter GF (2005) Principles of brain evolution. Sinauer, Sunderland
Symonds MR, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21. https://doi.org/10.1007/s00265-010-1037-6
Tardif SD, Carson RL, Gangaware BL (1992) Infant-care behavior of non-reproductive helpers in a communal-care primate, the cotton-top tamarin (Saguinus oedipus). Ethology 92:155–167. https://doi.org/10.1111/j.1439-0310.1992.tb00956.x
Tibbetts EA (2007) Dispersal decisions and predispersal behavior in Polistes paper wasp ‘workers’. Behav Ecol Sociobiol 61:1877–1883. https://doi.org/10.1007/s00265-007-0427-x
Tyler NJC (1987) Natural limitation of the abundance of the high arctic Svalbard reindeer. PhD dissertation, University of Cambridge
van Noordwijk MA, van Schaik CP (1999) The effects of dominance rank and group size on female lifetime reproductive success in wild long-tailed macaques, Macaca fascicularis. Primates 40:105–130. https://doi.org/10.1007/BF02557705
van Schaik CP, Burkart JM (2010) Mind the gap: cooperative breeding and the evolution of our unique features. In: Kappeler PM, Silk J (eds) Mind the gap: tracing the origins of human universals. Springer, Berlin, pp 477–496
van Schaik CP, Burkart JM (2011) Social learning and evolution: the cultural intelligence hypothesis. Philos Trans R Soc B 366:1008–1016. https://doi.org/10.1098/rstb.2010.0304
van Woerden JT, van Schaik CP, Isler K (2010) Effects of seasonality on brain size evolution: evidence from strepsirrhine primates. Am Nat 176:758–767. https://doi.org/10.1086/657045
van Woerden JT, Willems EP, van Schaik CP, Isler K (2012) Large brains buffer energetic effects of seasonal habitats in catarrhine primates. Evolution 66:191–199. https://doi.org/10.1111/j.1558-5646.2011.01434.x
van Woerden JT, van Schaik CP, Isler K (2014) Brief communication: seasonality of diet composition is related to brain size in new world monkeys. Am J Phys Anthropol 154:628–632. https://doi.org/10.1002/ajpa.22546
Veitschegger K (2017) The effect of body size evolution and ecology on encephalization in cave bears and extant relatives. BMC Evol Biol 17:124. https://doi.org/10.1186/s12862-017-0976-1
Wauters LA, Lens L (1995) Effects of food availability and density on red squirrel (Sciurus vulgaris) reproduction. Ecology 76:2460–2469. https://doi.org/10.2307/2265820
Weisbecker V, Blomberg S, Goldizen AW, Brown M, Fisher D (2015) The evolution of relative brain size in marsupials is energetically constrained but not driven by behavioral complexity. Brain Behav Evol 85:125–135. https://doi.org/10.1159/000377666
West RJ (2014) The evolution of large brain size in birds is related to social, not genetic, monogamy. Biol J Linn Soc 111:668–678. https://doi.org/10.1111/bij.12193
West HE, Capellini I (2016) Male care and life history traits in mammals. Nat Commun 7:11854. https://doi.org/10.1038/ncomms11854
Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W (2014) EltonTraits 1.0: species-level foraging attributes of the world’s birds and mammals. Ecology 95:2027–2027. https://doi.org/10.1890/13-1917.1
Woodroffe R, Vincent A (1994) Mother’s little helpers: patterns of male care in mammals. Trends Ecol Evol 9:294–297. https://doi.org/10.1016/0169-5347(94)90033-7
Wooldridge FL (1969) Behavior of the Abyssinian Colobus monkey, Colobus guereza, in captivity. Master’s Thesis, University of South Florida
Woxvold IA, Mulder RA, Magrath MJ (2006) Contributions to care vary with age, sex, breeding status and group size in the cooperatively breeding apostlebird. Anim Behav 72:63–73. https://doi.org/10.1016/j.anbehav.2005.08.016
Young AJ, Carlson AA, Clutton-Brock T (2005) Trade-offs between extraterritorial prospecting and helping in a cooperative mammal. Anim Behav 70:829–837. https://doi.org/10.1016/j.anbehav.2005.01.019
Yu X, Zhong MJ, Li DY, Jin L, Liao WB, Kotrschal A (2018) Large-brained frogs mature later and live longer. Evolution 72:1174–1183. https://doi.org/10.1111/evo.13478
Zenuto RR, Antinuchi CD, Busch C (2002) Bioenergetics of reproduction and pup development in a subterranean rodent (Ctenomys talarum). Physiol Biochem Zool 75:469–478. https://doi.org/10.1086/344739
Zöttl M, Chapuis L, Freiburghaus M, Taborsky M (2013) Strategic reduction of help before dispersal in a cooperative breeder. Biol Lett 9:20120878. https://doi.org/10.1098/rsbl.2012.0878
Zöttl M, Vullioud P, Goddard K, Torrents-Ticó M, Gaynor D, Bennett NC, Clutton-Brock T (2018) Allo-parental care in Damaraland mole-rats is female biased and age dependent, though independent of testosterone levels. Physiol Behav 193:149–153. https://doi.org/10.1016/j.physbeh.2018.03.021
Zucker EL, Kaplan J (1981) Allomaternal behavior in a group of free-ranging patas monkeys. Am J Primatol 1:57–64. https://doi.org/10.1002/ajp.1350010107
Acknowledgments
We are thankful to the editor and the two anonymous reviewers for their constructive and thoughtful comments on previous versions of the manuscript.
Funding
Financial support was provided by the Swiss National Science Foundation grant no. 31003A-144210, the A.H. Schultz Foundation and the University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical statement
All sources of data were from the literature or the web and did not involve ethical approval.
Data availability
The dataset and all additional analyses supporting the conclusions of this article are available in this published article and in the supplementary information files.
Additional information
Communicated by M. Raymond
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heldstab, S.A., Isler, K., Burkart, J.M. et al. Allomaternal care, brains and fertility in mammals: who cares matters. Behav Ecol Sociobiol 73, 71 (2019). https://doi.org/10.1007/s00265-019-2684-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2684-x