Abstract
In many territorial species, occasional movements beyond territory boundaries (extraterritorial forays) have been documented in many species. While many explanations for the occurrence of extraterritorial forays have been proposed, a logical and proposed function of extraterritorial forays is to engage in extra-pair copulations with extra-pair mates outside of their territories. We used an automated radio-telemetry system (ARTS) to examine the patterns and correlates of foray behavior of male and female field sparrows (Spizella pusilla) and investigated if forays were associated with extra-pair paternity (EPP) and cuckoldry. We found that male and female field sparrows regularly engaged in extraterritorial forays. In males, age and time of foraying (day vs. night) were important factors explaining foray rates (foray/h); older (ASY) males forayed more than younger (SY) males and, while we detected many nocturnal forays, most forays occurred during the day. For females, fertility stage and age appeared to be important in explaining foray rates; older females forayed more during pre-fertile period than fertile and post-fertile periods. Unlike foray rates, the duration of forays (min) was not explained by any of the variables examined. Surprisingly, despite the large number of forays documented (>3500), greater foray rates or duration of forays were not associated with higher probability of EPY for males or females or with cuckoldry in males. Forays may play a role in prospecting and acquiring information about their social and ecological environment, which ultimately may help them to achieve greater reproductive success, but not necessarily in the form of EPP.
Significance statement
Despite many territorial species are known to conduct extraterritorial forays (movements beyond their territory), very little is known about this behavior. We used an automated radio-telemetry system (ARTS) to examine the patterns, correlates, and paternity consequences of extraterritorial foray behavior in male and female field sparrows (Spizella pusilla). We documented more than 3500 forays and found that both male and female field sparrows regularly engaged in extraterritorial forays; however, different factors explain their foray rates (age, time of foraying (day vs. night), and fertility stage) but not the duration of forays. Surprisingly, greater foray rates or duration of forays were not associated with higher probability of EPY in males or females or with cuckoldry in males. Rather than exclusively acquiring extra-pair matings, forays likely serve multiple purposes, such as prospecting and acquiring information about their social and ecological environment, which ultimately may help individuals achieve greater reproductive success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most breeding birds are territorial, i.e., within a defined area, they monopolize and defend resources such as food, nesting sites, or access to mates, in order to survive and successfully reproduce (Brown 1964, 1969). Movements beyond territory boundaries (extraterritorial forays) however have been documented in many territorial species (Neudorf et al. 1997; Stutchbury et al. 2005; Chiver et al. 2008). Many socially monogamous birds are known to engage in extra-pair paternity (EPP); therefore, a logical and proposed function of extraterritorial forays (hereafter forays) is to engage in extra-pair copulations (EPC) with extra-pair mates settled away from their territories (Griffith et al. 2002; Westneat and Stewart 2003). While not mutually exclusive, other potential explanations for the occurrence of forays are to gather additional food outside their territories (Neudorf et al. 1997; Humbird and Neudorf 2008) and prospect for future dispersal, and breeding sites (Danchin et al. 2001; Pärt and Doligez 2003; Doligez et al. 2004). Despite these reasonable explanations, the relationship among forays and EPP are still in question. While some researchers have found that foray rates (foray/h) and the time spent off territory correlates with higher probability of obtaining extra-pair young (e.g., male reed buntings, Emberiza schoeniclus, Kleven et al. 2006; female hooded warblers, Wilsonia citrina, Chiver et al. 2008; male and female great tits, Parus major, Patrick et al. 2012), others have found a weak or non-existent relationship among foray behavior and EPP (e.g., male hooded warblers, Stutchbury et al. 2005; male American redstarts, Setophaga ruticila, Churchill and Hannon 2010). Conceivably, our limited ability to track birds and accurately describe these subtle and relatively rare behaviors is biasing our understanding of the patterns and correlates of foray behavior in birds and consequently, our view regarding the function of forays.
The most widely used approach for studying extraterritorial foray behaviors is tracking songbirds via manual radio-telemetry (e.g., Stutchbury et al. 2005; Kleven et al. 2006; Pedersen et al. 2006). While manual telemetry has enhanced our knowledge of extraterritorial forays, it has technical and logistical constraints. For example, it is difficult to track movements of individual birds if they move large distances, especially over short time periods. Consequently, the average total effort devoted to tracking foraying individuals is generally <20 h, which usually is achieved by tracking each bird a few hours a day every 2–3 days (e.g., Stutchbury et al. 2005; Pedersen et al. 2006; Evans et al. 2008). Additionally, recent studies have shown that some bird species make nocturnal forays (nightingale, Luscinia megarhynchos, Naguib et al. 2001; yellow-breasted chat, Ward et al. 2014), but most radio-telemetry studies are conducted during daylight hours. Manual telemetry, at best, provides a snapshot of foraying behavior and at worst biases our understanding of the patterns of foray behavior and its relative contribution to an individual’s overall reproductive performance (in the form of EPPs).
The relative use of forays and its related costs and benefits, however, may depend on numerous factors, such as sex, age, and body condition (Westneat and Stewart 2003). For instance, empirical data suggest that foraying may be an effective tactic for older males to acquire extra-pair mates (Weatherhead and Boag 1995; Stutchbury et al. 2005; Kleven et al. 2006), while younger males may stay in their territory and guard their female (Evans et al. 2008). The trade-off between the costs and benefits of foraying (in the form of mate guarding and cuckoldry) is one explanation of this pattern (Birkhead and Møller 1992). Forays allow males to acquire extra-pair mates, but during forays, they reduce mate guarding, resulting in an increased risk of cuckoldry (Westneat 1988; Sherman and Morton 1988). The risk of cuckoldry may differ between males of different age; older males are less often cuckolded than low-quality males possibly due to female preference for older males or traits that correlate with age and social dominance (Hoeschele et al. 2010) and/or because young males may be less experienced at assuring paternity by mate guarding (Weatherhead and Boag 1995). The difference in the likelihood of cuckoldry between individual males, therefore, predicts more foraying effort on older males than younger males.
Like males, females may use forays to search for extra-pair copulations in addition to or in lieu of accepting solicitations from males intruding on their mate’s territories (Kempenaers et al. 1997; Double and Cockburn 2008). Females searching for extra-pair matings via forays may improve a female’s probability of locating “preferred” mates in the population, potentially of high-quality (Mays and Hill 2004), or increasing genetic diversity of her brood (Arnold and Duvall 1994; Yasui 1998, 2001). A female’s ability to balance costs and benefits of foraying during reproductive activities, variation in female motivation and ability to invest in mate searching may vary depending on age (Jennions and Petrie 1997; Duval and Kappor 2015), potentially leading to differences in foraying behavior. In this scenario, female age is predicted to influence whether or not a female forays, how often and for how long she forays (Kempenaers and Dhondt 1993; Kleven et al. 2006; Chiver et al. 2008).
The patterns of foray behavior in males and females may also vary with body condition. For instance, differences in body condition, as it reflects an individual’s energy reserves, are known to influence physical activity in many avian species during mate search, reproductive, dispersal, stopover, and long-distance migration periods (Rintamaki et al. 1995; Deppe et al. 2015; Duval and Kapoor 2015). Therefore, body condition could influence how birds allocate their efforts toward searching for mates outside their territories, potentially influencing foray behavior. Changes in foray patterns in males and females may also occur across their different fertility stages. For instance, male and female song sparrows (Melospiza melodia) commonly remain together in the pre-fertile period, but afterwards males and females tend to foray separately (Akçay et al. 2012). Males tend to foray frequently during the post-fertile periods, as their role in reproductive activities with their mate is reduced (Akçay et al. 2012; Ward et al. 2014). In contrast, females tend to foray more during their fertile stage, and reduce foray effort when they need to attend to their offspring (Neudorf et al. 1997; Stutchbury et al. 2005).
Differences among individuals of different sex, age, or body condition may not only be reflected in the rate of foraying (number of forays/h) or duration of their forays (min), but also when they conduct their forays (Naguib et al. 2001; Roth et al. 2009). For example, older and better condition males may conduct more forays or spent more time during forays during the day because they can selectively intrude into territories of young and lower-condition males and obtain EPCs (Pedersen et al. 2006), while young and lower-condition males may stay in their territories and mate-guard due to their risk of intrusions from males (Westneat 1988; Griffith et al. 2002). Young and lower-condition males, however, may conduct forays at night because they can more easily “sneak” into neighboring territories to acquire matings without confronting resident males; making the best of a bad situation (Gross 1996). Benefits of foraying at night may also be extended to females (Roth et al. 2009). Females may opt to foray at night to avoid their social mates learning about their extraterritorial forays and the subsequent costs associated with foraying (Weatherhead et al. 1994) or because at night, there is a higher probability to find potential extra-pair males outside their territories. For instance, some species have been shown to foray before sunrise or nocturnally and seek EPCs (Malurus cyaneus, superb-fairy wrens, Double and Cockburn 2000; Icteria virens, yellow-breasted chat, Ward et al. 2014).
We used a combination of an automated radio-telemetry system (ARTS) and microsatellite DNA analyses to investigate the patterns and correlates of extraterritorial foray behavior in male and female field sparrows (Spizella pusilla) and how this behavior correlates with patterns of extra-pair paternity (EPP) and cuckoldry. First, we quantified foray behavior of field sparrows using ARTS. ARTS allowed us to track male and female field sparrows over 24-h periods across multiple breeding stages and over a large spatial extent (50–60 territories). Specifically, we explored relationships between male and female extraterritorial foray rates (forays/h) and mean duration of forays (min) and age, physical condition (as the residuals from a regression of mass [g] on tarsus length), fertility stage (pre-fertile, fertile and post-fertile) and time of forays (day vs. night). By taking advantage of our high-resolution spatio-temporal data obtained via automated telemetry, we also discuss other potential functions of foray behavior in the field sparrow. Second, we used a microsatellite analysis to estimate the patterns of paternity in our study population and examine whether males that foray more or for longer periods of time have more EPP (outside their nest), and whether females that foray more or for longer periods of time are more likely to have extra-pair young in their nests. Lastly, we examined whether males that foray more or for longer periods of time are more likely to be cuckolded by their mate.
Methods
Study species
Field sparrows, a socially monogamous songbird, are known to engage in extra-pair matings (at least 19% of offspring may be sired through extra-pair matings; Petter et al. 1990) and to be active at night (Walk et al. 2000; Celis-Murillo et al. 2016a, b). Field sparrows are sexually monomorphic with respect to plumage, but differ slightly in size (Carey et al. 1994); males are usually larger than females. Field sparrows are short-distance migrants, with some individuals remaining on the breeding grounds in winter (Carey et al. 1994). Females arrive on the breeding grounds between 15 April and 15 May (10–20 days after males), and pair formation usually occurs within a few days of their arrival. Field sparrows breed in successional old fields, brushy pastures, and woodland openings and edges (Carey et al. 1994). They typically place their nests at the base of woody vegetation, near the ground (Best 1977). Only females build the nest and incubate eggs, but males follow mates during the late stages of nest building. Both sexes provision young. Double brooding is common in field sparrows, and pairs usually re-nest immediately after nest failure.
Study site and general field methods
From May 1st to July 31st of 2012 to 2014, we studied male and female field sparrows at Kennekuk Cove County Park, IL, USA (40° 11.5″ N, 87° 42.9″ W). Kennekuk Park is composed of discreet grassland patches of varying sizes (2–10 ha) surrounded by oak-hickory forest. Each year 3–6 field sparrow males established territories in each of these grassland patches and formed clumped aggregations of territories separated by forest patches (hereafter “neighborhoods”). Each year, at the beginning of the season, and over the course of multiple visits (36–48 visits), we used spot-mapping (6–8 points per male per visit) to locate territorial males and behavioral observations of their dawn singing, counter-singing with their neighbors, or engaging in boundary disputes during the day. This facilitated the delineation of each male’s territory boundary in a map and aerial pictures and helped to delineate the territories of 50–60 territorial males distributed across 10 different neighborhoods of varying sizes (2–8 ha). We captured male and female birds using targeted mist netting, and banded sparrows with uniquely numbered USGS aluminum and colored plastic leg bands. Age (when possible), sex, tarsus length [mm], and mass [g] were recorded for all individuals. We estimated age as “second year” (SY, birds that were in their first breeding season) and “after second year” (ASY) by following Pyle (1997). Because most field sparrows start mating and nesting shortly after their arrival to their breeding grounds, we were able to reliably determine sex for individuals by observing cloacal protuberance in males and brood patches in females. We confirmed sex designations with subsequent behavioral observations (e.g., monitoring incubation and song). For adults and nestlings, we collected 30 μl of blood via brachial venipuncture for paternity analyses. We banded nestlings and collected a blood sample at 5 days of age. Blood was stored in Queen’s lysis buffer (Seutin et al. 1991) and was kept in the lab at ambient temperatures. We used DNeasy Blood kit (Qiagen, Valencia, CA, USA) to extract DNA from blood samples. Sparrows were fitted with radio-transmitters weighting 0.5 to 0.6 g (JDJC corp, Fisher, IL). This represented ~5% of birds’ average weight (12 g). Transmitters were glued on birds’ backs following Raim (1978). This method allowed the transmitters to fall off approximately by the end of the field season, potentially reducing stress and physiological impacts on birds. It was not possible to record data blind because our study involved focal animals in the field.
We conducted behavioral observations and visited territories every 3 days to monitor pairing and reproductive status. Birds were classified as paired but not nesting, building a nest, laying eggs, incubating eggs, nestlings, and fledglings. To minimize error in assigning reproductive stages, we later combined reproductive stages into more conservative periods following Akçay et al. (2012); pre-fertile period (paired but not nesting, and initial 4 days of building nest), fertile period (4 days before the first egg is laid and the period of laying eggs), and post-fertile period (incubating, feeding nestlings, and caring for fledglings). In addition to monitoring pairing status and reproductive behaviors across the season, we monitored territory ownership and establishment for each individual throughout the season. Based on our observations, the ownership of territories did not change throughout the season. In our study, we only observed two individuals per year switching territories to a different neighborhood, and these individuals were omitted from analyses.
Automated radio-telemetry and tracking birds
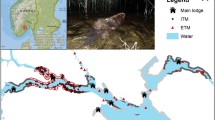
We documented foray behavior in mated male and female field sparrows using an automated radio-telemetry system (ARTS; Kays et al. 2011; Steiger et al. 2013; Ward et al. 2013, 2014). The ARTS was comprised of four towers with autonomous radio-telemetry receiving units (JDJC Corp., Fisher, IL) located 400–950 m apart and strategically placed in the study area to collect data from each radio-tagged bird of study (Fig. 1). The height of towers was 12–14 m. We connected each ARU to an array of six three-element Yagi antennas (Nighthawk model—JDJC Corp., Fisher, IL) attached to the top of towers. We positioned the six Yagi antennas at 0°, 60°, 120°, 180°, 240°, and 300° to 360° detection coverage. Receiving units collected up to three detections per minute per bird over the duration of the transmitter’s battery life (~29 days). The receiving units recorded the signal strength (in dB), electromagnetic noise (dB), and pulse width of the transmitter (milliseconds) of each radio transmitter mounted on birds. Custom scripts developed in R 2.15.2 software (R Development Core Team 2008) were used to estimate the locations of each bird via triangulation based on detection records collected from multiple receiving units (Ward et al. 2013, scripts available at http://ward.nres.illinois.edu/). As in previous studies (Ward et al. 2013, 2014), we used thresholds for signal strength, noise, and pulse width to remove potential locations caused by multipath effects (signals bouncing off manmade objects or spurious radio signals). After applying filters and removing spurious data, we estimated X and Y coordinates for each intersection of signals and used the harmonic mean of the X and Y values for each coordinate to estimate location. We plotted the locations of birds in Google Earth to facilitate interpretation of the data. Because it is important to quantify error when tracking birds via ARTS, multiple tests were previously conducted using ARTS in our study site and with the system used in this study (see Ward et al. 2013, 2014). In the core area of the study (the area within the four telemetry towers), the mean accuracy was 28.6 ± 12.6 m (mean difference of a radio transmitter attached to a tree and where the ARTS estimated the location of the radio transmitter).
Forays in other species have been identified by visually watching focal individuals leave their territories (Kleven et al. 2006; Dalziell and Cockburn 2008; Barron et al. 2015). However, we took a different approach and determined which utilization distribution (UD) best matched the territories of males. UDs are the relative frequency distributions of an animal’s occurrence in space and time (Keating and Cherry 2009). We estimated each individual’s UD from 20 to 95% by units of 5%. We estimated UDs using the dynamic Brownian bridge estimators in the R package “move” (Kranstauber et al. 2012). The contribution of each location was smoothed out to from a kernel estimate (polygon) (Silverman 1986). The data on UDs showed that 50–55% kernel estimates matched well in size and shape to the territories of the field sparrows delineated by our behavioral observations. We then used a conservative approach to identify forays. First, we used 60% UD as the individual’s territory (Fig. 2). Even though the 60% UD was slightly larger than the mapped territory, it allowed us to account for some of the error inherent in the ARTS. Communication radios, lightning strikes, and aircraft under certain situations can create signals that meet all the filters used in this study. Many of these very short forays were also biologically questionable (e.g., a foray in an inappropriate habitat hundreds of meters away from a territory for a period of less than a minute). Thus, we defined forays as three consecutive locations outside of an individual’s 60% UD, and each foray ended when the individual returned to within the 60% UD (Fig. 3). We collected data on 62 field sparrows but due to transmitters falling off and transmitter failure, we only analyzed data from 46 individuals all of which had at least 228 locations. Once we quantified forays for each bird, we estimated foray rates (forays per hour) and mean duration (minutes) of forays for each individual bird.
Extra-pair paternity analyses
To determine paternity, we used seven microsatellite loci identified from other species including five from Worthen’s Sparrow (Spizella wortheni) (Canales-Delgadillo et al. 2010) and three from Brown-headed Cowbird (Molothrus ater) (Strausberger and Ashley 2001, 2003) (Online resources, S1). We searched for cross-amplification of each locus for field sparrows first on a temperature gradient. Once we identified an optimal annealing temperature, we checked for allelic polymorphisms for each locus. PCR reaction conditions and cycles were variable by locus and are described in detail in the online supplement (Online resources, S2)
Each set of primers was tested on the full set of breeding field sparrows (n = 182) to assess allelic diversity, test for Hardy-Weinberg equilibrium, the frequency of null alleles [CERVUS, (Marshall et al. 1998)], and linkage disequilibrium [GENEPOP (Rousset 2008)]. All individuals (n = 424) were genotyped at more than four loci and the majority (> 99%) were genotyped at all seven loci (Online resource, S1). Allelic diversity ranged from four to 36 alleles and no locus deviated significantly from Hardy-Weinberg equilibrium (p > 0.05) nor did any loci show evidence of linkage disequilibrium. The frequency of null alleles from all but one locus (Sw09) ranged from 0.003 to 0.051, which is considered rare, and should not cause sufficient concern for paternity analyses (Dakin and Avise 2004). Because locus Sw09 had a relatively high (0.10) frequency of null alleles, we conducted our parentage analyses with and without this locus. The analyses did not differ in the identity of parents assigned to offspring so we included it in our analyses to maximize the potential to identify sires genetically. Because genotyping in this type of project is seldom 100% accurate, we reran 20% of samples to obtain an empirical measure of genotyping error (0.02) and used this rate with a likelihood-based approach implemented in application CERVUS 3.0 (Marshall et al. 1998; Kalinowski et al. 2007) to assign paternity. CERVUS uses the available data to calculate likelihood ratios for the possibility that the genotypes of parents and offspring are mistyped and to determine, via simulation, the level of confidence in the parentages it assigns. To determine the statistical significance of paternity assignments, we performed a simulation of 10,000 tests based on adult genotype frequencies using a genotyping error rate of 0.02 and assuming we sampled 75% of the candidate mothers and 90% of the candidate fathers. Our assumptions for the portion of males and females that were sampled were based on field observations. We used parentage assignments with ≥95% confidence, as determined by the likelihood-odds ratios (Kalinowski et al. 2007).
Statistical analyses
Extraterritorial foray behavior
We examined males and females separately because we expected males and females to differ in foraying behavior. We used generalized linear mixed models (GLMM, GLIMMIX procedure, SAS 9.3) with a negative binomial distribution and a log link function (Littell et al. 2006) to examine the factors influencing foray rates (forays/h.) and the average duration of forays (min) in male and female field sparrows. We used a negative binomial distribution to account for potential overdispersion of our data. We treated number of forays with a number of hours tracked as an offset and mean duration of forays (in minutes), as two different response variables and age, physical condition, as the residuals from a regression of mass [g] on tarsus length [mm], time of day (day or night), fertility stage (pre-fertile, fertile or post-fertile), ordinal date, year, and interactive combinations of these variables as fixed effects. We treated bird identity as a random effect to account for repeated sampling of individuals within years (i.e., during multiple fertility stages and during both day and night) and the tracking of one individual in two separate years. Prior to fitting models, we examined correlations among all variables and excluded variables that were highly correlated (|r| > 0.50). We evaluated a priori candidate models using Akaike’s information criterion adjusted for small sample sizes (AICc) (Burnham and Anderson 2002; Grueber et al. 2011; Symonds and Mousalli 2011). We considered models within 4 AICc of the top model to be competitive, as this represents a conservative inclusion of the most plausible models (Burnham et al. 2010). We also evaluated all models for their inclusion of uninformative parameters (i.e., “pretending variables”; Anderson 2008) by examining model deviance and the inclusion of zero in 85% confidence limits (Arnold 2010). In case of model selection uncertainty, we calculated model-averaged values as well as unconditional 85% confidence intervals for variables contained within competitive models (Burnham and Anderson 2002). We report means ± SE unless otherwise noted.
Extra-pair paternity
We used generalized linear mixed models (GLMM, GLIMMIX procedure, SAS 9.3) with a binomial distribution and a logit link function (Littell et al. 2006) to examine whether the probability of males obtaining EPY outside their nest increased with foray behavior and whether the probability of being cuckolded (EPY within their nest) increased with foray behavior. We included an interaction between male age and time of day as these were important predictors of foray rate in males. We also examined whether the probability of females obtaining EPY in their nest increased with foray behavior while controlling for female age and fertility period. We treated extra-pair paternity (coded as yes = 1 and no = 0) as the response variable, foray rate (forays/h.) as a fixed effect, and we treated bird identity as a random effect to account for sampling some individuals in more than 1 year. We also examined the influence of foray duration on EPY in males and females and cuckoldry in males using the same framework. We report means ± SE unless otherwise noted.
Results
We banded 567 field sparrows (161 males, 74 females, and 332 nestlings) over the course of the study. We documented foray behavior of 47 radio-tagged adult birds (25 males and 22 females) during 2012–2014. We tracked one male on two consecutive years. Across the study, eight birds were tracked in 2012, 14 birds in 2013, and 26 birds in 2014. We identified 3523 total forays over the 3-year study and tracked birds for 15,209 h (Table 1). We tracked each bird for an average of 317 h (~13 days) and acquired an average of 5394 locations per bird, with a maximum number of locations of 34,033 over the course of 28 days. The average number of forays per hour in males was 0.28 forays/h ± 0.34 SD (range = 0.05–1.89) and for females was 0.25 forays/h ± 0.37 SD (range = 0.01–2.68) (online resources, S3–4). The average duration of male forays was 87.27 min ± 166.10 SD and for females 90.17 min ± 116 min SD (online resources, S5–6).
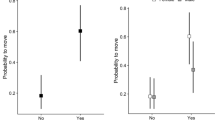
Foray rates (forays/h.) of males were best explained by one model with an interaction of age (ASY and SY) and time of day (Day_Night). This top model received nearly half the weight of evidence (∑w i = 0.49) (Table 2). ASY males forayed more than SY males and overall, more forays occurred during the day than at night (Fig. 4). The patterns from this top-ranked model were driven by the main effects of age and time of day, as the interaction effects were not strongly supported (ASY day = 0.12 ± 0.38). However, because this first model was not overwhelmingly supported, and two additional models were ranked within 4 AICc of the top model, we generated model-averaged estimates of the variables within our competitive models. The second ranked model, the global model, despite being ranked as competitive (∑w i = 0.38 and within 4 AICc of the top model; Burnham et al. 2010), was driven by the variables of age, day_night and year (age: ASY β = 0.51 ± 0.29; Day_Night: day β = 0.73 ± 0.18; year: 2012 β = 0.41 ± 0.33, 2013 β = 0.90 ± 0.36). The coefficients for fertility stage, condition and ordinal date were not informative (fertility stage: fertile β = 0.11 ± 0.23, post-fertile β = 0.12 ± 0.23; condition: β = −0.16 ± 0.13; ordinal date: β = 0.001 ± 0.008). The model of year alone was also a predictor of foray rates (year: 2012 β = 0.73 ± 0.0.27, 2013 β = 1.3 ± 0.25; birds forayed more in 2013 (0.46 forays/h ± 0.08) than in 2012 (0.26 forays/h ± 0.05) and 2014 (0.12 forays/h ± 0.02). We found little support for the other models containing fertility stage, ordinal date, condition, or interactions among these variables for influencing foray rates of males (Table 2). In contrast, we found no support for any of the models we tested (age, fertility stage, ordinal date, condition, or interactions among these variables) for influencing foray duration in males; null model was ranked within 4 AICc of the top model, suggesting that the models we tested were not competitive (Table 3).
Foray rate (forays/h) of females was best explained by fertility stage; this top model received nearly half the weight of evidence (∑w i = 0.42; Table 4); females forayed more during the pre-fertile stages than the fertile and post-fertile stages (fertility stage: fertile β = −0.93 ± 0.42; post-fertile β = −0.77 ± 0.40) (Fig. 5). Two additional models, the interaction of fertility stage and age (∑w i = 0.29; Table 4) and the interaction of fertility stage and time of day (∑w i = 0.10) were also ranked within 4 AICc of the top model; consequently, we generated model-averaged estimates of the variables within our competitive models. The model of the interaction of fertility stage and age, suggested that ASY females foray more than SY females but that the interaction terms were uninformative (fertile ASY β = 0.48 ± 0.57; post-fertile ASY β = −0.25 ± 0.52) (Fig. 5). The model of the interaction among fertility stage and time of day indicated that females foray more during the day than at night, but that the interaction terms were uninformative (fertile day β = 0.28 ± 0.55; post-fertile day β = −0.19 ± 0.49). Model-averaged values, however, suggested that the predominant influence was fertility stage and not time of day (Fig. 5). The additional models, global, condition, day vs. night, ordinal date, age, and interactive models were considered not competitive as they were ranked with ΔAICc > 4 (Table 4). Like in males, any of the models we tested (fertility stage, ordinal date, condition, or interactions among these variables) failed to explain foray duration in females; null model was ranked within 4 AICc of the top model suggesting that any of the models were competitive (Table 5).
We collected blood samples from 424 birds (143 males, 73 females, and 308 nestlings). We included the microsatellite genotypes of all known territorial adults for maximum-likelihood simulations and estimated the confidence of paternity assignments. We found at least one extra-pair offspring in 40.0% (32/80) of the broods and 9.7% (30/308) of all offspring sampled were sired by an extra-pair male. We identified 92% (24 of 26) of the extra-pair fathers. Four nests had EPY from two extra-pair fathers. Of the 47 radio-tagged birds (25 males and 22 females), we tracked and for which we estimated foray rates and duration of forays, and we had extra-pair paternity data for 30 (17 males and 13 females).
The probability of obtaining EPP in males and females (in males, outside their nest and in females within their nest) did not increase with foray rate; individuals that forayed more were not more likely to gain EPY (GLMM for males: n = 18, β = −0.64 ± 2.54, F 1,41 = 0.06, P = 0.80; GLMM for females: n = 13, β = −1.32 ± 3.35, F 1,28 = 0.16, P = 0.69). Similarly, the probability of obtaining EPP in males and females did not increase with foray duration; individuals that forayed for longer periods of time were not more likely to gain EPY (GLMM for males: n = 18, β = 0.004 ± 0.008, F 1,41 = 0.27, P = 0.60; GLMM for females: n = 13, β = −0.002 ± 0.009, F 1,28 = 0.08, P = 0.78). Furthermore, the probability of males being cuckolded (finding EPY within their nest) did not increase with foray rate (GLMM for males: n = 18, β = −4.12 ± 7.62, F 1,28 = 0.29, P = 0.59) or duration of forays (GLMM for males: n = 18, β = 0.004 ± 0.01, F 1,28 = 0.15, P = 0.70).
Discussion
With the high spatial and temporal resolution of movement data obtained via automated telemetry, we gained insight into the patterns, correlates, and paternity consequences of extraterritorial foray behavior in the field sparrow. We found that both male and female sparrows regularly engaged in extraterritorial forays and that factors such as age, time of day (day or night), and fertility stage influence foray rates but not duration of forays. Older males forayed more than younger males and, while we detected many nocturnal forays, most forays occurred during the day. These findings are consistent with Kleven et al. (2006) that specifically examined age differ-ences in foray effort and showed that older males of reed bunting (E. schoeniclus) foray seven times as frequently as young males. Based on Weatherhead and Boag (1995), older and more experienced males usually have higher success in extra-pair mating because older males are better at exploiting possibilities for EPCs and coercing females. In addition, females may be more likely to accept copulation from older males, as older males tend to have higher pairing success, clutch size, and fledgling success than SY males (Lemon et al. 1992). Although this pattern of older males foraying more than young males is consistent with several previous studies and correlates with patterns of extra-pair mating in socially monogamous birds (reviewed in Griffith et al. 2002), we did not find evidence for rate of forays and foray duration correlating with extra-pair paternity in our system.
For females, fertility stage and age appeared to be an important factor explaining foray rates; females forayed more during the pre-fertile period than the fertile and post-fertile periods and overall, ASY females forayed more than SY females. The pattern of foraying more during the pre-fertile period is consistent with studies of foray behavior in other species (song sparrows, Açkay et al. 2012; yellow-breasted chat, Ward et al. 2014). However, while in some species female forays stop after fertile periods (song sparrow, Açkay et al. 2012), our female sparrows, although less frequently, forayed throughout all fertility stages, including post-fertile stages, suggesting that seeking EPCs and acquiring EPPs may not be the only reason for foraying.
Male field sparrows forayed most often during the day and less often at night. Although this was not surprising, the fact that our field sparrows forayed greater than expected at night was interesting, especially given that field sparrows are primarily diurnal (Carey et al. 1994). Nocturnal activity (singing) in field sparrows, although rare, has been documented in our system; territorial mated males tend to vocalize at night throughout the breeding season, potentially advertising their location and willingness to copulate (Celis-Murillo et al. 2016a, b). These observations, therefore, reveal that field sparrows may employ both a passive (advertise) and active approach (foray) to seek and/or attract extra-pair mates, even at night.
Despite documenting >3500 forays, we failed to find a link between the foray rate or duration of forays and extra-pair paternity (in the form of EPY) in males and females. Furthermore, we failed to document a link between foray rate or duration of forays and cuckoldry in males. It is possible, however, that despite birds using forays to seek extra-pair mates they could be copulating frequently on forays. Thus, a correlation among foray effort and measurable EPYs could be difficult to obtain. This is consistent with many experimental studies that have shown that the occurrence of EPCs in nature is usually much greater than the observed number of EPY (Hunter et al. 1992; Fossøy et al. 2006) and that even females having no EPY may still be sexually promiscuous (Fossøy et al. 2006). Additionally, Sellaeg (2014), using a mathematical model, showed that while there might be a large number of EPCs in a population, the proportion of nests with EPY can be very small, making very difficult for investigators to find an effect of forays on EPP. EPCs could increase with more foray behavior of males and/or females; however, in our study we could not document the frequency of EPCs. Alternatively, it might be that just a few forays are necessary for an extra-pair fertilization to occur and consequently, the overall relationship between foray rate and/or duration and EPY or cuckoldry is normally weak.
Regardless of the purpose of forays, individuals appear to foray often. An alternative and not mutually exclusive explanation for the lack of relationship between forays and EPP is that forays may also serve for prospecting and acquiring information about their social (conspecific and heterospecific neighbors) and ecological environment (potential new territories, nesting sites, habitats, predators) (Dale et al. 1990; Pärt 1995; Giraldeu 1997; Pärt et al. 2011). For instance, prospecting in males may allow them to obtain information that could enhance their future breeding success (Doligez et al. 1994; Schjørring et al. 1999). The finding that male foray activity is constant across the breeding season rather than peaking during fertile periods of social mates or neighbors supports the idea that forays may serve many functions, including prospecting. Like males, females may foray to gather public information about future breeding habitat and for nest site selection (Eadie and Gauthier 1985; Betts et al. 2008; Pärt et al. 2011) and to assess potential males for future breeding attempts (Stutchbury et al. 1997). The increase in female forays during their pre-fertile and fertile stages is consistent with this conclusion. In some species, prospecting behavior is directly linked to the choice of a future breeding territory (Schjørring et al. 1999; Doligez et al. 2004). For example, up to 79% of northern wheatears (Oenanthe oenanthe) establish territories at their previous year’s prospecting sites (Pärt et al. 2011). Similarly, cavity-nesting ducks (common goldeneyes, Bucephala clangula, and barrow’s goldenyes, Bucephala islandica) search for nest sites at the end of the summer, in preparation for next breeding season (Eadie and Gauthier 1985). Foray behavior may help individuals gather social and ecological information allowing them to make informed decisions about where and/or with whom to breed in subsequent breeding attempts, within the same year (e.g., if a nest is depredated, or in cases of divorce or mate death) or in subsequent years.
Another alternative function of foraying is for foraging. Açkay et al. (2012), by simultaneously tracking paired male and female song sparrows, showed that females forayed more in the pre-fertile period, and suggested that these forays were for foraging, especially as most forays during the pre-fertile period were accompanied by their social mate. We conducted daily behavioral observations and described male-male and male-female interactions of birds within and outside their territories; however, we never observed birds foraging outside their territories. In the few cases where we saw males intruding into neighboring territories, intruders approached females in their territories and their mates showed aggressive behavior toward the intruder, but intruders were never seen foraging. Similar to our observations, other studies have also reported that territorial birds are rarely seen foraging outside of their territories (Yezerinac and Weatherhead 1997; Pitcher and Stutchbury 2000). In addition, Humbird and Neudorf (2008) experimentally tested if supplemented female northern cardinals (Cardinalis cardinalis) decreased their foraying effort. They found that supplemented females make more forays and spend more time off territory than unsupplemented females but suggested that forays may not have a role in foraging. Furthermore, if forays are related to foraging, females would be predicted to foray more during the post-fertile stages. In our study, however, females forayed more during pre-fertile and fertile stages than post-fertile stages. Although we cannot rule out foraging as a reason for foraying, we believe that other factors were more important. When we informally examined the spatial patterns of movements of individual birds, we did not see a clear spatial pattern of forays by individual birds, i.e., forays did not appear to be directed specifically at one or few particular territories or neighborhoods. Instead, forays appeared to be in random directions as if birds were prospecting (AC-M unpubl. data). For many species, visiting other patches or other neighborhoods can allow birds to gather information on potential mates in case their mates abandon the pair (Maness and Anderson 2008), local patch quality for potential nest sites (Eadie and Gauthier 1985; Doligez et al. 2004) and even nesting success of conspecifics (Schuett et al. 2012).
Overall, our findings suggest that extraterritorial forays are common in male and female of field sparrows; however, there is not a direct relationship of male and female foray rates and foray duration with extra-pair paternity or a direct relationship among male foray rates and foray duration and cuckoldry. While forays likely serves some function in acquiring EPCs and extra-pair matings, foray behavior may also have a major role in prospecting and gaining public information regarding their social and ecological environment, which ultimately may help them to achieve higher reproductive success but not necessarily in the form of EPP.
References
Akçay E, Searcy WA, Campbell SE, Reed VA, Templeton CN (2012) Who initiates extrapair mating in song sparrows? Behav Ecol 23:44–50
Anderson DR (2008) Model based inference in the life sciences: a primer on evidence. Springer, New York
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s information criterion. J Wildlife Manage 74:1175–1178
Arnold SJ, Duvall D (1994) Animal mating systems: a synthesis based on selection theory. Am Nat 143:317–348
Barron DG, Webster MH, Schwabl H (2015) Do androgens link morphology and behavior to produce phenotype-specific behavioural strategies? Anim Behav 1010:116–124
Best LB (1977) Territory quality and mating success in the field sparrow (Spizella pusilla). Condor 79:192–204
Betts MG, Hadley AS, Rodenhouse N, Nocera JJ (2008) Social information trumps vegetation structure in breeding-site selection by a migrant songbird. Proc R Soc Lond B 275:2257–2263
Birkhead TR, Møller AP (1992) Sperm competition in birds. Academic Press, London
Brown JL (1964) The evolution of diversity in avian territorial systems. Wilson Bull 76:160–169
Brown JL (1969) Territorial behavior and population regulation in birds. Wilson Bull 81:293–329
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Burnham KP, Anderson DR, Huyvaert KP (2010) AICc model selection in the ecological and behavioral sciences: some background, observations and comparisons. Behav Ecol Sociobiol 65:23–35
Canales-Delgadillo JC, Scott-Morales L, Niehuis O, Korb J (2010) Isolation and characterization of nine microsatellite loci in the endangered Worthen’s sparrow (Spizella wortheni). Conserv Genet Resour 2:151–153
Carey M, Burhans DE, Nelson DA (1994) Field sparrow (Spizella pusilla). In: Poole A,Gill F (eds) The birds of North America, No. 103. The Academy of Natural Sciences, Philadelphia, Pennsylvania and The American Ornithologists’ Union, Washington, DC
Celis-Murillo A, Stodola KW, Pappadopoli B, Burton JM, Ward MP (2016a) Seasonal and daily patterns of nocturnal singing in the field sparrow (Spizella pusilla). J Ornithol . doi:10.1007/s10336-015-1318-ypublished online
Celis-Murillo A, Benson TJ, Sosa-Lopez R, Ward MP (2016b) Nocturnal songs in a diurnal passerine: attracting mates or repelling intruders. Anim Behav 118:105–114
Chiver I, Stutchbury BJM, Morton ES (2008) Female foray behaviour correlates with male song and paternity in a socially monogamous bird. Behav Ecol Sociobiol 62:1981–1990
Churchill JL, Hannon SJ (2010) Off-territory movement of male American redstarts (Setophaga ruticilla) in a fragmented agricultural landscape is related to song rate, mating status and access to females. J Ornithol 151:33–44
Dakin EE, Avise JC (2004) Microsatellite null alleles in parentage analysis. Heredity 93:504–509
Dale S, Amundsen T, Lifjeld TJ, Slagsvold T (1990) Mate sampling behavior of female pied flycatchers: evidence for activemate choice. Behav Ecol Sociobiol 27:87–92
Dalziell AH, Cockburn A (2008) Dawn song in superb fairy-wrens: a bird that seeks extra-pair copulations during the dawn chorus. Anim Behav 75:489–500
Danchin E, Heg D, Doligez B (2001) Public information and breeding habitat selection. In: Clobert J, E. Danchin E, Dhondt AA, Nichols J (eds) Dispersal. Oxford University Press, Oxford, pp 243–258
Deppe JL, Ward MP, Bolus R et al (2015) Negotiating the Gulf of Mexico: fat, weather, and date affect migratory songbirds’ departure decisions, routes, and crossing times. P Natl Acad Sci USA 112:E6331–E6338
Doligez B, Pärt T, Danchin E (2004) Prospecting in the collared flycatcher: gathering public information for future breeding habitat selection? Anim Behav 67:457–466
Double M, Cockburn A (2000) Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proc R Soc Lond B 267:465–470
Duval EH, Kappor JA (2015) Causes and consequences of variation in female mate search investment in a lekking bird. Behav Ecol 26:1537–1547
Eadie JM, Gauthier G (1985) Prospecting for nest sites by cavity-nesting ducks of the genus Bucephala. Condor 87:528–534
Evans ML, Stutchbury BJM, Woofenden BE (2008) Off-territory forays and the genetic mating system of the wood thrush. Auk 125:67–75
Fossøy F, Johnsen A, Lifjeld JT (2006) Evidence of obligate female promiscuity in a socially monogamous passerine. Behav Ecol Sociobiol 60:255–259
Giraldeu LA (1997) The ecology of information use. In: Krebs JR, Davis NB (eds) Behavioural ecology. Blackwell Scientific, Oxford, pp 42–68
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212
Gross MR (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 11:92–98
Grueber CE, Nakagawa S, Laws RJ, Jamieson IG (2011) Multimodel inference in ecology and evolution: challenges and solutions. J Evol Biol 24:699–711
Hoeschele MMK, Moscicki KA, van Oort H, Fort KT, Farrell TM, Lee H, Robson SWJ, Sturdy CB (2010) Dominance signaled in acoustic ornament. Anim Behav 79:657–664
Humbird SK, Neuford DLH (2008) The effects of food supplementation on extraterritorial behavior in female northern cardinals. Condor 110:392–395
Hunter FM, Burke T, Watts SE (1992) Frequent copulation as a method of paternity assurance in the northern fulmar. Anim Behav 44:149–156
Jennions MD, Petrie M (1997) Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev 72:283–327
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
Kays R, Tilak S, Crofoot M, Fountain T, Obando D, Ortega A (2011) Tracking animal location and activity with an automated telemetry system in a tropical rainforest. Comput J 54:1931–1948
Keating KA, Cherry S (2009) Modeling utilization distributions in space and time. Ecology 90:1971–1980
Kempenaers B, Dhondt AA (1993) Why do females engage in extra-pair copulations? A review of hypotheses and predictions. Belg J Zool 123:93–103
Kempenaers B, Verheyren GR, Dhondt AA (1997) Extrapair paternity in the blue tit (Parus caeruleus): female choice, male characteristics, and offspring quality. Behav Ecol 8:481–492
Kleven O, Marthinsen G, Lifjeld JT (2006) Male extraterritorial forays, age and paternity in the socially monogamous reed bunting (Emberiza schoeniclus). J Ornithol 147:468–473
Kranstauber B, Kays R, LaPoint SD, Wikelski M, Safi K (2012) A dynamic Brownian bridge movement model to estimate utilization distributions for heterogeneous animal movement. J Anim Ecol 81:738–746
Lemon RE, Weary DM, Norris KJ (1992) Male morphology and behavior correlate with reproductive success in the American redstart (Setophaga ruticilla). Behav Ecol Sociobiol 29:399–403
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute Inc, Cary, NC
Maness TJ, Anderson DJ (2008) Mate rotation by female choice and coercive divorce in Nazca boobies, Sula granti. Anim Behav 76:1267–1277
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Mays HL, Hill GE (2004) Choosing mates: good genes versus genes that are a good fit. Trends Ecol Evol 19:554–559
Naguib M, Altenkamp R, Griebmann B (2001) Nightingales in space: song and extra-territorial forays of radio tagged song birds. J Ornithol 142:306–312
Neudorf DL, Stutchbury BJM, Piper WH (1997) Covert extraterritorial behavior of female hooded warblers. Behav Ecol 8:595–600
Pärt T (1995) The importance of local familiarity and search costs for age- and sex-biased philopatry in the collared flycatcher. Anim Behav 49:1029–1038
Pärt T, Doligez B (2003) Gathering public information for habitat selection: prospecting birds cue on parental activity. Proc R Soc Lond B 270:1809–1813
Pärt T, Arlt D, Doligez B, Low M, Qvarnström A (2011) Prospectors combine social and environmental information to improve habitat selection and breeding success in the subsequent year. J Anim Ecol 80:1227–1235
Patrick SC, Chapman JR, Dugdale HL, Quinn JL, Sheldon BC (2012) Promiscuity, paternity and personality in the great tit. Proc R Soc Lond B B279:1724–1730
Pedersen MC, Dunn PO, Whittingham LA (2006) Extraterritorial forays are related to a male ornamental trait in the common yellowthroat. Anim Behav 72:479–486
Petter SC, Miles DB, White MW (1990) Genetic evidence of mixed reproductive strategy in a monogamous bird. Condor 92:702–708
Pitcher TE, Stutchbury BJM (2000) Extraterritorial forays and male parental care in hooded warblers. Anim Behav 59:1261–1269
Pyle P (1997) Identification guide to north American birds, part 1. Slate Creek Press, Bolinas, CA
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org, Urbana, USA
Raim A (1978) A radio transmitter attachment for small passerine birds. Bird Band 49:326–332
Rintamaki PT, Alatalo RV, Hoglund J, Lundberg A (1995) Male territoriality and female choice on black grouse leks. Anim Behav 49:75–767
Roth T, Sprau P, Schmidt R, Naguib M, Amrhein V (2009) Sex-specific timing of mate searching and territory prospecting in the nightingale: nocturnal life of females. Proc R Soc Lond B 276:2045–2050
Rousset F (2008) Genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecology Resour 8:103–106
Schjørring S, Gregersen J, Bregnballe T (1999) Prospecting enhances breeding success of first-time breeders in the great cormorant, Phalacrocorax carbo sinensis. Anim Behav 57:647–654
Schuett W, Tregenza T, Dall SRX (2010) Sexual selection and animal personality. Biol. Rev. 85:217— 246
Sellaeg DE (2014) Inferring female extra-pair mating behaviour from observed patterns of extra-pair paternity with a process-based model. The University of Bergen, Bergen, Norway, MSc thesis
Seutin G, White BN, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analyses. Can J Zool 69:82–90
Sherman PW, Morton ML (1988) Extra-pair fertilizations in mountain white-crowned sparrows. Behav Ecol Sociobiol 22:413–420
Silverman BW (1986) Density estimation for statistics and data analysis. Chapman and Hall, Urbana, USA
Staiger SS, Valcu M, Spoelstra K, Helm B, Wikelski M, Kempenaers B (2013) When the sun never sets: diverse activity rhythms under continuous daylight in free-living Arctic-breeding birds. Proc R Soc B 280:20131016
Strausberger BM, Ashley MV (2001) Eggs yield nuclear DNA from egg-laying female cowbirds, their embryos and offspring. Conserv Genet 2:385–390
Strausberger BM, Ashley MV (2003) Breeding biology of brood parasitic brown-headed cowbirds (Molothrus ater) characterized by parent-offspring and sibling-group reconstruction. Auk 2:433–445
Stutchbury BJM, Piper WH, Neudorf DL, Tarof SA, Rhymer JM, Fuller G, Fleischer RC (1997) Correlates of extra-pair fertilization success in hooded warblers. Behav Ecol Sociobiol 40:119–126
Stutchbury BJM, Pitcher TE, Norris DR, Tuttle EM, Gonsar RA (2005) Does male extra-territory foray effort affect within and extra-pair fertilization success in hooded warblers. J Avian Biol 36:471–477
Symonds MRE, Musalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Walk JW, Kershner EL, Wagner RE (2000) Nocturnal singing in grassland birds. Wilson Bull 112:289–292
Ward MP, Sperry JH, Weatherhead PJ (2013) Evaluation of automated telemetry for quantifying movements and home ranges of snakes. J Herpetol 47:337–345
Ward MP, Alessi M, Benson TJ, Chiavacci SJ (2014) The active nightlife of diurnal birds: extraterritorial forays and nocturnal activity patterns. Anim Behav 88:175–184
Weatherhead PJ, Boag PT (1995) Pair and extra-pair mating success relative to male quality in red-winged blackbirds. Behav Ecol Sociobiol 37:81–91
Weatherhead PJ, Montgomerie R, Gibbs HL, Boag PT (1994) The cost of extra-pair fertilizations to female red-winged blackbirds. Proc R Soc Lond B 258:315–320
Westneat DF (1988) Male parental care and extrapair copulations in the indigo bunting. Auk 105:149–160
Westneat DF, Stewart IRK (2003) Extra-pair paternity in birds: causes, correlates, and conflict. Annu Rev Ecol Syst 34:365–396
Yasui Y (1998) The ‘genetic benefits’ of multiple mating reconsidered. Trends Ecol Evol 13:246–250
Yasui Y (2001) Female multiple mating as a genetic bet-hedging strategy when mate choice criteria are unreliable. Ecol Res 16:605–616
Yezerinac SM, Weatherhead PJ (1997) Extra-pair mating, male plumage coloration, and selection in yellow warblers (Dendroica petechia). Proc R Soc Lond B 264:527–532
Acknowledgments
We thank Jeff Brawn, Andy Suarez, and two anonymous reviewers for their comments on previous drafts of the manuscript. We thank Devin Kerr, Eric Swenson, Jessica Burton, Brian Pappadopoli, Zara John, Sarah Tomke, Ben Neece, Victor Zhang, Jonathon Jackson, Jill Deppe, Kyle Van den Bosch, and Scott Chiavacci for their help in the field and laboratory. We also thank the Vermilion County Conservation District for permission to conduct research at Kennekuk Cove County Park.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The National Science Foundation (DDIG-14070801), Animal Behavior Society Student Research Grant, American Ornithologist Union Research Award, Illinois Ornithological Society Grants, Department of Natural Resources and Environmental Sciences travel awards, University of Illinois at Urbana-Champaign student opportunity grants, and the Illinois Department of Natural Resources (W-154-R-6).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the University of Illinois’ Institutional Animal Care and Use Committee under the IACUC protocol #10127 and the USGS bird banding permit (23577).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by J. A. Graves
Electronic supplementary material
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Celis-Murillo, A., Schelsky, W., Benson, T.J. et al. Patterns, correlates, and paternity consequences of extraterritorial foray behavior in the field sparrow (Spizella pusilla): an automated telemetry approach. Behav Ecol Sociobiol 71, 45 (2017). https://doi.org/10.1007/s00265-017-2273-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2273-9