Abstract
Extra-pair fertilizations are common in many socially monogamous species, and paternity studies have indicated that females may use male vocal performance and plumage ornaments as cues to assess male quality. Female off-territory forays may represent a key component of female choice and male extra-pair mating success, and female foray behaviour is expected to be strongly influenced by indictors of male quality. In this study, we examined how male song and ornamentation affect how often females left their territories, which males they visited and extra-pair paternity in a socially monogamous passerine, the hooded warbler (Wilsonia citrina). We radiotracked 17 females during the fertile period and quantified male vocal performance (song output and rate) and plumage characteristics (size of the black melanin hood and colour of the black hood, yellow cheeks and breast areas). We obtained blood samples and determined paternity at 35 nests including those of 14 females that we radiotracked. Eleven (65%) of the 17 females forayed off-territory, whilst fertile and female foray rate was positively correlated with the number of extra-pair young in the nest. Females that left their territories more frequently were paired with males that sang at a low rate. In addition, extra-pair mates had higher song rates than the social mates they cuckolded (5.3 songs/min vs. 4.4 songs/min). Female off-territory forays or extra-pair paternity were not significantly related to male plumage characteristics. Our results indicate that a high song rate influences both the foray behaviour of a male’s social mate and the likelihood that he will sire extra-pair offspring with neighbouring females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extra-pair fertilisations are common among socially monogamous birds, and in the past decades, much has been learned about the extent of their occurrence (reviewed in Griffith et al. 2002; Westneat and Stewart 2003). Extra-pair fertilisations can represent a source of sexual selection on males (Webster et al. 1995; Stutchbury et al. 1997; Whittingham and Dunn 2005; Dolan et al. 2007; Webster et al. 2007), and paternity analysis in a number of species has revealed that certain male traits are associated with success at siring within- or extra-pair young (reviewed in Griffith et al. 2002). In a few species, male vocal performance as indicated by song output, repertoire, amplitude and consistency correlates with extra-pair paternity success (e.g. Hasselquist et al. 1996; Kempenaers et al. 1997; Otter et al. 1997; Forstmeier et al. 2002; Byers 2007). Similarly, male plumage ornaments such as larger melanin patches have been shown to be associated with the number of extra-pair young sired (Thusius et al. 2001; Yezerinac and Weatherhead 1997; but see Perreault et al. 1997).
Because paternity of the young represents the end result of the interactions that occur between the social male, the female and extra-pair males, many questions remain about the function of male ornaments in male–male competition for extra-pair fertilisations versus active female choice of extra-pair partners (Tarof et al. 2005). Males in many species are known to visit females on neighbouring territories (Westneat and Stewart 2003; Woolfenden et al. 2005), which often results in intense male–male displays and fights (Stutchbury 1998). If male ornaments are important in intra-sexual competition for extra-pair fertilizations, then more ornamented males may pursue extra-pair matings more intensely and may direct their efforts towards females mated to less ornamented males (e.g. Pedersen et al. 2006). Although male extra-territorial visits are common in many species, females are known to reject copulation attempts even if males are persistent (eastern bluebirds, Sialia sialis, Lifjeld and Robertson 1992; Venier et al. 1993; western bluebirds, Sialia mexicana, Dickinson 1997; serins, Serinus serinus, Mota and Hoi-Leitner 2003); nevertheless, male–male competition could be important in gaining access and displaying to a larger pool of fertile females. On the other hand, male foray effort is not necessarily related to fertilisation success. In hooded warblers, only 16% of the male visits to nearby territories were related to extra-pair fertilisations with the visited female (Stutchbury et al. 2005), suggesting that female choice and extra-pair tactics are important in determining male mating success.
Active female mate choice occurs when females foray outside their territory in search of extra-pair matings (reviewed in Stutchbury and Neudorf 1997; Double and Cockburn 2000; Mays and Ritchison 2004; Tarof and Ratcliffe 2000; Dunn and Whittingham 2007; Pedersen et al. 2006), and these off-territory forays can be a key determinant of male mating success. Females who make forays may benefit as a result of sampling a larger number of potential mates and possibly interacting with these males without interruption or harassment from their social mates. Females mated to low-quality males should be more likely to engage in off-territory forays, produce more extra-pair young and actively choose extra-pair males of higher quality than their social mates (Double and Cockburn 2000). To date, few studies have linked the behaviour of the female during the fertile period with indicators of male quality and resultant paternity in the nest.
In this study, we radiotracked female hooded warblers (Wilsonia citrina) to examine how male song and ornamentation affect how often females leave their territory, which males they visit and which males sire their offspring. In hooded warblers, female off-territory forays are common during a female’s fertile period (Neudorf et al. 1997). Males do not guard their mates (Fedy et al. 2002) and frequently foray off the territory to visit neighbouring females particularly when these females are vocalising during the fertile period (Stutchbury 1998). We measured male song rate and song output as well as the size and spectral reflectance of plumage ornaments.
Previous research suggests that females in other species may assess males based on song output and rate. Song rate may reflect male quality, as producing many songs in a sequence with short breaks in between songs may be physiologically demanding (Lambrechts and Dhondt 1988; Podos 1996, 1997; Poesel et al. 2001; Ballentine 2006). In black-capped chickadees (Poecile atricapilla), high-ranking males, preferred for extra-pair matings, start singing earlier in the dawn chorus and have a higher average and maximum song rate (Otter et al. 1997). In addition to being physically challenging to produce, male song features may become signals of social dominance or a ‘badge of status’ (reviewed in Gil and Ghar 2002). Similarly, plumage ornaments such as larger melanin patches have been shown to be important in extra-pair mating success (Thusius et al. 2001; Yezerinac and Weatherhead 1997; but see Perreault et al. 1997), whilst carotenoid ornaments are considered to reflect the male health status (McGraw and Hill 2000; Hill 2000, 2002). Male hooded warblers have a carotenoid-based bright yellow cheek patch and breast that contrasts with the melanin-based black hood and bib. If active female choice is important in determining male mating success, then we predicted that: (1) females mated to less vocal or less ornamented males should leave their territories more often to seek extra-pair copulations; (2) male song, but not plumage, should predict which males females choose to visit because in a forested habitat, females can readily assess the singing behaviour of neighbouring males but not their plumage; (3) the number of extra-pair young in the nest is positively correlated with female foray effort and (4) extra-pair sires are more ornamented and/or more vocal than cuckolded males.
Materials and methods
General methods
This study was conducted May to mid-July in 2004 and 2005 at the Hemlock Hill Biological Station (41°N, 79°W) near Cambridge Springs, Pennsylvania, USA. The study site is a 180-ha continuous forest composed of mature American beech (Fagus grandifolia), sugar maple (Acer saccharum), red oak (Quercus rubra), white ash (Fraxinus americana) and eastern hemlock (Tsuga canadensis). The forest is gridded with points placed 50 m apart in each direction. Hooded warblers are abundant at the study site, and we monitored a subset of approximately 30 contiguous territories occupied by pairs and unmated males over an area of approximately 56 ha.
In early May, unbanded individuals were captured using mist nets and, for males, playbacks of conspecific song. Adults were banded with one numbered aluminium US Fish and Wildlife Service band and three colour bands for individual identification. Mass (to the nearest 0.1 g), tarsus length (to the nearest 0.1 mm) and flat wing chord (to the nearest 0.5 mm) were measured, and a small amount of blood (30 μl) was taken from the brachial vein and stored in Queens lysis buffer at 4°C (Seutin et al. 1991). Individuals were sexed in the field using singing behaviour and plumage features; only males sing, and sexes are dichromatic. Age was determined by examining the shape of the outer retrix feathers [as SY or ASY (after second year) Pyle 1997]. For females, the amount of black pigmentation in the crown and bib area was also used to age the bird, as this varies between first year and after first year birds (Morton 1989). For males, there is no delayed plumage maturation.
Radiotracking
To determine the occurrence and frequency of female extra-territorial forays, we radiotracked 17 female hooded warblers during their fertile period to obtain a total of 178 h of radiotracking observations. Females are known to leave their territories chiefly during the fertile period, defined as the period of time between 5 days before the first egg was laid until the penultimate egg was laid (Neudorf et al. 1997). Female hooded warblers lay one egg everyday for a total of three to four eggs per clutch, so the fertile period lasted for 7 or 8 days. We radiotracked females from May 20 through June 12 in 2004 and from May 19 through June 11 in 2005. All females laid their first clutches within the first 2.5 weeks of arrival. Four of the females we radiotracked were laying their second clutches, as their first clutches were depredated either during egg laying or early incubation.
We monitored each female daily during the pre-fertile stages and noted nest building behaviour. Females may abandon their nest if captured before the nest is almost complete (Neudorf et al. 1997), so we waited until females started lining the interior of the nest cup before capturing them using mist nets. Captured females were fitted with a BD-2B radio transmitter (Holohil Systems, Woodlawn, Ontario) weighing 0.67 g, approximately 5–6.7% of the average female body mass. These transmitters do not influence female physical condition or locomotion (Neudorf and Pitcher 1997). To attach the transmitters, we used two-ply cotton thread in a figure-eight harness that fits around the legs (Neudorf et al. 1997). Females were allowed at least 24 h to get accustomed to the radio tags before we began to observe them.
Radiotracking sessions took place between 0530 and 1400 hours daily except when it rained, as the equipment is sensitive to water. We radiotracked each female for 2–3 h per day for a total of 10.5 h per female (range from 8.5–13 h/female). We used an R-1000 (Communications Specialists) receiver and a handheld three-element antenna. During each radiotracking session, we located the female and then followed her from approximately 20–25 m away. The location of the female was recorded continuously using a compass and the 50-m grid markers available at the study site. We also recorded the following: (1) the presence of singing males within a 100-m radius around the female and (2) any female vocalisations or interactions with another conspecific male or female. An off-territory foray was defined as an intrusion by at least 50 m into a neighbouring territory, and foray rate was calculated as the number of off-territory forays per hour.
Male singing behaviour
Male hooded warblers defend all-purpose territories during the breeding season. As males settled on the breeding territories, we mapped territory boundaries by following singing males and noting boundary disputes with neighbours. To standardise the song observations, we observed all males during the same period of the breeding cycle, namely while females were fertile and none of the pairs were feeding young. We only included paired males in our observations, as unpaired males typically sing throughout the day and tend to use a limited repertoire (Wiley et al. 1994; personal observations). We visited each male daily to determine his mating and nesting status. Once paired, early in the breeding season when the female started nest building but before any males were feeding young, we made observations of male singing behaviour. Over the two seasons, we observed males on 35 territories, consisting of 25 different males, ten of which were observed during both 2004 and 2005 field seasons. Observations of these ten males were considered independent as their mates were different between years. At our study site, 40% (8/20) of the females banded in 2004 returned in 2005 and two out of these females settled on the same or neighbouring territory, whereas the other six settled at least two territories away from their previous year’s location. Low return rates of females to the same neighbourhood suggest that females likely sample males anew each year and that male behaviour may be considered independent from year to year.
Observations of male singing behaviour took place from 0630 to 1500 hours Eastern Standard Time. In order to have independent observations of male song, only non-neighbouring males were observed on the same day. On consecutive days, different males were chosen for sampling early in the morning in order to increase the range of times at which each male was sampled. Males were observed for two to four 30- to 60-min time intervals (average of 2.4 observations per male; 22 males during two time intervals, 11 males during three intervals and two males during four intervals) for an average of 114 min/male (range 60–201 min/male). We used a timer and a counter to record the duration of each song bout (the interval of time the male spent singing including up to 10 s of silence between consecutive songs) and the total number of songs uttered during each song bout. We determined male song rate (as the number of songs sung per minute during a song bout) and song output (minutes spent singing out of the total time observed). Multiple observation periods of the same male showed that male singing behaviour was very consistent (Pearson correlation n = 35, r = 0.843, p < 0.01 for song rate; r = 0.40, p = 0.017 for song output). Male song rate or song output were not related to time of day (n = 85, r = 0.05 for song rate, and r = 0.19 for song output).
Plumage reflectance measurements
Plumage spectral information was collected for 46 males captured at the study site during the two seasons. We collected plumage spectral data using an X-Rite Digital Swatchbook spectrophotometer (X-Rite, MI, USA). The spectrophotometer covers the wavelengths from 400 to 700 nm with a resolution of 10 nm. Measurements were given as the proportion of light reflected from the plumage patch relative to a pure white patch (100% reflectance). The spectrophotometer was calibrated before each measurement, and spectral data were recorded for breast area (immediately underneath the black bib), cheeks (yellow face mask), black crown (top of head) and bib (black throat patch). Three measurements of each area were taken by moving the eye of the unit at least 5 mm from the previously measured spot, and the average reflectance for each plumage patch was computed. We calculated repeatability of colour measurements as estimated by the segment method (see below) and found that within-male measurements were highly repeatable for the yellow plumage patches (r = 0.78 and r = 0.88 for breast and cheek areas, respectively) but not for the black plumage patches (r < 0.60 for the bib and hood plumage). Thus, we removed the spectral reflectance of the black bib and hood (brightness and colour) from further analysis and focussed on the size of these ornaments.
Principal components analysis of spectral data
Principal component analysis was performed using the raw reflectance data (% reflectance values at each 10-nm interval from 400 to 700 nm) to determine the wavelengths where most variation in plumage reflectance occurred (Grill and Rush 2000). The first two principal components explained 97.8% to 99.9% of the variance among males, and only these two were used in subsequent analyses. The first principal component (PC1) represents the brightness of the plumage patch, whereas PC2 is interpreted as the hue and chroma of the plumage (Grill and Rush 2000). Principal component scores were subsequently used in analysis of male plumage colour characteristics.
Segment classification analysis
In addition, we examined variation in plumage brightness and colouration using segments of the reflectance spectrum corresponding to the reflectance properties of the pigments in the feathers of the birds (Endler 1990). In this case, ‘long’ (510–700 nm) and ‘short’ (400–510 nm) segments of the spectrum were chosen to correspond to the reflectance properties of lutein pigments in the feathers of passerines (McGraw et al. 2003; Mays et al. 2004). Lutein products are expected to affect colour parameters by absorbing light in the short wavelength part of the spectrum (390–510 nm, λ max = 448 and 476 nm), thus increasing relative reflectance in the long-wavelength spectrum and reducing overall brightness.
Brightness, R t, was calculated as the total reflectance from 400 to 700 nm. To estimate hue, we defined segment contrast as:
where, R l and R s are the sum of the reflectance in the long (510–700 nm) and short (400–510 nm) part of the spectrum. The two methods—principal components and segment classification—were in agreement in estimating brightness and colour of each plumage patch (r > 0.99, p < 0.0001 for correlations between brightness values and colour values as estimated by the two methods).
Male hood size
We quantified male variation in the area of the black crown and bib plumage patches for 43 males captured at the study site during the two seasons. Digital video recordings were made using a Sony 8-mm digital camcorder set on a tripod at a standard distance from the ground with the lens oriented parallel to the surface. A ruler was placed on the background surface for later calibration of the pictures. Video recordings were taken of the birds held in standard positions. To estimate bib area, the male was placed on its back with the beak pointed up; for the crown area measurement, the males were positioned flat on their ventral side. During video recordings, the males were repositioned, and three independent still pictures of each of the two plumage patches were taken. These were imported in an image analysis program (ImageJ 1.28), and after scaling the image using the ruler in the background, the area of each patch was outlined using a Wacom digitizing tablet. Measurements were given in cm2. The patch area was outlined three times for each picture and an average measurement was computed per picture. Within-male measurements were highly repeatable (r = 0.90, r = 0.95 for the bib and crown measurements, respectively; Lessells and Boag 1987). The average area measurement for each plumage patch per male was computed. Within-male bib and crown size were highly correlated (Pearson correlation, r = 0.663, n = 43, p = 0.001), and therefore, we computed ‘hood’ size as the sum of the bib and crown patch size.
Paternity analysis
To determine genetic parentage, we conducted paternity analyses using multilocus microsatellite analysis. Blood samples were obtained from 81 adults and 124 nestlings representing 35 family groups. Blood samples were stored at 4°C in Queen’s lysis buffer (Seutin et al. 1991). DNA extraction involved cell lysis and the use of ammonium acetate and isopropyl to precipitate (L. de Souza, unpublished protocol). We determined paternity by using allelic variation at three hypervariable microsatellite loci (Dpu 01, Dpu 15, Dpu 16) previously isolated from yellow warblers (Dendroica petechia, Dawson et al. 1997).
DNA was amplified using a 10 μl polymerase chain reaction (PCR) containing 2.5 μl DNA solution (at 20 ng/μl), 2.9 μl water, 1.0 μl PCR buffer (Bio Basic, Toronto, Ontario), 3.0 μl of 20 mM MgSO4 (Amersham Biosciences, Baie d’Urfe, PQ), 0.2 μl of 10 μM dNTPs, 0.2 μl of 10 μM fluorescently labelled forward primer, 0.2 μl of 10 μM reverse primer and 0.05 μl of 5 U/μl TSG (Bio Basic). PCR was performed using the following protocol: 2 min denaturing step at 94°C followed by 35 cycles of 20 s at 72°C, 20 s at the annealing temperature (53–50°C for Dpu 01, 51–48°C for Dpu 15 and 60–58°C for Dpu 16) and 30 s at 72°C.
Individuals were genotyped using the Beckman Coulter CEQ 8000 Genetic Analysis System. PCR products from the three reactions were multiplexed for size estimation. Each well contained 35 μl formamyde (Sigma Aldrich, Oakville, Ontario, Canada), 0.5 μl size standard (400 bp, Applied Biosystems) and 0.5, 1.0 and 2.0 μl of amplified product (using Dpu 15, Dpu 01 and Dpu 16, respectively). A drop of mineral oil was added to prevent sample evaporation. Fragment sizes were analysed using the readings relative to the size standard and was done using the automatic allele identification software (using the 400 fragment length). Each allele assignment was visually double checked by one of the authors (IC). We calculated the frequency of each allele (x i) in the population of all adults, and for each locus, we calculated the expected and observed frequency of heterozygotes [H e = 1−Σ(x i)2 and H o, respectively]. H e and H o were within 8% of each other, and we concluded that null alleles were unlikely to be present (chi-square tests, χ 3 = 6.0, p = 0.20).Variation was high at each of the three loci (28, 22 and 19 alleles for Dpu 01, 15 and 16, respectively). Individual probabilities of exclusion for the three loci were high, from 0.84 to 0.90, with a cumulative probability of exclusion of 0.997, indicating that we had a high probability that a non-parental individual would be excluded as the genetic father of the young (Chakraborty et al. 1988).
To assign paternity, we compared the genotype of the nestlings with that of their social parents. The female attending the nest was assumed to be the social mother. The social father was defined as the male feeding the young at the nest. Adults were excluded as the genetic parents of the young if adults and nestling alleles mismatched by more than 2 bp at more than one locus. Of the total 124 nestlings genotyped, all nestlings matched the social mother at all three loci except for four nestlings that mismatched with the mother at one locus; similarly, nine nestlings mismatched with the social father at only one locus. In all cases of a mismatch at a single locus, the mismatches were within 2 bp and likely represent mutations or laboratory artefacts. In these 13 instances, we calculated the probability of resemblance (P RA; Ibarguchi et al. 2004; Albrecht et al. 2007), which represents the probability that the nestlings shared alleles with the social mother or father at the two matching loci by chance alone. We considered nestlings with a P RA < 0.01 as being sired by that adult because of the low likelihood that two individuals would share those alleles by chance alone. In all 13 cases, the P RA of social parents and the young that mismatched at a single locus was less than 0.004 (0.0008 ± 0.0002); thus, the social parent was assigned as the genetic parent of the nestlings.
For 35 of the total 124 chicks, the social father mismatched with the nestlings at two (n = 6) or three loci (n = 29) and were therefore defined as extra-pair offspring. For each of these nestlings, their genotype was compared to that of all possible male–female pairs in the population. We have sampled 100% of the males in the focal area (i.e. there were no unbanded territory holders), and around the edges of the focal area, we have banded males at least one, sometimes two territories away (so at least 75% of the candidate males for the edge territories). In previous studies of this population, extra-pair fathers have been identified as neighbouring males, so we inferred that we sampled a very high percentage of all potential extra-pair fathers in the neighbourhood. For seven of the extra-pair chicks, we could not identify the genetic father in the population of sampled males, indicating that the father was a male from outside the sampled area. For the other 28 chicks, 14 matched at all three loci with only one male in the population and 14 others matched with a male at two loci, and the third locus was within 2 bp (n = 12) or 4 bp (n = 2). These last two nestlings were omitted from further analysis. For the other 12 nestlings, the calculated P RA was less than 0.003 (0.0014 ± 0.0004), indicating that the probability of the putative extra-pair sire matching the nestling at two loci by chance alone was extremely low.
To verify our assignments, we used the program CERVUS 2.0 (Marshall et al. 1998; Webster et al. 2004), which uses a maximum likelihood approach to assign paternity. CERVUS provides confidence intervals based on a simulation of possible nestling genotypes and their correct assignment to the parents (given the allele frequency of adults in the population). CERVUS assigned the same extra-pair father for the 26 nestlings (within the 95% confidence interval). For the nine nestlings that we classified as unassigned, CERVUS provided a potential sire, but the confidence level was lower than 95% (i.e. there was another male in the population that closely fit the non-maternal alleles, or the mismatch was larger than 2 bp or at more than one locus). In all cases where a genetic sire was assigned, the extra-pair father identified was an immediate neighbour, which is consistent with previous studies in this population (e.g. Stutchbury et al. 2005).
Results
Female off-territory forays and extra-pair young
Eleven out of the 17 (65%) females made extra-territorial forays to neighbouring territories during their fertile period (Table 1). We obtained paternity data for 14 of the 17 females that we radiotracked (three nests were depredated before the eggs hatched). Thirty-four percent (17/50) of the young produced were sired by a male other than the social mate. Eight of the 14 females produced extra-pair young, five of which were observed to foray onto neighbouring territories, whereas the other three were never observed to do so. The number of extra-pair young in the nest was positively related to female extra-territorial foray rate (Fig. 1, Spearman correlation, n = 14, r s = 0.538, p = 0.039). Similarly, females that spent more time off territory had more of their young sired by extra-pair males (Spearman correlation, n = 14, r s = 0.527, p = 0.043).
Females visited from one to four different territories (average of 2.2 territories per female) at a rate of 0.33 forays per hour (Table 1). Seven of the 11 females revisited neighbouring territories on subsequent forays. On average, female off-territory forays lasted 9.7 min (range of 6–14 min), and during this time, females moved through the lower shrub layer (1- to 2.5-m-tall vegetation). In 31% (12/38) of the forays, the females gave ‘chip’ calls, and during four of these forays, the female was observed interacting with the territorial male—they were seen within 5 m of each other, and in one case, the female crouched in a pre-copulatory position, although when the male approached, she flew and a chase ensued. Females were quiet during the remainder (26 out of 38) of the forays.
In total, the radiotracked females produced 17 extra-pair young fathered by 13 different males. Eleven (85%) of the extra-pair sires were identified, four of which were visited by radio-tagged females, whereas females were not observed to visit the other seven (Table 1). Males receiving many visits from a female were not more likely to sire young with that female (binary logistic regression, \(\chi {\kern 1pt} _1^2 \) = 0.219, p = 0.64). Similarly, the time the female spent on a male’s territory was not indicative of his probability of gaining extra-pair fertilisation with that female (binary logistic regression, \(\chi {\kern 1pt} _1^2 \) = 0.086, p = 0.77).
Female forays, male characteristics and extra-pair paternity
To determine whether some male characteristics are associated with female tendency to foray off territory, we tested if female foray rate was negatively correlated with their social mate’s song or plumage characteristics. A summary of male characteristics is presented in Table 2. Female extra-territorial foray rate was negatively correlated with their mate’s song rate (Pearson correlation, n = 17, r = −0.568, p = 0.017; Fig. 2). Female extra-territorial foray rate was not related to their mate’s song output (Pearson correlation, n = 17, r = 0.245, p = 0.44) or to their plumage characteristics (all p > 0.25).
We also asked the question of whether female forays took place more often while the visited male was singing. The male being visited sang during 26 out of the 38 (68%) extra-territorial forays observed. On average, males spend 50.4% of their time singing, and female forays onto neighbouring territories did not occur while the male was singing more than expected by chance (Fisher’s exact test, n = 78, df = 1, p = 0.12).
For each female that forayed off territory, we compared the mate with the visited male(s) to determine whether visited males were different in song or plumage characteristics. Because some females visited more than one male (seven out of 11), for these females, we randomly chose one of the visited males. We repeated this process 100 times (100 iterations), and each time, we computed the average difference between the mate and the visited male. This method allowed for all visited males to be in the pool of resampled males. The p value was determined as the number of times out of 100 that this difference was less than or equal to a 5% difference between the mate and the visited male. We found no significant difference between the mates and the visited males (all p > 0.20).
Males sired anywhere from zero to six nestlings, although all males had females that laid eggs on their territories (average clutch size is three to four eggs). On average, males that sired extra-pair young produced 3.6 ± 1.2 young (n = 8), whereas males that did not produced 2.6 ± 1.3 young (n = 27).
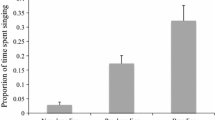
To determine whether male extra-pair fertilisation success is predicted by male characteristics, we conducted a paired comparison of the extra-pair male and the cuckolded social mate. We identified the genetic father for 26 of the 35 extra-pair nestlings at 16 nests. We found that extra-pair males had a higher song rate than the social male they cuckolded (paired samples t test, t 16 = 2.467, p = 0.025, Fig. 3), but that the cuckolded and extra-pair males did not differ in the amount of time they spent singing (song output, paired samples t test, t 16 = 0.395, p = 0.68, Fig. 3). Extra-pair males also did not differ in yellow plumage colouration or black hood size from social mates (Table 3).
Discussion
Our study shows that female off-territory forays play a key role in extra-pair matings. Female hooded warblers made frequent off-territory forays, whilst fertile and off-territory forays were positively related to extra-pair fertilisations. Females may benefit from visiting many males because they can sample more males and assess male quality more accurately (Gibson and Langen 1996). Almost half the females who made off-territory forays visited three or four different neighbouring males, in effect sampling over half of the adjacent neighbours that were available. We were only able to follow radio-equipped females for a fraction of the time they were fertile; nevertheless, the positive correlation between foray rate and extra-pair paternity suggests that females that left their territories more frequently may be able to assess more males and choose among these males than females that left their territories less frequently.
Female extra-territorial forays were correlated with male singing behaviour. Females mated to males with lower song rates left their territory more frequently. In a pairwise comparison of social and extra-pair males, we also found that extra-pair males had significantly higher song rates than the males they cuckolded, indicating that females may prefer extra-pair mates with higher song rates (i.e. produce more songs per minute). Our results show that although extra-pair males had higher song rates than social mates, female visits were not directed solely towards neighbours that had a higher song rate. Some of the off-territory forays included more than one territory and it is not clear whether all males on the route were of interest to the visiting females or whether females avoided interactions with resident females. In addition, females may visit a large proportion of their neighbours and assess these males, possibly not only based on song characteristics, as male traits may reflect quality differently over time. Six of the 17 (35%) extra-pair young produced were sired by males that were visited by radio-tagged females, suggesting that not all visits may result in copulations or fertilisations. Female off-territory forays are likely part of an ensemble of tactics which include male forays that likely aid in assessing male quality and extra-pair mate choice.
Although our results indicate that female forays are important in extra-pair mate choice, we cannot rule out the possibility that females use off-territory forays in part to forage for food, as we did witness females foraging during forays. Nevertheless, off-territory forays are performed almost exclusively by fertile females (Neudorf et al. 1997), and foray effort was correlated with extra-pair young and social mate singing behaviour, strongly suggesting that forays function primarily in the mating system. Furthermore, in our population, it is unlikely that male quality is strongly correlated with that of his territory. Males occupied the same territories from year to year, and territory owners, if they failed to return from their wintering grounds (50% of males), were always replaced by newcomers to the site as opposed to neighbouring males. Nevertheless, female forays for food, and female forays to assess extra-pair mates may not be exclusive activities (also see Gray 1996). However, if females’ off-territory forays were driven by food availability, we would expect that females would leave their territories as often at other periods of their nesting cycle, but we know that females almost exclusively foray off-territory during the fertile period (Neudorf et al. 1997).
More studies need to be done to clarify the prevalence of female off-territory forays and their relationship to male mating success and ornamentation. In the superb fairy-wren (Malurus cyaneus), all female visits to neighbouring territories resulted in extra-pair young being sired by the visited male(s) (Double and Cockburn 2000). In this species, males often visit neighbouring females and perform a display, although copulations do not occur at this time (Green et al. 2000). In the hooded warbler, female visits to neighbouring males may serve to assess males and/or gain copulations and could be a strong source of sexual selection (Stutchbury et al. 1997) on male song and, to a lesser extent, male colouration. Documenting female foray behaviour and active mate choice should be a top priority for future studies of the role of extra-pair matings in the evolution of male ornamentation and singing behaviour.
References
Albrecht T, Schnitzer J, Kreisinger J, Exnerova A, Bryja J, Munclinger P (2007) Extrapair paternity and the opportunity for sexual selection in long-distant migratory passerines. Behav Ecol 18:477–486
Ballentine B (2006) Morphological adaptation influences the evolution of a mating signal. Evolution 60:1936–1944
Byers BE (2007) Extrapair paternity in chestnut-sided warblers is correlated with consistent vocal performance. Behav Ecol 18:130–136
Chakraborty R, Meagher TR, Smouse PE (1988) Parentage analysis with genetic markers in natural populations I. The expected proportion of offspring with unambiguous paternity. Genetics 118:527–536
Dawson RJG, Gibbs HL, Hobson KA, Yezerinac SM (1997) Isolation of microsatellite DNA markers from a passerine bird, Dendroica petechia (the yellow warbler), and their use in population studies. Heredity 79:506–514
Dickinson JL (1997) Male detention affects extra-pair copulation frequency and pair behaviour in western bluebirds. Anim Behav 53:561–571
Dolan AC, Murphy MT, Redmond LJ, Sexton K, Duffield D (2007) Extrapair paternity and the opportunity for sexual selection in a socially monogamous passerine. Behav Ecol 18:985–993
Double M, Cockburn A (2000) Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proc R Soc Lond, B Biol Sci 267:465–470
Dunn PO, Whittingham LA (2007) Search costs influence the spatial distribution, but not the level, of extra-pair mating in tree swallows. Behav Ecol Sociobiol 61:449–454
Endler JA (1990) On the measurement and classification of color in studies of animal color patterns. Biol J Linn Soc 41:315–352
Fedy BC, Norris DR, Stutchbury BJM (2002) Do male hooded warblers guard their mates when their paternity is most at risk? J Field Ornithol 73:420–426
Forstmeier W, Kempenaers B, Meyer A, Leisler B (2002) A novel song parameter correlates with extra-pair paternity and reflects male longevity. Proc R Soc Lond, B Biol Sci 269:1479–1485
Gibson RM, Langen TA (1996) How do animals choose their mates? Trends Ecol Evol 11:468–470
Gil D, Gahr M (2002) The honesty of bird song: multiple constraints for multiple traits. Trends Ecol Evol 17:133–141
Gray EM (1996) Female control of offspring paternity in a western population of red-winged blackbirds (Agelaius phoeniceus). Behav Ecol Sociobiol 38:267–278
Green DJ, Osmond HL, Double MC, Cockburn A (2000) Display rate by male fairy-wrens (Malurus cyaneus) during the fertile period of females has little influence on extra-pair mate choice. Behav Ecol Sociobiol 48:438–446
Griffith SC, Owens IPF, Thuman KA (2002) Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol 11:2195–2212
Grill CP, Rush VN (2000) Analysing spectral data: comparison and application of two techniques. Biol J Linn Soc 69:121–138
Hasselquist D, Bensch S, vonSchantz T (1996) Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature 381:229–232
Hill GE (2000) Energetic constraints on expression of carotenoid-based plumage coloration. J Avian Biol 31:559–566
Hill GE (2002) A Red Bird in a Brown Bag: The Function and Evolution of Ornamental Plumage Coloration in the House Finch. Oxford University Press, Oxford
Ibarguchi G, Gissing GJ, Gaston AJ, Boag PT, Friesen VL (2004) Male-biased mutation rates and the overestimation of extrapair paternity: Problem, solution, and illustration using thick-billed murres (Uria lomvia, Alcidae). J Heredity 95:209–216
Kempenaers B, Verheyren GR, Dhondt AA (1997) Extrapair paternity in the blue tit (Parus caeruleus): female choice, male characteristics, and offspring quality. Behav Ecol 8:481–492
Lambrechts M, Dhondt AA (1988) The anti-exhaustion hypothesis—a new hypothesis to explain song performance and song switching in the great tit. Anim Behav 36:327–334
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lifjeld JT, Robertson RJ (1992) Female control of extra-pair fertilization in tree swallows. Behav Ecol Sociobiol 31:89–96
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Mays HL, Ritchison G (2004) The effect of vegetation density on male mate guarding and extra-territorial forays in the yellow-breasted chat (Icteria virens). Naturwissenschaften 91:195–198
Mays HL, McGraw KJ, Ritchison G, Cooper S, Rush V, Parker RS (2004) Sexual dichromatism in the yellow-breasted chat Icteria virens: spectrophotometric analysis and biochemical basis. J Avian Biol 35:125–134
McGraw KJ, Hill GE (2000) Differential effects of endoparasitism on the expression of carotenoid- and melanin-based ornamental coloration. Proc R Soc Lond Series B Biol Sci 267:1525–1531
McGraw KJ, Beebee MD, Hill GE, Parker RS (2003) Lutein-based plumage coloration in songbirds is a consequence of selective pigment incorporation into feathers. Comp Biochem Physiol Part B Biochem Mol Biol 135:689–696
Morton ES (1989) Female hooded warbler plumage does not become more male-like with age. Wilson Bull 101:460–462
Mota PG, Hoi-Leitner M (2003) Intense extrapair behaviour in a semicolonial passerine does not result in extrapair fertilizations. Anim Behav 66:1019–1026
Neudorf DL, Pitcher TE (1997) Radio transmitters do not affect nestling feeding rates by female hooded warblers. J Field Ornithol 68:64–68
Neudorf DL, Stutchbury BJM, Piper WH (1997) Covert extraterritorial behavior of female hooded warblers. Behav Ecol 8:595–600
Otter K, Chruszcz B, Ratcliffe L (1997) Honest advertisement and song output during the dawn chorus of black-capped chickadees. Behav Ecol 8:167–173
Pedersen MC, Dunn PO, Whittingham LA (2006) Extraterritorial forays are related to a male ornamental trait in the common yellowthroat. Anim Behav 72:479–486
Perreault S, Lemon RE, Kuhnlein U (1997) Patterns and correlates of extrapair paternity in American redstarts (Setophaga ruticilla). Behav Ecol 8:612–621
Podos J (1996) Motor constraints on vocal development in a songbird. Anim Behav 51:1061–1070
Podos J (1997) A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes:Emberizidae). Evolution 51:537–551
Poesel A, Foerster K, Kempenaers B (2001) The dawn song of the blue tit Parus caeruleus and its role in sexual selection. Ethology 107:521–531
Pyle P (1997) Identification guide to North American birds. Part I. Bolinas. Slate Creek Press, California
Seutin G, White BN, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analyses. Can J Zool -Revue Canadienne De Zoologie 69:82–90
Stutchbury BJM (1998) Extra-pair mating effort of male hooded warblers, Wilsonia citrina. Anim Behav 55:553–561
Stutchbury BJ, Neudorf DL (1997) Female control, breeding synchrony and the evolution of extra-pair mating systems. Ornithol Monogr 49:103–129
Stutchbury BJM, Piper WH, Neudorf DL, Tarof SA, Rhymer JM, Fuller G, Fleischer RC (1997) Correlates of extra-pair fertilization success in hooded warblers. Behav Ecol Sociobiol 40:119–126
Stutchbury BJM, Pitcher TE, Norris DR, Tuttle EM, Gonser RA (2005) Does male extra-territory foray effort affect fertilization success in hooded warblers Wilsonia citrine? J Avian Biol 36:471–477
Tarof SA, Ratcliffe LM (2000) Pair formation and copulation behavior in least flycatcher clusters. Condor 102:832–837
Tarof SA, Dunn PO, Whittingham LA (2005) Dual functions of a melanin-based ornament in the common yellowthroat. Proc R Soc B-Biol Sci 272:1121–1127
Thusius KJ, Peterson KA, Dunn PO, Whittingham LA (2001) Male mask size is correlated with mating success in the common yellowthroat. Anim Behav 62:435–446
Venier LA, Dunn PO, Lifjeld JT, Robertson RJ (1993) Behavioral-patterns of extra-pair copulation in tree swallows. Anim Behav 45:412–415
Webster MS, PruettJones S, Westneat DF, Arnold SJ (1995) Measuring the effects of pairing success, extra-pair copulations and mate quality on the opportunity for sexual selection. Evolution 49:1147–1157
Webster MS, Tarvin KA, Tuttle EM, Pruett-Jones S (2004) Reproductive promiscuity in the splendid fairy-wren: effects of group size and auxiliary reproduction. Behav Ecol 15:907–915
Webster MS, Tarvin KA, Tuttle EM, Pruett-Jones S (2007) Promiscuity drives sexual selection in a socially monogamous bird. Evolution 61:2205–2211
Westneat DF, Stewart IRK (2003) Extra-pair paternity in birds: causes, correlates, and conflict. Annu Rev Ecolog Evol Syst 34:365–396
Whittingham LA, Dunn PO (2005) Effects of extra-pair and within-pair reproductive success on the opportunity for selection in birds. Behav Ecol 16:138–144
Wiley RH, Godard R, Thompson AD (1994) Use of 2 singing modes by hooded warblers as adaptations for signaling. Behaviour 129:243–278
Woolfenden BE, Stutchbury BJM, Morton ES (2005) Male acadian flycatchers, Empidonax virescens, obtain extrapair fertilizations with distant females. Anim Behav 69:921–929
Yezerinac SM, Weatherhead PJ (1997) Extra-pair mating, male plumage coloration and sexual selection in yellow warblers (Dendroica petechia). Proc R Soc Lond, B Biol Sci 264:527–532
Acknowledgment
We would like to thank Stefanie LaZerte and Elizabeth Gow for their enthusiastic help in the field as well as Bonnie Woolfenden for much help with the genetic analysis. This research was supported by a Natural Science and Engineering Research Council of Canada (NSERC) scholarship and York University Faculty of Graduate Studies grants to IC as well as NSERC grants to BJMS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Podos
Rights and permissions
About this article
Cite this article
Chiver, I., Stutchbury, B.J.M. & Morton, E.S. Do male plumage and song characteristics influence female off-territory forays and paternity in the hooded warbler?. Behav Ecol Sociobiol 62, 1981–1990 (2008). https://doi.org/10.1007/s00265-008-0629-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0629-x