Abstract
Recognising predators accurately is key to making fine-scale adjustments to behaviour that enhance survival and maximise overall fitness for prey. Prey incorporate information from specific predator features in order to recognise predators and the risk they pose. For olfactory cues, prey can use both predator odour and diet cues to recognise predators. The role of diet cues in predator recognition has only been tested when they provide information about risk and act as an unconditioned stimulus. Thus, it is unclear whether prey use diet cues in the development of more general predator recognition templates. Here, we tested whether diet cues that contain no apparent information about the prey’s vulnerability to the predator are used by prey when they learn to recognise predators. We trained predator-naive wood frog tadpoles (Lithobates sylvaticus) to recognise the odour of a novel crayfish (Orconectes virilis) as risky by pairing tadpole alarm cues with the odour of crayfish fed one of two diets: alfalfa pellets or earthworms (Lumbricus sp.). We tested tadpoles from each group for their response to one of the two crayfish diet odour combinations or a water control. Tadpoles displayed antipredator responses to crayfish odour, irrespective of diet. However, their responses were stronger when tadpoles were exposed to crayfish fed the same diet as during training. Such results demonstrate that diet cues play a previously unrecognised but subtle role in predator recognition and suggest that flexibility in prey choice can lead to an advantage for the predator.
Significance statement
Recognising predators and the threat they pose is critical for prey to adjust their behaviour in response to fluctuations in predation risk. There is therefore a need to understand how prey use different cues to develop effective recognition templates that allow for threat-sensitive adjustments to behaviour. Here, we demonstrate that diet cues of predators contribute to the development of predator recognition templates by prey. These results provide new information about how prey develop recognition templates for predators and that, by incorporating diet cues, they are able to adjust their responses to variable risk posed by different predators within a population. Additionally, we suggest that generalist diets may provide unrecognised benefits to predators when switching between prey types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many prey are able to adapt and respond to predation risk as it changes in intensity and frequency through time and space (Lima and Bednekoff 1999; Sih et al. 2000). Those individuals that are better able to recognise risky situations and effectively implement the appropriate antipredator response should enhance their chances of surviving to reproduce (Helfman 1989; Lima and Bednekoff 1999). The fact that prey are exposed to predators that come and go over time or target specific life stages means that learning about predators and their associated risks is an adaptive response to variability in predation risk (Griffin 2004; Kelley and Magurran 2006). Learning often occurs via associative conditioning, where prey encounter the sight or smell of an unknown predator (conditioned stimulus) paired with a conspecific alarm call or a chemical alarm cue (unconditioned stimulus), both of which elicit a fright response and label the predator a threat (Kelley and Magurran 2006; Ferrari et al. 2010a). By learning about predators, prey are able to adjust their antipredator responses as the risk associated with the predator changes through time (Lima and Bednekoff 1999; Brown et al. 2001). This includes the extinction of responses (forgetting) to predators that are no longer a threat (Ferrari et al. 2010a, 2012).
Acquiring pertinent information is key for prey to develop optimal responses to predators as individuals with more information about variables within their environment will be able to make better-informed decisions about how to manage danger (Dall 2010). Whilst we know much about how prey develop effective responses to risk (Lima and Bednekoff 1999; Sih et al. 2000; Kelley and Magurran 2006; Ferrari et al. 2010a; Brown et al. 2013), less is known about the specific features prey use when learning to recognise predators. A look at the predator recognition literature suggests that not all information available from predators is equal and that different species focus on certain cues whilst ignoring others. For visual features, prey appear to preferentially focus on those that specifically relate to predatory functions, such as the morphology of eyes and their position on the head (Karplus et al. 1982; Beránková et al. 2014), size and shape of the mouth (Karplus et al. 1982), size/shape/silhouette of the body (Engstrom-Ost and Lehtiniemi 2004; Stankowich and Coss 2007; Brown et al. 2011) and posture/orientation (Helfman 1989; Cooper 1998; Schluessel et al. 2014). Experimentally modifying these features can significantly impact antipredator responses of prey, whilst modifying non-functional features has little or no effect (Karplus et al. 1982; Beránková et al. 2014). Furthermore, these features can be used to recognise novel predators that share similar features with a known predator (Griffin et al. 2001; Kullberg and Lind 2002; Stankowich and Coss 2007; Brown et al. 2011). In aquatic systems, olfactory cues are a primary means by which prey recognise predators, and both the predator-specific odour (the odour unique to that species) and diet cues (cues that are emitted as a by-product of digestion) have been shown to play important roles in how prey learn to recognise predators. Generally, the role of these two cues in predator recognition have been considered independently, and it is unclear how the two cue types are used when prey learn to recognise predators.
Diet cues are thought to be a distinct set of cues from predator odours. Consequently, they provide independent information through which prey are able to assess predation risk and recognise predators (Chivers and Mirza 2001; Ferrari et al. 2010a). Prey innately respond to a wide range of diet cues that vary in the type information they provide about predation risk (reviewed in Scherer and Smee 2016). For example, some cues are very general in the information they provide and simply label an unknown species as carnivore (Fendt 2006; Ferrero et al. 2011). Other diet cues are more specific, allowing prey to recognise whether a predator has recently eaten a conspecific (Chivers et al. 1996), closely related species (Schoeppner and Relyea 2009) or a prey guild member (Pollock et al. 2003). Whilst these cues provide different types of information, the response they elicit in prey species will depend on their capacity to predict future attacks by predators (Scherer and Smee 2016). Beyond their role as direct indicators of predation risk, diet cues can also be used by prey when learning to recognise novel predator odours (Mathis and Smith 1993; Chivers et al. 1996). Interestingly, diet cues may still influence how prey assess risk even if they contain no innately recognisable information regarding predation risk. For example, lemon damselfish (Pomacentrus moluccensis) used diet cues to distinguish between novel predators and non-predators when generalising recognition from a known predator (Mitchell et al. 2015). Irrespective of their actual predatory status, damselfish only recognised novel species that had been fed the same diet as the known predator. This was despite the fact that the diet cues (distantly related fish species and squid) were novel and contained no known information about predation risk. The results suggested that damselfish use both diet cues and predator odours when learning to recognise predators.
If prey also learn about novel diet cues when learning to recognise predators, then to what extent does the presence or absence of those same diet cues alter prey responses during future encounters with those predators? Diet cues have been shown to inhibit recognition of novel predators via generalisation in damselfish when the novel predator was fed a different novel diet from the known predator (Mitchell et al. 2015). It seems unlikely that changes to a known predator’s diet should have such dramatic effects on prey antipredator responses during future encounters with the predator. However, diet cues might alter recognition of the predator in a way that allows prey to fine-tune antipredator responses and enhance their chances of survival. Diet cues may also provide a mechanism through which predators are able to mask or manipulate their odours and, therefore, levels of vigilance in their prey. Indeed, it has been suggested that switching between prey types may provide benefits to generalist predators via the manipulation of diet cue-related risk assessment by prey (Lima et al. 2003).
Diet cues clearly influence predator–prey dynamics, yet it is unclear whether they are also used by prey when learning about predators. Here, we tested whether the diet of a predator during a learning event altered antipredator responses during subsequent encounters with the predator when fed a different diet. To do this, we conditioned tadpoles to recognise novel predatory crayfish (Orconectes virilise) that had been fed one of two diets (earthworms, Lumbricus sp., or alfalfa pellets) by pairing crayfish odour with a tadpole alarm cue. We then tested tadpoles conditioned to one of the two crayfish diet combinations for their behavioural response to one of three odours (crayfish fed earthworms, crayfish fed alfalfa pellets or a water control). We predicted that if tadpoles used both diet cues and kairomones to learn about predators, then they should display a greater response to the odour of the predator fed the learned diet than to the odour of the predator fed the alternative diet. If tadpoles only learn about predator odour, then different predator diet cues will have no effect on the way prey respond to the predator.
Methods
Larval rearing and predator maintenance
Freshly laid egg clutches were collected from local ponds and divided between four 380-L troughs filled with conditioned well water that had been seeded with algae and plankton. This water contained odours from natural ponds, but lacked any predator odours. Once the tadpoles hatched, alfalfa pellets and Tetramin flakes were provided to the tadpoles to supplement the algae present in the pools. Tadpoles were left to develop for 3 weeks, at which point they were removed from the pools, mixed together and randomly assigned to one of twenty 7.4-L pails containing 5 L of conditioned well water and some food (Tetramin flakes and alfalfa pellets).
Crayfish were selected as the novel predator as they are a non-native species that local wood frog population have no experience with (Hanson et al. 1990) and are known to eat tadpoles (Gherardi et al. 2001; Gomez-Mestre and Diaz-Paniagua 2011). Crayfish were fed either earthworms or alfalfa pellets. Diets were chosen to represent two ecologically distinct diets (invertebrates and plant detritus) that are commonly consumed by omnivorous crayfish (Parkyn et al. 2001) and that lacked cues about predation risk to which tadpoles respond innately. Crayfish were housed in individual 10-L aquaria and fed pellets once a day prior to the onset of the experiment protocol.
Cue production
The crushed tadpole cues were prepared by first euthanizing tadpoles with a rapid blow to the head (UCACS protocol 2015031), pulverising their bodies with a mortar and pestle, and filtering and diluting the solution in 20 mL of water. The solution was then used shortly after preparation during conditioning. For the conditioning phase, crushed tadpole cues were produced at a concentration of three tadpoles per 20 mL.
To produce the crayfish odours, four crayfish (two per diet) were kept in individual 3.5-L pails containing 2 L of conditioned well water and fed equal amounts of either alfalfa pellets or earthworms every day for 4 days prior to making the cues for conditioning. Crayfish are able to clear their foregut within 9 h (Loya-Javellana et al. 1995), suggesting that crayfish would have completely evacuated their guts over the 4 days and removed any residual diet cues from previous diets. One hour after the final feeding, crayfish were moved to a clean pail containing 1 L of water and were left for 24 h. Prior to conditioning or testing, equal amounts of water were removed from each of the four crayfish pails and the water from crayfish fed the same diet was mixed together for our stock solution.
Experimental protocol
Tadpoles were left to acclimate in pails (15 tadpoles per pail) for 24 h prior to the conditioning phase. Pails were assigned to one of two conditioning treatments for the conditioning phase, which consisted of an exposure to 20 mL of crushed tadpole cues paired with either 20 mL of worm-fed crayfish odour or 20 mL of alfalfa-fed crayfish odour. Tadpoles were then left undisturbed for 1 h (between ∼1600 and 1700 hours), after which, each pail received a complete water change. To demonstrate that learning has occurred, a conditioning control is normally required (a false or pseudo-group). However, we have published numerous papers demonstrating that this population do not innately recognise natural or invasive predators, but learn to recognise predators using the protocol described (Ferrari et al. 2010b, 2012; Chivers and Ferrari 2013, 2014; Ferrari and Chivers 2013; Chivers et al. 2015). To reduce the number of tadpoles used, we excluded the conditioning control group in this instance. A total of 149 tadpoles were conditioned.
The day following conditioning, tadpoles from both conditioning treatments were tested for their response to one of three odours: worm-fed crayfish odour, alfalfa-fed crayfish odour or a water control (this produced six test groups; two conditioning treatments × three test odours). Individual tadpoles were placed into 0.5-L cups and left to acclimate for an hour prior to testing. Behavioural observations were conducted during a 4-min pre- and a 4-min post-stimulus injection observation. Following the pre-stimulus observation, 5 mL of one randomly assigned odour was carefully injected on the side of the cup and the post-stimulus observation began immediately. Each tadpole was exposed to one cue only. The observer was blind to both conditioning treatment and the testing odour (n = 16–28 tadpoles per test group). During observations, tadpole activity (number of times they crossed the midline of the cup) was measured. Activity levels are a well-established measure of antipredator behaviour in larval amphibians as they reduce activity in response to predation cues (Ferrari et al. 2009, 2010a).
Statistical analysis
We used a two-way repeated measures analysis of variance (ANOVA) to test the effects of conditioning (crayfish fed worms vs. crayfish fed alfalfa pellets) and test odour (crayfish fed worms vs. crayfish fed alfalfa pellets vs. water) on the activity of tadpoles through time (pre- and post-behavioural observations as repeated measures). As tadpoles were conditioned in groups within each pail, we included pail as a nested factor to account for the lack of independence amongst the tadpoles from the same conditioning group.
Due to a significant interaction between conditioning, test odour and time, we split the analysis by conditioning treatment and ran two one-way repeated measures ANOVAs testing for the effects of test odour on activity levels through time, with pail included as a nested factor. Paired t tests with a Bonferroni correction were used to explore significant effects of cue. Data met parametric assumptions.
Results
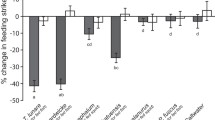
The behaviours of tadpoles were affected by an interaction between conditioning and test odour and time (F 2,129 = 3.225, p < 0.05; Supplementary Table 1). Further analysis showed that the test odour had a significant effect on the activity levels of tadpole conditioned with crayfish fed earthworms (one-way repeated measures ANOVA: F 2,61 = 16.6, p < 0.0001) and tadpoles conditioned with crayfish fed alfalfa pellets (F 2,68 = 8.05, p < 0.001). Tadpoles from both conditioning groups showed a greater reduction in activity (∼45–60 %) after being exposed to either of the crayfish odours compared to tadpoles exposed to water (<10 % reduction, all p < 0.005; Fig. 1). The interactions stem from the fact that tadpoles conditioned with crayfish fed earthworms showed a greater reduction in activity when exposed to crayfish fed earthworm odour compared to crayfish fed alfalfa pellets (F 1,46 = 5.13, p < 0.05), whilst tadpoles conditioned with crayfish fed alfalfa pellets showed a different pattern, displaying similar reduction in activity to both odours (F 1,45 = 0.64, p = 0.43).
Mean activity (±SE) of wood frog (Lithobates sylvaticus) tadpoles before and after exposure to the odour of crayfish (Orconectes virilis) fed alfalfa pellets (black bars), crayfish fed earthworms, (Lumbricus sp.; dark grey bars) or a water control (light grey bars). Tadpoles were initially conditioned to recognise crayfish as a predator by pairing injured tadpole cues with odour from crayfish fed either alfalfa (a) pellets or earthworms (b)
Discussion
Recognising predators and the risk they pose is a fundamental prerequisite for effective predator evasion. Here, we demonstrate that prey use both predator odour and diet cues when learning to recognise predators and show that changes in a predator’s diet can alter how prey respond to predators during future encounters, even when the diets appear to contain no relevant information to the prey about predation risk. Tadpoles displayed a strong antipredator response (reduction in activity) when exposed to either crayfish odour, irrespective of the crayfish diet cues experienced during conditioning. Tadpoles did not respond to the water controls. Importantly, tadpoles showed a greater reduction in activity (∼20 %) to odours of crayfish fed the known diet (i.e. the one they were conditioned to recognise) compared to the odours from crayfish fed the alternative diet; however, the differences in response to crayfish diet cues were only significant for tadpoles conditioned with crayfish fed earthworms. These results suggest that tadpoles incorporate information from diet cues when learning about novel predators. Furthermore, the results suggest that prey may be able to obtain pertinent information about the current threat posed by a predator and that prey use such cues to refine their antipredator responses.
Studies have demonstrated that prey can learn to recognise almost any novel odour as a risk, including ecologically irrelevant odours, when paired with a chemical alarm cue (Leduc et al. 2007; Ferrari et al. 2010a). We might have expected prey to incorporate diet cues into the characteristics of a predator signature they learn. However, rather than using all available cues, prey may be predisposed to use cues that directly provide information about predation risk (Karplus et al. 1982; Beránková et al. 2014). These studies suggest that prey have undergone selection to only use cues that enhance decision-making about predation risk and ignore cues that do not offer relevant information. The presence of conspecifics in diet cues clearly provides information about predation risk (Chivers and Mirza 2001), but as shown here and in a previous study (Mitchell et al. 2015), diet cues still affect how prey respond to predators, even when diet cues contain no predation risk cues that are innately recognised by prey. This suggests that in the absence of direct predation cues, diet cues provide ecologically relevant information about risk. The information from such diet cues may allow prey to adjust responses based on intraspecific variation in the level of risk posed by predators (Scherer and Smee 2016). Many species that are considered generalist predators actually comprised individual specialist predators that only target a subset of prey species consumed by the population as a whole (Bolnick et al. 2003). For example, sea otters (Enhydra lutris) show intraspecific diet preferences that are maintained along matrilines (Estes et al. 2003). Similarly, wolf spiders (Hogna helluo) show a greater preference for the odour of prey that it has recently consumed (Persons and Rypstra 2000). Such preferences for certain prey can last for several months (Bryan and Larkin 1972) and suggest that the risk of being attacked by a given predator within the population may not be equal amongst predators with different diet preferences. Altering antipredator behaviours in response to changes in predator diets represents an adaptive response to predator foraging strategies and provides a fitness benefit to the prey. The influence of diet cues on the antipredator responses of prey should, therefore, be directly dependant on the frequency with which predators switch between different prey types. For example, diet cues may have a large effect on prey responses to predators if predators show strong preferences for specific prey and only occasionally switch between different prey species. Conversely, diet cues may have little effect on antipredator responses if predators frequently/opportunistically switch between different prey species.
From the predators’ perspective, the presence of diet cues might have a negative impact on optimal foraging strategies and capture success rate. To counter this, predators should develop behaviours/mechanisms that minimise detection or manipulate the information available from diet cues. For example, by manipulating their diet cues, predators may gain significant advantage during interactions with prey, where the outcome of predation events can be determined by a few milliseconds difference in prey responses (Domenici 2010; Domenici et al. 2011). In fact, optimal foraging models that include responsive prey show that switching between different prey allows generalist predators to reduce prey vigilance and increase foraging success to the point where they outperform specialist predators (Lima et al. 2003). Lima et al. (2003) suggested that altering information from diet cues might be a primary means through which predators could manage prey vigilance. In support of this idea, Northern pike (Esox lucius) have been shown to selectively defecate away from their foraging sites in order to avoid detection by prey (Brown et al. 1995). Studies have also shown that certain predators may be able to prevent prey from recognising them through olfactory cues, but as yet, it is unknown how they are able to do so (Lonnstedt and McCormick 2013; Resetarits and Binckley 2013).
The fact that alterations to diet cues reduced antipredator responses but did not prevent recognition suggests that recognition is based primarily on predator kairomones and the diet cues add secondary information that modifies responses along a generalised gradient, as suggested by Mitchell et al. (2015). Understanding how diet cues alter antipredator responses may depend on how predators process the metabolites of their prey. If the diet directly alters the predators’ odour, then prey will learn diet cues and kairomones as a single cue. In subsequent encounters, responses will depend on the prey’s ability to generalise recognition from a known odour to a similar odour. Alternatively, diet cues may be independent of predator kairomones, simply passing through the digestive system without altering the predators’ odour. The fact that prey are able to recognise heterospecific alarm cues learnt by detecting them in the diet of predators suggests that such cues may pass through the digestive system unchanged (Mirza and Chivers 2003). In this instance, diet cues and predator odours may act as independent conditioned stimuli and provide independent information about risk.
A long-standing assumption about chemical cues is that they are harder to manipulate than visual cues and, thus, provide reliable information (Munoz and Blumstein 2012). Yet, there is a growing number of studies suggesting that both predators and prey alter the chemical cues that they release to avoid detection or even camouflage themselves simply by altering their diets (Fishlyn and Phillips 1980; Ruxton 2009; Resetarits and Binckley 2013; Brooker et al. 2014). Such findings suggest it may not be as hard as previously thought to manipulate chemical cues and that such modifications may significantly alter the dynamics of predator–prey interactions. Certainly, pike appear to actively manipulate prey behaviour by defecating away from their prey (Brown et al. 1995), but it is unclear whether both predators and prey actively manipulate the chemical composition diet cues to deceive others. Further work is needed to understand the extent to which both predators and prey can deceive each other by manipulating different components of their chemical cues.
References
Beránková J, Veselý P, Sýkorová J, Fuchs R (2014) The role of key features in predator recognition by untrained birds. Anim Cogn 17:963–971
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Brooker RM, Munday PL, Chivers DP, Jones GP (2014) You are what you eat: diet-induced chemical crypsis in a coral-feeding reef fish. Proc R Soc B 282:20141887
Brown GE, Chivers DP, Smith RJF (1995) Localized defecation by pike: a response to labelling by cyprinid alarm pheromone? Behav Ecol Sociobiol 36:105–110
Brown GE, Ferrari MCO, Elvidge CK, Ramnarine I, Chivers DP (2013) Phenotypically plastic neophobia: a response to variable predation risk. Proc R Soc B 280:20122712
Brown GE, Ferrari MCO, Malka PH, Russo S, Tressider M, Chivers DP (2011) Generalization of predators and nonpredators by juvenile rainbow trout: learning what is and is not a threat. Anim Behav 81:1249–1256
Brown GE, LeBlanc VJ, Porter LE (2001) Ontogenetic changes in the response of largemouth bass (Micropterus salmoides, Centrarchidae, Perciformes) to heterospecific alarm pheromones. Ethology 107:401–414
Bryan JE, Larkin PA (1972) Food specialization by individual trout. J Fish Res Board Can 29:1615–1624
Chivers DP, Ferrari MCO (2013) Tadpole antipredator responses change over time: what is the role of learning and generalization? Behav Ecol 24:1114–1121
Chivers DP, Ferrari MCO (2014) Social learning of predators by tadpoles: does food restriction alter the efficacy of tutors as information sources? Anim Behav 89:93–97
Chivers DP, Mathiron A, Sloychuk JR, Ferrari MCO (2015) Responses of tadpoles to hybrid predator odours: strong maternal signatures and the potential risk/response mismatch. Proc R Soc B 282:20150365
Chivers DP, Mirza RS (2001) Predator diet cues and the assessment of predation risk by aquatic vertebrates: a review and prospectus. In: Marchlewska-Koj A, Lepri JJ, Müller-Schwarze D (eds) Chemical signals in vertebrates, vol 9. Plenum, New York, pp 277–284
Chivers DP, Wisenden BD, Smith R (1996) Damselfly larvae learn to recognize predators from chemical cues in the predator’s diet. Anim Behav 52:315–320
Cooper WE (1998) Direction of predator turning, a neglected cue to predation risk. Behaviour 135:55–64
Dall SR (2010) Managing risk: the perils of uncertainty. In: Westneat DF, Fox CW (eds) Evolutionary behavioral ecology. Oxford University Press, New York, pp 194–206
Domenici P (2010) Context-dependent variability in the components of fish escape response: integrating locomotor performance and behavior. J Exp Zool Part A 313:59–79
Domenici P, Blagburn JM, Bacon JP (2011) Animal escapology I: theoretical issues and emerging trends in escape trajectories. J Exp Biol 214:2463–2473
Engstrom-Ost J, Lehtiniemi M (2004) Threat-sensitive predator avoidance by pike larvae. J Fish Biol 65:251–261
Estes JA, Riedman ML, Staedler MM, Tinker MT, Lyon BE (2003) Individual variation in prey selection by sea otters: patterns, causes and implications. J Anim Ecol 72:144–155
Fendt M (2006) Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. J Chem Ecol 32:2617–2627
Ferrari MCO, Brown GE, Bortolotti GR, Chivers DP (2010a) Linking predator risk and uncertainty to adaptive forgetting: a theoretical framework and empirical test using tadpoles. Proc R Soc Lond B 277:2205–2210
Ferrari MCO, Brown GE, Chivers DP (2012) Temperature-mediated changes in rates of predator forgetting in wood frog tadpoles. PLoS One 7:e51143
Ferrari MCO, Brown GE, Messier F, Chivers DP (2009) Threat-sensitive generalization of predator recognition by larval amphibians. Behav Ecol Sociobiol 63:1369–1375
Ferrari MCO, Chivers DP (2013) Temporal dynamics of information use in learning and retention of predator-related information in tadpoles. Anim Cogn 16:667–676
Ferrari MCO, Chivers DP, Wisenden BD (2010b) Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Ferrero DM, Lemon JK, Fluegge D, Pashkovski SL, Korzan WJ, Datta SR, Spehr M, Fendt M, Liberles SD (2011) Detection and avoidance of a carnivore odor by prey. Proc Natl Acad Sci U S A 108:11235–11240
Fishlyn DA, Phillips DW (1980) Chemical camouflaging and behavioral defences against a predatory seastar by three species of gastropods from the surfgrass Phyllospadix community. Biol Bull 158:34–48
Gherardi F, Renai B, Corti C (2001) Crayfish predation on tadpoles: a comparison between a native (Austropotamobius pallipes) and an alien species (Procambarus clarkii). B Fr Peche Piscic 361:659–668
Gomez-Mestre I, Diaz-Paniagua C (2011) Invasive predatory crayfish do not trigger inducible defences in tadpoles. Proc R Soc Lond B 278:3364–3370
Griffin AS (2004) Social learning about predators: a review and prospectus. Anim Learn Behav 32:131–140
Griffin AS, Evans CS, Blumstein DT (2001) Learning specificity in acquired predator recognition. Anim Behav 62:577–589
Hanson J, Chambers PA, Prepas EE (1990) Selective foraging by the crayfish Orconectes virilis and its impact on macroinvertebrates. Freshw Biol 24:69–80
Helfman GS (1989) Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behav Ecol Sociobiol 24:47–58
Karplus I, Goren M, Algom D (1982) A preliminary experimental analysis of predator face recognition by Chromis caeruleus (Pisces, Pomacentridae). Z Tierpsychol 58:53–65
Kelley JL, Magurran AE (2006) Learned defences and counter-defences in predator‐prey interactions. In: Brown C, Laland KN, Krause J (eds) Fish cognition and behaviour. Blackwell, Oxford, pp 28–48
Kullberg C, Lind J (2002) An experimental study of predator recognition in great tit fledglings. Ethology 108:429–441
Leduc AOHC, Roh E, Breau C, Brown GE (2007) Learned recognition of a novel odour by wild juvenile Atlantic salmon, Salmo salar, under fully natural conditions. Anim Behav 73:471–477
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior—the predation risk allocation hypothesis. Am Nat 153:649–659
Lima SL, Mitchell WA, Roth TC (2003) Predators feeding on behaviourally responsive prey: some implications for classical models of optimal diet choice. Evol Ecol Res 5:1083–1102
Lonnstedt OM, McCormick MI (2013) Ultimate predators: lionfish have evolved to circumvent prey risk assessment abilities. PLoS One 8:e75781
Loya-Javellana GN, Fielder DR, Thorne MJ (1995) Foregut evacuation, return of appetite and gastric fluid secretion in the tropical freshwater crayfish, Cherax quadricarinatus. Aquaculture 134:295–306
Mathis A, Smith RJF (1993) Fathead minnows, Pimephales promelas, learn to recognize nothern pike, Esox lucius, as predators on the basis of chemical stimuli from minnows in the pike’s diet. Anim Behav 46:645–656
Mirza RS, Chivers DP (2003) Fathead minnows learn to recognize heterospecific alarm cues they detect in the diet of a known predator. Behaviour 140:1359–1370
Mitchell MD, Chivers DP, McCormick MI, Ferrari MCO (2015) Learning to distinguish between predators and non-predators: understanding the critical role of diet cues and predator odours in generalisation. Sci Rep 5:13918
Munoz NE, Blumstein DT (2012) Multisensory perception in uncertain environments. Behav Ecol 23:457–462
Parkyn SM, Collier KJ, Hicks BJ (2001) New Zealand stream crayfish: functional omnivores but trophic predators? Freshw Biol 46:641–652
Persons MH, Rypstra AL (2000) Preference for chemical cues associated with recent prey in the wolf spider Hogna helluo (Araneae: Lycosidae). Ethology 106:27–35
Pollock MS, Chivers DP, Mirza RS, Wisenden BD (2003) Fathead minnows, Pimephales promelas, learn to recognize chemical alarm cues of introduced brook stickleback, Culaea inconstans. Environ Biol Fish 66:313–319
Resetarits WJ, Binckley CA (2013) Is the pirate really a ghost? Evidence for generalized chemical camouflage in an aquatic predator, pirate perch Aphredoderus sayanus. Am Nat 181:690–699
Ruxton GD (2009) Non-visual crypsis: a review of the empirical evidence for camouflage to senses other than vision. Philos Trans R Soc B 364:549–557
Scherer AE, Smee DL (2016) A review of predator diet effects on prey defensive responses. Chemoecology 26:83–100
Schluessel V, Kraniotakes H, Bleckmann H (2014) Visual discrimination of rotated 3D objects in Malawi cichlids (Pseudotropheus sp.): a first indication for form constancy in fishes. Anim Cogn 17:359–371
Schoeppner N, Relyea R (2009) When should prey respond to consumed heterospecifics? Testing hypotheses of perceived risk. Copeia 2009:190–194
Sih A, Ziemba R, Harding KC (2000) New insights on how temporal variation in predation risk shapes prey behavior. Trends Ecol Evol 15:3–4
Stankowich T, Coss RG (2007) The re-emergence of felid camouflage with the decay of predator recognition in deer under relaxed selection. Proc R Soc Lond B 274:175–182
Acknowledgments
We would like to thank Jean and Glen for providing housing and access to the field site and Adam Crane for supplying the crayfish used in the study. We would also like to thank the reviewers for their insightful comments. Funding was provided by NSERC (DPC and MCOF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All work was done in accordance with the University of Saskatchewan animal care protocol 2015031. Care was taken to minimise handling stress in tadpoles and crayfish during the experiment. All animals were housed in low densities and fed daily. Following the completion of the experiment, all tadpoles were returned to the ponds from which they were collected.
Additional information
Communicated by D. W. Pfennig
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Mitchell, M.D., Ferrari, M.C.O., Lucon-Xiccato, T. et al. Diet cues alter the development of predator recognition templates in tadpoles. Behav Ecol Sociobiol 70, 1707–1713 (2016). https://doi.org/10.1007/s00265-016-2176-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2176-1