Abstract
Upon leaving the hive, foragers carry a small amount of honey, which they subsequently consume to generate energy for flight. We investigated the relationship between waggle-phase duration and crop volume in foragers (both dancers and dance followers) leaving the hive. Our findings indicate that these variables were positively correlated in the two types of bee, suggesting that they were able to adjust the amount of food that they carry depending on the distance to a food source. We also found that dance followers left the hive with a larger amount of honey than dancers. We suggest two possible explanations: (1) dance followers have less information about the location of the food source than dancers, who have a better knowledge of the surrounding area; or (2) honeybees lack a precise calibration method for estimating energy needs from waggle-run duration. The effect of foraging experience was confirmed: bees decreased their honey load at departure with repeated trips to a sugar-syrup feeder. Honeybees showed a different pattern of change when the feeder provided soybean flour as a pollen substitute, possibly because honeybees use honey not only as an energy source but also as “glue” to form “balls” of pollen on their hind legs. Based on our observations that followers of sugar-syrup foragers carry a different amount of honey in their crop than followers of soybean-followers, we suggest that waggle dancers also convey information concerning food type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flying animals consume much energy (Peters 1983). Honeybee foragers fly great distances from their hive even though they have limited energy reserves, such as fat and glycogen (Panzenböck and Crailsheim 1996). As an energy source, honeybee foragers take small amounts of honey from nestmates through trophallaxis shortly before leaving the hive (von Frisch 1967). The honey is stored in the crop (honey stomach) and is consumed to provide energy during a foraging trip (Gmeinbauer and Crailsheim 1993). Males leaving the hive on a mating flight (Free 1957) and workers preparing to leave in a swarm (Combs 1972) are also known to fill their crops with honey.
Although a large honey load enables a worker to fly a long distance, there may be costs associated with carrying the load. For example, increased body weight due to the honey load might increase flight energy cost (Wolf et al. 1989; Feuerbacher et al. 2003; but also see Balderrama et al. 1992; Moffatt 2000). A large honey load might also reduce maneuverability and agility, and consequently increase predation risk. These costs have been reported in birds in association with fat accumulation (Witter and Cuthill 1993). In addition, there may be additional costs specific to bees. Since bees transport liquid loads (nectar and water) in the crop, carrying honey limits space for these loads. Visscher et al. (1996) argued that this is why water-collecting bees carry only a little fuel when leaving the hive.
Considering these costs, the amount of honey loaded before departing the nest should be regulated in an adaptive fashion. Beutler (1950, 1951) investigated the relationship between the crop contents of foragers leaving the nest and the distances to their food sources. She found that foragers collecting sugar syrup at a distant feeder left the nest with larger amounts of honey than those foraging at a feeder nearby, suggesting that bees adjust their fuel load as a function of the expected energy requirement of a foraging trip.
It is well known that honeybees communicate the locations of food sources to nestmates by means of dance communication (von Frisch 1967). The distance and direction to a food source are expressed in the duration and direction, respectively, of the waggle run in waggle dances. Nestmates can obtain this information by following the dances (von Frisch 1967; Michelsen et al. 1992; Riley et al. 2005). Therefore, it is possible that dance followers, that is, potential recruits, determine the amount of fuel needed based on the dance information, as previously mentioned by Beutler (1950).
Conversely, dance followers may be expected to have a larger honey load than dancers because waggle dances indicate only the approximate location of a food source. Followers may therefore require more energy to find the food source than dancers that have already learnt the route from the hive. Esch and Bastian (1970) reported that indeed recruits need more time to reach a feeder than dancers. Other studies have shown that the dance language contains a certain amount of intrinsic inaccuracy in terms of the indication of distance and direction to a food source (von Frisch 1967; De Marco et al. 2008). This inaccuracy in the encoding of spatial information is, at least partially, due to a sensory constraint in dancers (Tanner and Visscher 2010). The waggle dance recruits bees to a relatively wide area of 25–100 m in radius around a food source (von Frisch 1967; Gould 1975a, b, 1976; Towne and Gould 1988). The fact that a considerable proportion of followers fail to find a food source (Seeley 1983; Biesmeijer and Seeley 2005) also suggests that dance information does not provide sufficient guidance to a food source and that recruits need to search the target after arriving at the general area indicated by the dance.
To examine whether foragers use distance information in waggle dances to determine the amount of fuel loaded at departure and how this determination is influenced by foraging experience, we investigated the relationship between waggle-run duration and crop contents at departure from the nest in both dancers and dance followers. We also evaluated the effect of foraging experience in another experiment in which bees were allowed to visit an identical feeder multiple times.
Honeybees collect not only nectar but also pollen as food. Typically, individual workers tend to collect either—but not both—of these substances (Free 1960). Beutler (1950) showed that pollen foragers carried a greater amount of honey than did sugar-syrup foragers working on feeders and explained this finding by arguing that pollen foragers need additional honey for collecting pollen. Pollen foragers typically regurgitate a bit of honey or nectar and then mix it with collected pollen to ensure that the grains are sufficiently sticky to form pollen balls (Hodges 1952). If Beutler’s argument is correct, pollen foragers may adjust the amount of honey carried at departure differently from nectar foragers because the honey will be used for an additional purpose, not just for fuel. In our study, we compared the regulation of honey crop content at departure between foragers collecting a pollen substitute and those collecting sugar syrup.
Methods
Bees and hive
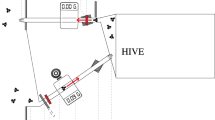
A colony of honeybee Apis mellifera L. housed in a glass-walled observation hive was used for our experiments (Fig.1a). The colony consisted of a queen and 3,000–4,000 worker bees with two frames of comb. Each frame contained a large area of brood in the center, both sealed and open cells of honey in the periphery, and pollen stores in a belt-like zone between these two areas. The conditions were maintained as above during observation in some experiments, but brood and food stores gradually decreased in the other experiments carried out in a flight cage. In the latter experiments, the colony was fed 500 ml of 50 % sugar syrup using a feeder placed at the entrance of the hive and with a patty of pollen substitute (Feed Bee; Bee Processing Enterprises Ltd., Canton ON, Canada) placed in the hive about once every 2 weeks. The hive was placed in an air-conditioned (27 ± 2 °C) room.
Experimental apparatus. a Horizontal view of experimental colony housed in a two-frame observation hive: a1 nylon screen covering dance floor, a2 sampling apparatus, a3 colony entrance. b Vertical view of experimental set-up in a flight cage. c Sampling apparatus. Departing bees were confined in a chamber by inserting two pieces of plastic board vertically. Each chamber can be used separately. d Crop exposed by separating thorax and abdomen. e Sugar-syrup feeder. f Soybean-flour feeder
The hive was connected either to the field or to an outdoor flight cage (3.7 × 3.7 × 2.4 m; Fig. 1b) with a transparent corridor (9.5 × 33 × 3 cm) made of polyvinyl chloride except for the wooden floor. The corridor was equipped with a sampling apparatus to catch workers returning to or leaving the hive (Fig. 1a, c). The sampling apparatus consisted of three tube-like chambers that were open at both ends. These were placed at the middle of the corridor so bees had to pass through one of them when exiting or entering the hive. Bees were captured by closing the chamber with two pieces of plastic board after they had entered the chamber (Fig.1c).
Bees returning from foraging were guided to one side of the lower frame by a wedge-shaped wooden block placed in the entrance of the hive (Seeley 1995) so that one observer could observe all of the dances performed. An area of comb adjacent to the entrance (“dance floor”) was covered with a nylon screen (18 × 20 cm, 4.2 mesh; Fig.1a) through which the bees were marked by the observer as described below.
Marking bees
The thoraces of bees performing a waggle dance or those following a dance were marked with a small amount of colored chalk powder using a fine paint brush (no. 0; Sakura Co. Ltd., Tokyo, Japan) inserted through the nylon screen covering the dance floor. We adopted this method to minimize disturbance to the bee upon marking. In many studies (e.g., von Frisch 1967; Seeley 1995), bees were marked with artists’ paint. However, while such markings have advantages in terms of visibility and persistency, the method sometimes induces intensive self-grooming that interrupts bee behavior, including dance following. Bees marked with chalk powder seldom show this undesirable response. Markings with chalk powder tend to persist for only a few hours, but this length of time was adequate for our purpose.
Measurement of crop contents
Marked bees leaving the hive were captured using the sampling apparatus and fixed on a dissection dish by an insect pin. Each bee’s abdomen was gently pulled from the thorax using tweezers to expose the crop (Fig. 1d). The content of the crop was then quantified in a 50-μL microsyringe (Hamilton, Reno, NE). When the contents were too small to be collected, even though a small amount of liquid was seen in the crop, the quantity was considered to be 0.25 μL since the quantification limit of this method was 0.5 μL.
Relationship between waggle-run duration and crop contents
To examine the effects of distance to a food source on the amount of honey in a forager’s crop upon departure, we investigated the relationship between waggle-run duration in the dances performed (N = 54) or followed (N = 44) by bees and the crop contents of these bees as they left the hive. The experiment was carried out from late June to early August, 2011 with a colony in which bees were allowed to forage freely in the field. The duration of the waggle run in waggle dances was recorded with a digital voice recorder (model ICR-PS401RM; Sanyo, Tokyo, Japan). Three to six waggle runs were recorded per dance, and the average duration was calculated from the record. We focused only on dances performed without pollen loads.
Dance followers were sampled only when they went to the hive entrance within 3 min after following a dance. A dance was usually followed by several bees. We sampled the bee that most actively followed a given dance.
Determination of crop contents in relation to foraging experience in a flight cage
To investigate the effect of foraging experience on the crop content of bees departing for foraging, we allowed bees to visit a feeder multiple times before we measured the crop contents. In this series of experiments, an observation hive was connected to a flight cage, and bees were prevented from foraging on natural food sources. The experiments were carried out from September to November, 2010.
A sugar-syrup feeder (Fig. 1e) was placed in the flight cage on a table that was 2.8 m distant from the entrance of the observation hive and 1.2 m above the ground (Fig. 1b). The feeder consisted of a plastic board (12 × 12 × 0.2 cm), a piece of polyvinyl chloride screen (diameter 9.5 cm, thickness 2 mm, mesh size 5) placed on the board, and a glass vessel (diameter 7.5 cm, height 6 cm) which was filled with 40 % sucrose solution and placed upside down on the screen. Bees fed on the sugar syrup that leaked from the gap formed by the screen between the glass vessel and the plastic board (Fig.1e).
To recruit the first bee to the feeder, a cotton ball soaked in sugar syrup was initially placed at the entrance of the hive; once bees were sucking the syrup, the cotton ball (with bees) was moved to the feeder. Some of these “priming” bees began to commute between the feeder and the hive and subsequently recruited other bees. When some of the bees had been trained to visit the feeder in previous days, they appeared at the feeder and recruited other bees a short time after the feeder was provided in the morning of the experimental day. In the absence of these priming or previously trained bees, the feeder was often not found for several hours. We did not use the priming or previously trained bees for analysis.
When bees arrived at a feeder, we marked each one on the thorax and/or abdomen with different colors of acrylic paint (Acryla Gouache; Holbein Works Ltd., Osaka, Japan) for individual identification. These bees were captured on departure from the hive after they had visited the feeder one to four times and sacrificed for the determination of crop contents (19–23 bees each). Only a small number of bees (<10) were allowed to forage at any one time to avoid crowding at the feeder. In addition, a few bees were allowed to forage continuously so that naive bees were always being recruited to the feeder. Excess bees arriving at the feeder were captured and not allowed to return to the colony. When unmarked bees were going to leave the hive after following a waggle dance, they were sampled as followers; crop content was measured in 37 followers. These bees were considered to be making their first trip to a feeder; it was always certain that these bees had never visited the feeder before being captured because (1) the feeder was provided only during an observation period and (2) all bees that had previously visited a feeder were marked. We also determined the crop contents at departure after bees were allowed to forage on the feeder continuously for 1–5 h. In this experiment, multiple bees were allowed to visit the feeder at the same time and marked with paint upon the first visit. Measurement of crop content was started 1 h after the last bee was marked and ended within 5 h after the first bee appeared at the feeder. Most of these bees were not individually identified, but some were in order to record the number and rate of their visits during the foraging period. Forty-nine bees were sampled for the measurement of crop content.
In other experiments, dry soybean flour (CanDo Co. Ltd., Tokyo, Japan) was provided in a Petri dish (diameter 9 cm, height 1.5 cm) placed on a plastic board (Fig. 1f) to compare honey crop contents at departure between bees collecting this material and those collecting the sucrose solution. Soybean flour is commonly substituted for pollen in beekeeping practice. Sampling of bees and determination of crop contents were carried out (N = 21–49 each) as described in the experiments with a sugar-syrup feeder. The experiment was repeated twice in different weeks using the same colony. The amount of remaining honey in the bees’ crops was also measured when foragers returned to the hive after collecting soybean flour to estimate the consumption of honey during a foraging trip. In addition, the size of soybean-flour loads collected by those bees was measured by weighing the loads after drying at 100 °C overnight.
Crop contents in followers depending on the food type collected by dancers
To test whether dance followers regulate the amount of honey load at departure depending on the food type collected by the dancers that they followed, both sugar-syrup and soybean-flour feeders were placed 20 cm apart on a table at the same time. Bees visiting one of the two feeders were marked with paint of different colors to identify which food they were collecting. Most bees solely foraged on one feeder, and bees that changed feeders were eliminated from the analysis. When these bees performed a waggle dance in a hive, 40 or 50 followers were captured for determination of crop contents upon departure. The contents were compared between followers that paid attention to a sugar-syrup forager (N = 40) and those that watched a soybean-flour forager (N = 50).
Statistics
The degree of correlation between waggle-run duration and honey crop content at departure from the hive was determined using Spearman’s correlation coefficient (r s ) for both dancers and followers. After obtaining regression equations for the data, fitness of the equations to plots was compared between dancers and followers. Differences between actual data and expected values based on the regressions were calculated for crop contents as well as between the two types of bees using Mann–Whitney’s U test. Analysis of covariance (ANCOVA) was applied to test the difference in crop contents between the two types of bees under consideration on the effects of waggle-run duration.
The effect of the number of visits to a feeder on the size of the honey load at departure from the hive was tested by Steel–Dwass multiple comparison after Kruskal–Wallis test. The Mann–Whitney U test was used to examine whether dance followers adjusted their honey load size at departure depending on the food type that a dancer had collected. We adopted non-parametric methods because inequality of variance was detected for some data sets. All statistical analyses except for ANCOVA were carried out using Excel add-in software Ekuseru-Toukei ver. 2007 (SSRI Co. Ltd., Tokyo, Japan). ANCOVA was conducted with JMP ver. 9.0.2 (SAS Institute, Cary, NC). All statistical tests were performed at the 5 % significance level.
Results
Correlations between waggle run durations and honey crop contents
When bees were allowed to forage on flowers in the field, dancers showed a positive correlation between the waggle-run duration of their dances and the size of the crop content at departure (Spearman’s rank correlation r s = 0.74, N = 54, P < 0.001; Fig. 2). A similar correlation was found in followers which significantly increased the amount of honey taken from the hive with increases in waggle-run duration in a dance that they followed (r s = 0.58, N = 44, P < 0.001; Fig. 2). When the amounts of crop content were compared between dancers and followers taking into account the effects of waggle-run duration, the values were generally lower in the former than in the latter (ANCOVA F 1, 95 = 23.26, N = 54 and 44, respectively, P < 0.001).
Relationships between waggle-run duration in a dance performed or followed and crop contents in waggle dancers and followers upon departure from the hive. Solid and dashed lines Regression lines for dancers (y = 1.5399x + 0.7879, P < 0.001) and followers (y = 3.7244x + 2.3053, P < 0.001), respectively, numbers in parenthesis sample size
From these data, regression equations were obtained as y = 1.5399x + 0.7879 in dancers and y = 3.7244x + 2.3053 in followers (P < 0.001 each), where y = honey crop content upon departure from the hive and x = waggle-run duration. When the variances of the actual data from the expected value were calculated for crop contents in the two bee groups, these were statistically smaller in dancers [1.76 ± 1.82 μL (mean ± standard deviation, SD), N = 54] than in followers (3.57 ± 3.32 μL, N = 44) (Mann–Whitney U test Z = 3.89, P < 0.001), indicating that the regression equation fit better in the former group.
Effects of foraging experience in sugar-syrup foragers
With repeated trips to the sugar-syrup feeder, bees decreased their crop contents at departure (Kruskal–Wallis test H 5 = 91.09, N = 169, P < 0.001; Fig. 3). A sharp decline was found between the first and third trip, but the decline appeared to continue after this point because the mean value had decreased still further after 1–5 h of continuous foraging (Steel–Dwass test P < 0.01). In the continuous foraging experiment, bees visited the feeder a mean of 11.2 ± 2.8 times (N = 16) during the first hour, but we could not record the number of their visits in the later period because we had to leave the feeder for the measurement of crop contents (see Methods).
Effects of foraging experience in soybean-flour foragers
In the soybean-flour experiment, bees briefly landed on the mound of soybean flour once at the soybean-flour feeder and made balls of the soybean flour on their hind legs while making hovering flights. The soybean-flour loads were brought back to the hive and unloaded to a cell in a similar fashion as observed in pollen foragers coming back from flowers.
The crop content in bees leaving the hive after following a waggle dance was smaller than that of other bees (Fig. 4). It is possible that being marked with chalk powder disturbed followers so that they did not receive a sufficient amount of honey from nestmates. We tested this possibility by marking experienced foragers on the dance floor with chalk. Bees leaving the nest for a second trip were marked with chalk powder before they received honey from nestmates; this was also done in followers. We detected no statistical difference in crop contents at departure between second-trip foragers marked with chalk and those without chalk marking (with marking, 5.9 ± 1.6 μL, N = 29; without marking, 6.4 ± 1.9 μL, N = 20; Mann–Whitney U test Z = 0.47, P = 0.64). The chalk-marked second-trip foragers left the nest with statistically significant larger amounts of honey than chalk-marked followers (4.1 ± 1.7 μL, N = 30) (Steel–Dwass test P < 0.01). Therefore, the smaller crop content in followers was not attributed to the handling effect of marking.
Honey crop contents in bees leaving for the soybean-flour feeder changed depending on foraging experience, but the pattern of change was different from that obtained with sugar-syrup foragers (Fig. 4). After returning back from their first successful trip, the bees left the hive with an increased amount of honey in the crop (Steel-Dwass test P < 0.01; Fig. 4). The amount of honey load did not significantly change thereafter, even when the bees continuously foraged for 1–5 h (P > 0.05). This pattern was confirmed in a replicated experiment (Fig. 4b). In the continuous foraging experiment, bees visited the soybean-flour feeder 11.2 ± 2.5 times (mean ± SD, N = 10) during the first 1 h.
Effects of food type that dancers collected on dance followers
When the results described above were compared between sugar-syrup and soybean-flour foragers, the followers of soybean-flour foragers appeared to load a larger amount of honey at departure than sugar-syrup foragers (Mann–Whitney U-test Z = 3.51, N = 37and 67, respectively, P < 0.001; Figs. 3 and 4). This difference was confirmed in another experiment in which the two feeders were provided during the same period so that data were collected under the same conditions (Z = 3.46, P < 0.001; Fig. 5).
Honey load remaining at arrival at the nest and size of soybean-flour load
To estimate how much honey was consumed during a single trip in soybean-flour foraging, crop contents were measured in bees arriving at the hive. These bees still had 2.0 ± 1.3 μL (mean ± SD, N = 51) of honey in their crops. Because bees foraging on soybean flour loaded 6.7 ± 1.9 μL of honey at departure (N = 198, bees that had visited a feeder once or more), we estimated that an average of 4.7 μL was used during a round trip. In this measurement, bees returning from the first successful trip were not sampled because they left the hive with a smaller amount of honey than the others.
We also sampled loads of soybean flour from the bees’ hind legs upon arrival at the hive. Figure 6 shows a negative correlation between dry weight of the load and amount of remaining honey in their crop on arrival (Spearman’s rank correlation r s = −0.51, N = 35, P < 0.01).
Discussion
The results of our study confirm those of Beutler (1950) that foragers load a certain amount of honey at departure depending on the distance to a food source. We further found that the amount of honey loaded was influenced by dance communication, foraging experience, and food type. These results demonstrate that honey bees possess a complicated and finely tuned mechanism through which they are able to regulate the amount of honey loaded before departure from the hive.
One question is whether the amount of honey loaded corresponds to the energy expended in flight to the food source. A regression obtained for dancers (Fig. 2) predicts that dancers showing a waggle run for 1.3 s, which indicates a distance of 1 km from the hive to the food source in our bee strain (M. Sasaki, unpublished data), have a honey load of 2.8 μL at departure. Assuming that the concentration of honey is 20–80 % (Gary 1992; Seeley 1995), the sugar content of the load is calculated to be 0.5–2.2 mg. According to an estimation by Visscher et al. (1996) based on Gmeinbauer and Crailsheim (1993), workers can fly 2 km on 1 mg of sugar. Therefore, bees can fly 1–4.5 km with 2.8 μL of honey. When the waggle-run duration increased by 1 s, which corresponds to an increase in flight distance of 1,087 m (M. Sasaki, unpublished data), dancers increased the crop load by approximately 1.5 μL of honey (Fig. 2). Following the estimation described above, the additional honey increases the distance to which the bees can fly by 0.6–2.4 km. Although the concentration of honey that foragers load at departure was not closely investigated, it would appear from our results that dancers adjust their crop loads in relation to the energy requirement in the foraging trip.
Do dance followers determine the amount of honey load at departure based on dance information?
The crop contents of dance followers leaving a hive were positively correlated with the respective waggle-run durations of the dances that they followed. This relationship may reflect a determination of the amount of fuel load based on dance information. Although this explanation appears to be reasonable because followers can use the dance information to locate the advertised food source (von Frisch 1967; Michelsen et al. 1992; Riley et al. 2005), our results might exaggerate the effect of dance communication on the regulation of honey load in followers. In our study, we did not control the foraging experience of dance followers, and when dancers advertised food sources by dancing, some followers may have already visited the sources. In this case, followers attending the dancer could adjust their crop load size without any reference to the information provided by the dance on location because a flower scent carried by dancers would be sufficient to re-activate them to visit a previously visited food source based on their navigational memory (Reinhard et al. 2004).
It is known that some followers ignore dance information and that they visit a previously visited food source other than an advertised site under certain conditions (von Frisch 1968; Grüter et al. 2008). These bees may load a quantity of fuel based on their own memory independent of the distance indicated by dances. In our results, however, the sizes of crop loads correlated well with waggle-run durations in the dances that had been followed. We sampled the most active followers among many followers attending to a dancer. This method might have helped us to avoid sampling bees that would ignore dance information because bees that have followed waggle runs many times (and so appear to be especially active in dance following) tend to use dance information (Wray et al. 2011).
When discussing how followers determine the size of a honey load by referencing dance information, we should note that waggle dances do not convey explicit information on energy needs for a foraging trip. Srinivasan et al. (2000) demonstrated that the waggle-run duration encodes amounts of optic flow in the route, but not absolute distances, to a food source. Since the rate of optic flow per unit distance varies depending on the visual features of the ground en route to the food source (Esch et al. 2001), waggle-run durations do not always show a certain correlation with energy needs for flight. How followers estimate the energy needs and determine the amounts of fuel required based on waggle-run duration is still an open question. One possible strategy of followers is to fill the crop with the maximum amount of fuel when they are activated to visit unfamiliar food source by waggle dances. Although in our study the crop content was generally correlated with waggle-run duration in followers, the dataset does contain a number of extreme outliers with large crop contents. If we assume that these outliers only represent naïve followers (followers that had not visited a food source indicated by the dance), the presence of these outliers could be explained by this hypothesis. Another hypothesis is that naïve followers use a gross calibration method, which may be innate or produced by averaging optic-flow rate under the specific circumstances, to estimate energy needs based on waggle-run duration. The calibration would subsequently be modified to match the actual energy expenditure after making flights to the food source. If this is the case, dancers are expected to estimate the energy needs more precisely than followers. In accordance with this prediction, the variance of the plot of the respective regressions was statistically smaller in dancers than in followers (Fig. 2; also see Results). These hypotheses should be examined closely in future studies.
The effects of foraging experience
Followers had larger amounts of honey at departure than dancers. We demonstrated that this difference resulted from a difference in foraging experience between the two groups of bees by showing a gradual decrease in crop contents at departure as bees repeatedly visited a syrup feeder. Consistent with these results, Brandstetter et al. (1988) reported that the total sugar content of a bee’s whole body (including the crop) was larger in foragers that visited a feeder only 6–12 times relative to those that had foraged there for 2–5 days. These data suggest that foragers change the amount of fuel taken from the nest depending on their information state.
Followers may leave the nest with additional fuel for searching flights because waggle dances only indicate the approximate location of a food source. Once they find a food source and learn the route from the hive, they would reduce the fuel loaded at the nest. However, this is not the only explanation for a decline in load size at departure with foraging experience. Followers may load a lot of fuel because they do not have a precise calibration method to estimate energy needs from waggle-run duration. Upon making several flights, they may decrease fuel to actual needs. However, there are no data to exclude either hypothesis at present.
Difference between nectar and pollen foragers
In our study, bees collecting soybean flour were characterized by a large honey load at departure. This result is consistent with the findings of Beutler (1950), who observed larger amounts of honey in departing pollen foragers than in departing sugar-syrup foragers. In our study, bees needed to take some honey from the hive to make balls of collected soybean flour because our feeder did not provide any liquid food. The need for “glue honey” should be a major cause of the increase of honey load at departure in soybean-flour foragers, as suggested by Beutler (1950). A possible increase in the metabolic rate due to a burden of soybean-flour load during a return trip is unlikely to account for the increased honey load in soybean-flour foragers because this load was estimated to increase only by 10 % compared with that of nectar foragers (Feuerbacher et al. 2003) and the feeder was located very close to the hive in our experiment.
Assuming that foragers did not consume honey during a foraging trip to the feeder in our experiments, the amount of glue honey used to make a pair of soybean-flour balls was estimated to be approximately 5 μL on average—that is, 70 % of the total honey load taken from the hive. However, the honey remaining in their crops at arrival at the hive was negatively correlated with the size of the soybean-flour loads, and the bees that returned with large loads had used up almost all of the honey that they had brought (6.7 μL on average). Considering these results, soybean-flour foragers would appear to have had a sufficient amount of honey for making the largest possible pollen balls but that some bees returned to the hive before collecting this much food, suggesting that honey load is not always the limiting factor in pollen collecting.
Soybean-flour foragers did not decrease the honey load at departure over a course of consecutive foraging trips, unlike sugar-syrup foragers. An increase was found after bees visited a feeder once, but no significant change was observed in subsequent foraging trips. These honeybees may have learned the actual requirement for glue honey during the first visit and loaded this amount of honey before subsequent departures. Whether pollen foragers working on flowers also show this increase is unknown, but our results suggest that foraging experience differently influences the amount of honey taken when it is used for glue versus fuel.
The regulation of honey load at departure, including a response to the distance to a food source, is little understood in pollen foragers. The amount might be affected by the availability of nectar on pollen-source plants because bees could use nectar collected from the flowers as glue for pollen loads. Honeybees, however, often forage on flowers providing pollen without nectar (Shuel 1992). Parker (1926) suggested that pollen foragers working on such flowers take larger amounts of honey from the hive than those working on flowers that also provide nectar, although this tendency was not observed by Beutler (1950). Thus, the regulation of honey load at departure might be more complicated in pollen foragers than in nectar foragers.
Do waggle dancers convey information on food type?
In our study, followers left the hive with significantly larger amounts of honey when a waggle dance was performed by soybean-flour foragers than by sugar-syrup foragers. This difference suggests that waggle dancers convey information on food type because the followers had never visited any feeder. This may not be surprising because pollen foragers usually perform waggle dances with pollen loads on their hind legs, whereas nectar foragers do not. Díaz et al. (2007) reported differences in dance-following behavior depending on the presence or absence of pollen loads on dancers. It is also known that recruits tend to collect the same type of food as has been collected by dancers that they follow (Lindauer 1953). These observations agree with our point of view that followers can learn which type of food is available at a food source through a dance.
The results of our study show that the size of the honey load at departure changed depending on various factors, such as the distance to the food source, the type of food to be collected, and the informational state of the foragers. These findings support the hypothesis that a honey load taken from the hive is associated with certain costs and that bees have evolved to optimize foraging efficiency by regulating the size of the honey load. It remains unclear which costs are produced by the honey load. An increase of energy requirement for flight seems to be one cost, but Balderrama et al. (1992) and Moffat (2000) suggest that the amount of nectar load does not affect metabolic cost in honeybees. Both the costs and the benefits of the honey load need to be determined if the ecological significance of the regulation of honey load at departure is to be elucidated.
References
Balderrama NM, de Almeida LOB, Núñez JA (1992) Metabolic rate during foraging in the honeybee. J Comp Physiol B 162:440–447
Beutler R (1950) Zeit und Raum im Leben der Sammelbiene. Naturwissenschaften 37:102–105
Beutler R (1951) Time and distance in the life of the foraging bee. Bee World 32:25–27
Biesmeijer JC, Seeley TD (2005) The use of waggle dance information by honey bees throughout their foraging careers. Behav Ecol Sociobiol 59:133–142
Brandstetter M, Crailsheim K, Heran H (1988) Provisioning of food in the honeybee before foraging. BIONA Rep 6:129–148
Combs GF (1972) The engorgement of swarming worker honeybees. J Apic Res 11:121–128
De Marco RJ, Gurevitz JM, Menzel R (2008) Variability in the encoding of spatial information by dancing bees. J Exp Biol 211:1635–1644
Díaz PC, Grüter C, Farina WM (2007) Floral scents affect the distribution of hive bees around dancers. Behav Ecol Sociobiol 61:1589–1597
Esch H, Bastian JA (1970) How do newly recruited honey bees approach a food site? Z Vergl Physiol 68:175–181
Esch HE, Zhang S, Srinivasan MV, Tautz J (2001) Honeybee dances communicate distances measured by optic flow. Nature 411:581–583
Feuerbacher E, Fewell JH, Roberts SP, Smith EF, Harrison JF (2003) Effects of load type (pollen or nectar) and load mass on hovering metabolic rate and mechanical power output in the honey bee Apis mellifera. J Exp Biol 206:1855–1865
Free JB (1957) The food of adult drone honeybees (Apis mellifera). Anim Behav 5:7–11
Free JB (1960) The behaviour of honeybees visiting the flowers of fruit trees. J Anim Ecol 29:385–395
Gary NE (1992) Activity and behavior of honey bees. In: Graham JM (ed) The hive and the honey bee. Dadant and Sons, Hamilton, pp 269–373
Gmeinbauer R, Crailsheim K (1993) Glucose utilization during flight of honeybee (Apis mellifera) workers, drones and queens. J Insect Physiol 39:959–967
Grüter C, Balbuena MS, Farina WM (2008) Informational conflicts created by the waggle dance. Proc R Soc B 275:1321–1327
Gould JL (1975a) Honey bee recruitment: the dance-language controversy. Science 189:685–693
Gould JL (1975b) Communication of distance information by honey bees. J Comp Physiol 104:161–173
Gould JL (1976) The dance-language controversy. Q Rev Biol 51:211–244
Hodges D (1952) The pollen loads of the honeybee: a guide to their identification by colour and form. Bee Research Association, London
Lindauer M (1953) Division of labour in the honeybee colony. Bee World 34(63–73):85–90
Michelsen A, Andersen BB, Storm J, Kirchner WH, Lindauer M (1992) How honeybees perceive communication dances, studied by means of a mechanical model. Behav Ecol Sociobiol 30:143–150
Moffatt L (2000) Changes in the metabolic rate of the foraging honeybee: effect of the carried weight or of the reward rate? J Comp Physiol A 186:299–306
Panzenböck U, Crailsheim K (1996) Glycogen in honeybee queens, workers and drones. J Insect Physiol 43:155–165
Parker RL (1926) The collection and utilization of pollen by the honeybee. Mem Cornell Univ Agric Exp Sta 98:1–55
Peters RH (1983) The ecological implications of body size. Cambridge University Press, New York
Reinhard J, Srinivasan MV, Zhang S (2004) Scent-triggered navigation in honeybees. Nature 427:411
Riley JR, Greggers U, Smith AD, Reynolds DR, Menzel R (2005) The flight paths of honeybees recruited by the waggle dance. Nature 435:205–207
Seeley TD (1983) Division of labor between scouts and recruits in honeybee foraging. Behav Ecol Sociobiol 12:253–259
Seeley TD (1995) The wisdom of the hive. Harvard University Press, Cambridge
Shuel RW (1992) The production of nectar and pollen. In: Graham JM (ed) The hive and the honey bee. Dadant and Sons, Hamilton, pp 401–436
Srinivasan MV, Zhang S, Altwein M, Tautz J (2000) Honeybee Navigation: Nature and Calibration of the “Odometer”. Science 287:851–853
Tanner DA, Visscher PK (2010) Adaptation or constraint? Reference-dependent scatter in honey bee dances. Behav Ecol Sociobiol 64:1081–1086
Towne WF, Gould JL (1988) The spatial precision of the honey bees’ dance communication. J Insect Behav 1:129–155
Visscher PK, Crailsheim K, Sherman G (1996) How do honey bee (Apis mellifera) fuel their water foraging flight? J Insect Physiol 42:1089–1094
von Frisch K (1967) The dance language and orientation of bees. Harvard University Press, Cambridge
von Frisch K (1968) The role of dances in recruiting bees to familiar sites. Anim Behav 16:531–533
Witter MS, Cuthill IC (1993) The ecological costs of avian fat storage. Phil Trans R Soc Lond B 340:73–92
Wray MK, Klein BA, Seeley TD (2011) Honey bees use social information in waggle dances more fully when foraging errors are more costly. Behav Ecol 23:125–131
Wolf TJ, Schmid-Hempel P, Ellington CP, Stevenson RD (1989) Physiological correlates of foraging efforts in honey-bees: Oxygen consumption and nectar load. Funct Ecol 3:417–424
Acknowledgments
We would like to thank Prof. T. D. Seeley of Cornell University for his valuable comments and kind suggestions on this manuscript. We are grateful to Dr. Y. Sakai of the Brain Science Institute, Tamagawa University for stimulating discussions. Thanks are also due to Ms. K. Tsuruta and Ms. K. Matsuoka for experimental assistance in the preliminary studies. Two anonymous reviewers improved the manuscript greatly. This work was partially supported by a Strategic Research Center Establishment Program of Tamagawa University [S0901017] funded by the Ministry of Education, Culture, Sport, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Naug
Rights and permissions
About this article
Cite this article
Harano, Ki., Mitsuhata-Asai, A., Konishi, T. et al. Honeybee foragers adjust crop contents before leaving the hive. Behav Ecol Sociobiol 67, 1169–1178 (2013). https://doi.org/10.1007/s00265-013-1542-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1542-5