Abstract
There is plenty of evidence that resource value is one of the most important non-strategic variables in animal fighting behavior. Here, we tested whether the past ownership of a shelter might modify the agonistic behavior of the crayfish Austropotamobius pallipes, eventually increasing its probability to win when it reencounters a previously met conspecific away from that resource. The agonistic behavior of familiar pairs composed of size-matched males was observed for an hour; after that, the two contestants had been kept in isolation for 2 days, either in the presence or in the absence of a shelter. Specifically, in the isolation phase, a shelter was offered to (1) both crayfish, (2) no crayfish, (3) the dominant crayfish only, and (4) the subordinate crayfish only. The following combat was conducted in the absence of any refuge. The crayfish that previously owned a shelter showed a higher aggressive motivation to fight than the individuals kept without a shelter. Particularly, in the pairs (4), subordinate crayfish were even more aggressive than dominants but were never able to invert hierarchies. Taken together, our results confirm the role played by shelters as determinants of agonism and also show, for the first time, how the behavior of crayfish and their internal state may be affected by their past ownership of a resource.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most animals engage fights for the access of crucial but limited resources, such as mate, food, or shelter (Hack et al. 1997; Bridge et al. 2000; Lindström and Pampoulie 2005). A multiplicity of factors governs the intensity and duration of such fights (Enquist and Jakobsson 1986), but the value of the resource at stake (RV) is likely to be the most important non-strategic variable in fighting behavior (Enquist and Leimar 1987; Parker 1974; Riechert 1998). RV results from the combination between the inherent quality of the resource (the absolute resource value) and the value that an animal assigns to it as the effect of its internal state (the subjective or relative resource value; McNamara and Houston 1989). Absolute and relative RVs together affect an animal's motivation to fight. So, this is generally higher in hungry rather than in satiated individuals (Griffiths 1992; Hazlett et al. 1975; Lawton 1987; Stocker and Huber 2001; Wilcox and Ruckdeschel 1982) or in reproductive males in the presence of a female when the probability to find another mate is low (Keeley and Grant 1993).
Several empirical studies have investigated the effects of RV on the outcome of fights, most often confirming the hypothesis that a contestant that expects a greater benefit from winning is generally more likely to do so (e.g., Austad 1983; Bridge et al. 2000; Cant et al. 2006; Smith et al. 1994; Tibbetts 2008). The influence that the perceived ownership of a resource (through the phenomena of “prior exposure” or “prior residence”) has on the behavior of a contestant has been shown in several invertebrates and vertebrates (Austad 1983; Beaugrand and Zayan 1985; Bentley et al. 2009; Chellappa et al. 1999; Enquist and Leimar 1987; Fayed et al. 2008; Figler et al. 1976; Hack et al. 1997; Humphries et al. 2006; Peeke et al. 1995; Riechert 1984). However, in all the above-listed studies (except Hack 1997), the resource was “physically” present during the contests and in close proximity to the contestants. On the contrary, our interest here is to understand whether the past ownership of a shelter has still an influence on the agonistic behavior of its owner even in its absence.
Since Bovbjerg (1953, 1956), crayfish have been often used as model organisms to understand several relevant aspects of the agonistic behavior in invertebrates. Many species form, at least in confined environments, stable dominance hierarchies that secure prior access to a given resource (in Orconectes virilis: Bovbjerg 1953; Cambarellus shufeldtii: Lowe 1956; Procambarus clarkii: Copp 1986; Procambarus acutus acutus: Gherardi and Daniels 2003; Austropotamobius pallipes: Tricarico et al. 2005). Among the different resources, shelters are usually limited in the habitat and serve multiple functions (Bovbjerg 1970; Capelli and Magnuson 1983; Lodge and Hill 1994). They minimize the risks of predation (DiDonato and Lodge 1993; Englund 1999; Englund and Krupa 2000; Garvey et al. 1994; Hill and Lodge 1999; Lodge and Hill 1994; Olsen et al. 1991), allow for the successful completion of reproduction (Figler et al. 2001, 2005), and, in some species, even attract mates (Bergman and Moore 2003). The important role that a shelter plays in the lifecycle of crayfish explains its strong effect on agonism; in its presence, the intensity of fights increases (Bergman and Moore 2003; Edsman and Jonsson 1996), and shelter occupancy makes the owner more likely to win (Martin and Moore 2008).
A large number of studies has focused on the association between dominance and the use of a shelter (e.g., Capelli and Hamilton 1984; Fero et al. 2007; Herberholz et al. 2003; Martin and Moore 2008) and on the intra- and interspecific competition for its access (e.g., Gherardi and Cioni 2004; Gherardi and Daniels 2004; Figler et al. 2005; Peeke et al. 1995), but none have ever analyzed whether its past ownership influences the agonistic behavior of an individual when it is absent during the contest.
The white-clawed crayfish, A. pallipes, offers an ideal opportunity to investigate this issue. This species forms stable dominance hierarchies in the laboratory (Tricarico et al. 2005). The completion of its life cycle also depends on the available hiding places. It is not considered an active burrower such as other species (e.g., Pacifastacus leniusculus, P. clarkii, many Orconectes species; Hobbs 1988; but see Holdich 2003) but uses crevices in the river banks, stones, roots, and decaying wood as refuges (Bernardo et al. 1997; Garcìa-Arberas and Ralo 2000; Grandjean et al. 1996; Renai et al. 2006). The importance of shelters for A. pallipes has been confirmed by Gherardi and Cioni (2004), who showed their more extensive use by this species with respect to other freshwater decapods (the crayfish P. clarkii and the river crab Potamon fluviatile).
Based on the above premises, our hypothesis was that the past ownership of a shelter makes A. pallipes males more prone to combat and more able to win even in its absence.
Material and methods
Subjects, collection, and housing
To eliminate any factor that could induce an obvious bias to our experiments, only sexually mature, hard-shelled A. pallipes males in good conditions (no mutilations or visible parasites) were collected by hand from the streams Gattaia and Corsalone (northern Tuscany, Italy) in July 2008. In the laboratory, we measured their cephalothorax length (from 2.5 to 4.5 cm) and the width and length of both chelae. Crayfish were maintained in PVC aquaria (50 × 75 cm) containing constantly aerated water at the temperature of 18°C (±1°C), under a natural 14:10 h light/dark cycle regime and were fed every second day with a 0.1 g larva of Sarcophaga calliphora. The maintenance phase lasted for a maximum of 2 weeks. After the experiment, crayfish were returned to their collection site.
Experimental design
Experiments were conducted between 10:00 and 18:00 h. The experiments were composed of four phases, as follows.
-
Phase 1:
isolation (1 week). One week was sufficient to remove any effect of previous social experience (Guiasu and Dunham 1997; Zulandt Schneider et al. 2001). Each individual was numbered on the cephalothorax using a white typing correction fluid for its recognition by the observer. Crayfish were kept isolated in opaque PVC aquaria (30 × 16 cm) with constantly aerated water and with a shelter; a shelter consisted of a brick (20 × 10 × 5 cm) that preliminary observations had shown to be used by crayfish and preferred over other types of refuge. Crayfish were fed every second day as in the maintenance phase.
-
Phase 2:
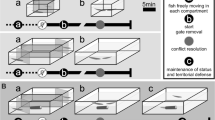
familiarization (25 h). We formed a total of 41 size-matched pairs (maximum difference in the cephalothorax length and in the length of both chelae, 5%; in the width of both chelae, 2%). The two opponents were kept in an experimental tank (a circular opaque PVC container, diameter, 30 cm) without a shelter. Previous studies on this and other crayfish species (Gherardi and Daniels 2003; Tricarico et al. 2005; Tricarico and Gherardi 2007b) have shown that a 1-h contest, also in the absence of a shelter, does not cause injury to the experimental subjects. Additionally, when encounters appeared to escalate, yielding to the potential damage of at least one combatant, individuals were thus separated and the observation was considered over. In our study, one encounter only was interrupted and thus was discarded from the analysis. The tank was initially divided into two equal compartments separated by an opaque PVC divider for a 10-min acclimatization. Experiments started with the removal of the divider.
Crayfish behavior was video-recorded for 1 h using a Sony DCR-TRV33E for the analysis. Simultaneously, an experienced observer (E.T.) recorded the number of interactions and the winner of each interaction; winners were deemed the crayfish that did not retreat or that retreated after the opponent showed a motionless posture, typical of subordinates (Bruski and Dunham 1987). Dominants or alphas (and subordinates or betas) were defined as the crayfish winning more (and less) than 50% of the total interactions. No ties were ever recorded. Dominance averaged 78%. At the end of the observations, the pairs were left in the experimental aquaria for 24 h with an aerator.
-
Phase 3:
maintenance with/without a shelter. Each crayfish from the 40 familiarized pairs were placed back into the same individual aquarium as used in isolation and were randomly assigned to one of the four categories of pairs that differed for the presence/absence of the shelter: (1) α+β+ pairs (n = 10): both crayfish had the shelter; (2) α−β− pairs (n = 10): no crayfish had the shelter; (3) α+β− pairs (n = 10): only alphas had the shelter; and (4) α−β+ pairs (n = 10): only betas had the shelter.
The crayfish were left undisturbed for 2 days in their respective aquaria and fed as in the maintenance phase. Previous observations (Tricarico and Gherardi, in prep.) had shown that, after 2 days of isolation, A. pallipes has not been stressed by the absence of a shelter and still recognizes the status of the former opponent.
-
Phase 4:
reconstitution of the original pairs. For each pair, the original opponents were inserted into a novel experimental tank, following the same procedures as phase 2. After a 10-min acclimatization, their behavior was video-recorded for 1 h. No shelter was offered during the trials. A number code was given to each videotape for the subsequent reading by an observer, extraneous to the experimental design and to the authors' expectations but experienced in the description of crayfish behavior.
Data recorded
Along with dominance (the number of interactions won by a crayfish over the total interactions in percentage; see phase 2), during phase 4 we also recorded the parameters as follows:
-
1.
Latency (in seconds), the time elapsed between the divider removal and the first interaction between the two opponents. One interaction begins when one crayfish approaches the rival and ends when one of them retreats to a distance of 10 cm for at least 10 s (Gherardi and Daniels 2003).
-
2.
Percentage, and
-
3.
Total duration (in seconds) of strong fights. Strong fights are here defined as the interactions in which at least one strong contact (see below) is executed.
-
4.
Percentage of the approaches and of the strong contacts (i.e., chelae strikes and interlocked; Bruski and Dunham 1987), both indices of a high motivation to fight, performed by each of the two contestants.
We compared latency, percentage and duration of strong fights, dominance, and percentage of the strong contacts (these latter were considered as the sum of contacts performed by alphas and betas) among the different pairs; the percentage of the approaches and of the strong contacts were also analyzed per individual.
Statistical analyses
The analyses were conducted on the parameters recorded in phase 4. Data were tested for normality using the Kolmogorov–Smirnov test and for homogeneity of variance using the Levene test. Percentages were first normalized using the arcsine square root transformation. A one-way multivariate analysis of variance (MANOVA: statistic: F) was then used to compare all the recorded parameters among the different pairs; a two-way MANOVA was performed to compare the percentages of the approaches and of the strong contacts among the pairs and between alphas and betas. When we obtained significant F-ratios, we applied the Student–Newman–Keuls (SNK) multiple comparisons tests or the paired samples Student's t test (statistic: t) for the comparison between alphas and betas.
Figures give means (and SE). The level of significance at which the null hypothesis was rejected is α = 0.05.
Results
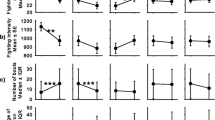
The pairs differed significantly with respect to the dependent variables (F = 17.34, df = 5,34, p < 0.0001). In α−β− pairs, latency was longer than the other pairs (F = 10.44, df = 3,40, p < 0.0001; α−β− > α−β+ > α+β− = α+β+), and strong fights were less numerous, shorter, and characterized by few strong contacts (number, F = 4.11, df = 3.40, p = 0.01; α+β+ = α+β− > α−β+ = α−β−; duration, F = 14.44, df = 3,40, p < 0.0001; α+β+ > α−β+ > α+β− = α−β−; strong contacts, F = 14.32, df = 3,40, p < 0.0001; α+β+ > α+β− > α−β+ > α−β−; Fig. 1). Dominance was similar among pairs (F = 0.30, df = 3,40, p = 0.83), and no inversion of hierarchies was observed. The two-way MANOVA showed significant differences among pairs and between hierarchical status (F = 17.41, df = 3,75, p < 0.0001) with respect to the dependent variables. A significant interaction was also found between pairs and hierarchical status (Table 1). In all pairs, except α−β−, the percentage of approaches and strong contacts significantly differed between alphas and betas, with these latter performing more approaches and strong contacts in α−β+ pairs (t between 4.27 and 8.54, df = 9, p between 0.0001 and 0.002; for α−β−: t between 1.29 and 1.50, df = 9, p between 0.17 and 0.23; Fig. 2).
Comparisons among the four categories of pairs (α+β+=both crayfish with shelter; α−β−= no crayfish with shelter; α+β− = only alphas had the shelter; α−β+=only betas had the shelter; n = 10 for each pair) for a latency, b percentage of strong fights, (c) duration of strong fights, and (d) percentage of strong contacts. Letters over bars denote the hierarchy among pairs after Student Newman Keuls Multiple comparisons. Bars are means (+SE)
Means (+SE) of the frequency of strong contacts in percentage distinguished between alphas and betas (α+β+ = both crayfish with shelter; α−β− = no crayfish with shelter; α+β− = only alphas had the shelter; α−β+ = only betas had the shelter; n = 10 for each pair). Asterisk denotes a significant difference between alphas and betas after the paired samples Student's t test
Discussion
Our study clearly shows that the agonistic behavior of A. pallipes is affected by the past ownership of a shelter. In fact, previous owners showed a higher motivation to fight than the individuals that had been maintained in its absence, as indicated by the more numerous approaches and strong contacts they executed.
Previous studies had shown that prior access to a resource and its ownership can affect behavior in different ways and in a multitude of taxa. In the hermit crab Pagurus longicarpus, individuals that had been subject to a worsening in the quality of their shell are more willing to initiate and escalate fights (Gherardi 2006; Tricarico and Gherardi 2007a). Insects may increase their foraging efficiency according to their past experience at particular sites (Ohashi and Thomson 2005). Prior experience, in concert with appropriate physiological conditions, also influences an insect's reproductive behavior. For example, male seaweed flies are more likely to mount females if they have had prior exposure to seaweed (Dunn et al. 2002); in the walnut fly, Rhagoletis juglandis, experience with a limiting resource such as a host plant or territory influences its mating behavior (Carsten and Papaj 2005); and the preference of the black field cricket females for given male traits depends on their prior exposure to a high- or a low-protein diet (Hunt et al. 2005). Males of the sparrow Melospiza melodia that previously held territories on a site, regardless of whether they were holding the same territory as the previous year, show higher levels of territory defense than males that are new to that site (Hyman et al. 2004). In the swordtail fish Xiphophorus birchmanni, food-deprived females are more motivated to explore the environment, displaying a stronger preference for chemical cues associated with a male nutritional state (Fisher and Rosenthal 2006), while in meadow voles Microtus pennsylvanicus an interruption in food availability of only 6 h inhibits the female interactions with males (Pierce et al. 2005).
In our study, both alphas and betas maintained in isolation without a shelter were less prone to attack and showed less numerous and lasting fights with few strong contacts. On the contrary, they were more aggressive when they have had previous access to a shelter: in α−β+ pairs betas even displayed more strong contacts than alphas. However, as a confirmation that hierarchies may also depend on asymmetries in the intrinsic characteristics and social experience of the opponents (Tricarico and Gherardi 2007b), betas did not succeed in changing its rank. Similarly, the cichlid Cichlasoma nigrofasciatum subordinates, after having had access to a mate, tended to bite first in the subsequent contest, even though they did not always win (Keeley and Grant 1993).
Prior residence (or exposure) to a resource is known to overcome the inferior fighting ability of some species: it increases an animal's resource holding power (Fayed et al. 2008; Tricarico et al. 2008), thus affecting the agonistic behavior and the probable wins of the resident but always in the presence of that resource or of visual/chemical stimuli produced by it. On the contrary, in our study, the effects that a resource has on the agonistic behavior of the owner are evident also in its absence. Before our study, a similar phenomenon was observed only in the cricket Acheta domesticus: males that owned a burrow continued to win more encounters also when combated in an open arena, away from the burrow (Hack 1997). In both this and our case, the “mechanical” advantage of possessing a resource (Fayed et al. 2008) cannot be an explanation of the phenomenon, being the contestants away from it. It thus seems that the former condition of “resource owner” not only alters the internal state of the contestant, increasing the subjective value it assigns to that resource (and so its motivation to fight), but it is possibly memorized by an animal and alters its subsequent behavior without the need of being again exposed to the shelter. Indeed, crayfish have well-demonstrated memory capabilities: they recognize an opponent after 2 weeks of isolation (Hemsworth et al. 2007), establish associations between odors and predation risks (Hazlett et al. 2002), and remember the spatial configuration of a previously explored area (Barbaresi and Gherardi 2006).
In conclusion, our study pinpoints the relevant role of shelter as determinants of agonism in crayfish, and shows, for the first time, how the past ownership of these critical resources alters the behavior and the internal state of crayfish, even in their absence. In essence, our results here raise new and stimulating questions about the cognitive abilities of this taxon.
References
Austad SN (1983) A game theoretical interpretation of male combat in the bowl and doily spider (Frontinella pyramitela). Anim Behav 31:59–73
Barbaresi S, Gherardi F (2006) Experimental evidence for homing in the red swamp crayfish, Procambarus clarkii. Bull Fr Pêche Piscic 380–81:1145–1153
Beaugrand JP, Zayan R (1985) An experimental-model of aggressive dominance in Xiphophorus helleri (Pisces, Poeciliidae). Behav Process 10:1–52
Bentley T, Hull TT, Hardy ICW, Goubault M (2009) The elusive paradox: owner–intruder roles, strategies, and outcomes in parasitoid contests. Behav Ecol 20:296–304
Bergman DA, Moore PA (2003) Field observations of intraspecific agonistic behaviour of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol Bull 205:26–35
Bernardo JM, Ilheu M, Costa AM (1997) Distribution, population structure and conservation of Austropotamobius pallipes in Portugal. Bull Fr Pêche Piscic 347:617–624
Bovbjerg RV (1953) Dominance order in the crayfish Orconectes virilis (Hagen). Physiol Zool 26:173–178
Bovbjerg RV (1956) Some factors affecting aggressive behavior in crayfish. Physiol Zool 29:127–136
Bovbjerg RV (1970) Ecological isolation and competitive exclusion in two crayfish (Orconectes virilis and Orconectes immunis). Ecology 51:225–236
Bridge AP, Elwood RE, Dick JTA (2000) Imperfect assessment and limited information preclude optimal strategies in male–male fights in the orb-weaving spider Metellina mengei. Proc R Soc B 267:273–279
Bruski C, Dunham DW (1987) The importance of vision in agonistic communication of the crayfish Orconectes rusticus. An analysis of bout dynamics. Behaviour 103:83–107
Cant MA, English S, Reeve HK, Field J (2006) Escalated conflict in a social hierarchy. Proc R Soc B 273:2977–2984
Capelli GM, Hamilton PA (1984) Effects of food and shelter on aggressive activity in the crayfish Orconectes rusticus (Girard). J Crustac Biol 4:252–260
Capelli GM, Magnuson JJ (1983) Morphoedaphic and biogeographic analysis of crayfish distribution in northern Wisconsin. J Crustac Biol 3:548–564
Carsten LD, Papaj DR (2005) Effects of reproductive state and host resource on mating decisions in a walnut fly. Behav Ecol 16:528–533
Chellappa S, Yamamoto ME, Cacho MSRF, Huntingford FA (1999) Prior residence, body size and the dynamics of territorial disputes between male freshwater angelfish. J Fish Biol 55:1163–1170
Copp N (1986) Dominance hierarchies in the crayfish Procambarus clarkii and the question of learned individual recognition. Crustaceana (Leiden) 51:9–24
DiDonato GT, Lodge DM (1993) Species replacements among Orconectes crayfishes in Wisconsin lakes: the role of predation by fish. Can J Fish Aquat Sci 50:1484–1488
Dunn DW, Crean CS, Gilburn AS (2002) The effects of exposure to seaweed on willingness to mate, oviposition, and longevity in seaweed flies. Ecol Entomol 27:544–564
Edsman L, Jonsson A (1996) The effect of size, antennal injury, ownership, and ownership duration on fighting success in male signal crayfish, Pacifastacus leniusculus (Dana). Nord J Freshw Res 72:80–87
Englund G (1999) Effects of fish on the local abundance of crayfish in stream pools. Oikos 87:48–56
Englund G, Krupa JJ (2000) Habitat use by crayfish in stream pools: influence of predators, depth and body size. Freshw Biol 43:75–83
Enquist M, Jakobsson S (1986) Decision making and assessment in the fighting behaviour of Nannacara anomala (Cichlidae, Pisces). Ethology 72:143–153
Enquist M, Leimar O (1987) Evolution of fighting behaviour: the effect of variation in resource value. J Theor Biol 127:187–205
Fayed SA, Jennions MD, Backwell PRY (2008) What factors contribute to an ownership advantage? Biol Lett 4:143–145
Fero K, Simon JS, Jourdie V, Moore PA (2007) Consequences of social dominance on crayfish resource use. Behaviour 144:61–82
Figler MH, Klein RM, Peeke HVS (1976) The establishment and reversibility of dominance relationships in jewel fish, Hemichromis bimaculatus Gill (Pisces, Cichlidae): effects of prior exposure and prior residence situations. Behaviour 58:254–271
Figler MH, Blank GS, Peeke HVS (2001) Maternal territoriality as an offspring defense strategy in red swamp crayfish (Procambarus clarkii, Girard). Aggress Behav 27:391–403
Figler MH, Blank GS, Peeke HVS (2005) Shelter competition between resident male red swamp crayfish Procambarus clarkii (Girard) and conspecific intruders varying by sex and reproductive status. Mar Freshw Behav Physiol 38:237–248
Fisher HS, Rosenthal GG (2006) Hungry females show stronger mating preferences. Behav Ecol 17:979–981
Garcìa-Arberas L, Ralo A (2000) Survival of natural populations of Austropotamobius pallipes in rivers in Bizcaia, Basque Country (North Iberian Peninsula). Bull Fr Pêche Piscic 356:17–30
Garvey JE, Stein RA, Thomas HM (1994) Assessing how fish predation and interspecific prey competition influence a crayfish assemblage. Ecology 75:532–547
Gherardi F (2006) Fighting behavior in hermit crabs: the combined effect of resource holding potential and resource value in Pagurus longicarpus. Behav Ecol Sociobiol 59:500–510
Gherardi F, Cioni A (2004) Agonism interference and competition in freshwater decapods. Behaviour 141:1297–1324
Gherardi F, Daniels WH (2003) Dominance hierarchies and status recognition in the crayfish Procambarus acutus acutus. Can J Zool 81:1269–1281
Gherardi F, Daniels WH (2004) Agonism and shelter competition between invasive and indigenous crayfish species. Can J Zool 82:1923–1932
Grandjean F, Bramard M, Souty-Grosset C (1996) Distribution and proposal for the conservation of the indigenous freshwater crayfish species, Austropotamobius pallipes pallipes, in a French department. Freshw Crayfish 11:655–664
Griffiths D (1992) Interference competition in ant–lion (Macroleon quinquemaculatus) larvae. Ecol Entomol 17:219–226
Guiasu RC, Dunham DW (1997) Agonistic contests in male form I Cambarus bartoni (Fabricius, 1789) (Decapoda, Cambaridae) crayfish and comparison with contests of the same type in Cambarus robustus Girard, 1852. Crustaceana 72:1079–1091
Hack MA (1997) Assessment strategies in the contests of male crickets, Acheta domesticus (L.). Anim Behav 53:733–747
Hack MA, Thompson DJ, Fernandes DM (1997) Fighting in males of the autumn spider, Metellina segmentata: effects of relative body size, prior residency and female value on contest outcome and duration. Ethology 103:488–498
Hazlett BD, Rubenstein D, Rittschof D (1975) Starvation, energy reserves, and aggression in the crayfish, Orconectes virilis (Hagen). Crustaceana 28:11–16
Hazlett BD, Acquistapace P, Gherardi F (2002) Differences in memory capabilities in invasive and native crayfish. J Crustac Biol 22:439–448
Hemsworth R, Villareal W, Patullo BW, MacMillan DL (2007) Crustacean social behavioral changes in response to isolation. Biol Bull 213:187–195
Herberholz J, Sen MM, Edwards DH (2003) Parallel changes in agonistic and nonagonistic behaviors during dominance hierarchy formation in crayfish. J Comp Physiol A 189:321–325
Hill AM, Lodge DM (1999) Replacement of resident crayfishes by an exotic crayfish: the roles of competition and predation. Ecol Appl 9:678–690
Hobbs HH Jr (1988) Crayfish distribution, adaptive radiation, and evolution. In: Holdich DM, Lowery RS (eds) Freshwater crayfish: biology, management, and exploitation. Timber Press, Portland, Oregon, pp 52–81
Holdich DM (2003) Ecology of the white-clawed crayfish. Conserving Natura 2000, Rivers Ecology Series no. 1. English Nature, Peterborough
Humphries EL, Hebblethwaite AJ, Batchelor TP, Hardy ICW (2006) The importance of valuing resources: host weight and contender age as determinants of parasitoid wasp contest outcomes. Anim Behav 72:891–898
Hunt J, Brooks R, Jennions MD (2005) Female mate choice as a condition-dependent life-history trait. Am Nat 166:79–92
Hyman J, Hughes M, Searcy WA, Nowicki S (2004) Individual variation in the strength of territory defense in male song sparrows: correlates of age, territory tenure, and neighbor aggressiveness. Behaviour 141:15–27
Keeley RE, Grant JWA (1993) Visual information, resource value, and sequential assessment in convict cichlid (Cichlasoma nigrofasciatum) contests. Behav Ecol 4:345–349
Lawton P (1987) Diel activity and foraging behavior of juvenile American lobsters Homarus americanus. Can J Fish Aquat Sci 44:1195–1205
Lindström K, Pampoulie C (2005) Effects of resource holding potential and resource value on tenure at nest sites in sand gobies. Behav Ecol 16:70–74
Lodge DM, Hill AM (1994) Factors governing species composition, population size, and productivity of coolwater crayfishes. Nord J Freshw Res 69:111–136
Lowe ME (1956) Dominance-subordinate relationships in the crawfish Cambarellus shufeldtii. Tulane Stud Zool 4:139–170
Martin AL, Moore PA (2008) The influence of dominance on shelter preference and eviction rates in the crayfish, Orconectes rusticus. Ethology 114:351–360
McNamara JM, Houston AI (1989) State dependent contests for food. J Theor Biol 137:457–479
Ohashi K, Thomson JD (2005) Efficient harvesting of renewing resource. Behav Ecol 16:592–605
Olsen TM, Lodge DM, Capelli GM, Houlihan RJ (1991) Mechanisms of impact of an introduced crayfish (Orconectes rusticus) on littoral congeners, snails, and macrophytes. Can J Fish Aquat Sci 48:1853–1861
Parker GA (1974) Assessment strategy and the evolution of animal conflicts. J Theor Biol 84:93–101
Peeke HVS, Sippel J, Figler MH (1995) Prior residence effects in shelter defense in adult signal crayfish (Pacifastacus leniusculus (Dana)): results in same- and mixed-sex dyads. Crustaceana 68:873–881
Pierce A, Ferkin M, Williams T (2005) Food deprivation induced changes in sexual behavior of meadow voles, Microtus pennsylvanicus. Anim Behav 70:339–348
Renai B, Bertocchi S, Brusconi S, Grandjean F, Lebboroni M, Parinet B, Souty Grosset C, Trouilhe MC, Gherardi F (2006) Ecological characterisation of streams in Tuscany (Italy) for the management of the threatened crayfish Austropotamobius pallipes complex. Bull Fr Pêche Piscic 380–381:1095–1114
Riechert SE (1984) Game spider play. III: Cues underlying context-associated changes in agonistic behaviour. Anim Behav 32:1–15
Riechert SE (1998) Game theory and animal contests. In: Dugatkin LA, Reeve HK (eds) Game theory and animal behaviour. Oxford University Press, Oxford, UK, pp 64–93
Smith IP, Huntingford FA, Atkinson RJA, Taylor AC (1994) Strategic decisions during agonistic behavior in the velvet swimming crab, Necora puber (L.). Anim Behav 47:885–894
Stocker AM, Huber R (2001) Fighting strategies in crayfish Orconectes rusticus (Decapoda, Cambaridae) differ with hunger state and the presence of food cues. Ethology 107:727–736
Tibbetts EA (2008) Resource value and the context dependence of receiver behaviour. Proc R Soc B 275:2201–2206
Tricarico E, Gherardi F (2007a) The past ownership of a resource affects the agonistic behavior of hermit crabs. Behav Ecol Sociobiol 61:1945–1953
Tricarico E, Gherardi F (2007b) Biogenic amines influence aggressiveness in crayfish but not their force or hierarchical rank. Anim Behav 74:1715–1724
Tricarico E, Renai B, Gherardi F (2005) Dominance hierarchies and status recognition in the threatened crayfish Austropotamobius italicus. Bull Fr Pêche Piscic 376–377:655–664
Tricarico E, Benvenuto C, Buccianti A, Gherardi F (2008) Morphological traits determine the winner of “symmetric” fights in hermit crabs. J Exp Mar Biol Ecol 354:150–159
Wilcox RS, Ruckdeschel T (1982) Food threshold territoriality in a water strider (Gerris remigis). Behav Ecol Sociobiol 11:85–90
Zulandt Schneider RA, Huber R, Moore PA (2001) Individual and status recognition in the crayfish, Orconectes rusticus: the effects of urine release on fight dynamics. Behaviour 138:137–153
Acknowledgments
We thank Silvia Bertocchi and Carmen Trunfio for their help in conducting the experiments, and Laura Aquiloni and Giuseppe Mazza for their help in collecting crayfish. We also thank Dr. Paul Moore and one anonymous reviewer for their helpful suggestions. No license was required for this work within Italy. All animals recovered from the experiments and were returned to the rivers. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Moore
Rights and permissions
About this article
Cite this article
Tricarico, E., Gherardi, F. Past ownership makes crayfish more aggressive. Behav Ecol Sociobiol 64, 575–581 (2010). https://doi.org/10.1007/s00265-009-0873-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0873-8