Abstract
In the habitat of desert ants, Cataglyphis fortis, a constant wind is usually blowing during the daytime. When visiting a familiar food source, the ants steer some distance downwind of the feeder, rather than attempting a direct approach that might miss small food sources, in particular. In the downwind area, the ants pick up the odor plume emanating from the food and follow it upwind to the prey. This strategy saves considerable walking distance and time. The additional path necessitated by the downwind strategy is only about 0.75 to 2 m, depending on nest–feeder distance, while missing the food on the upwind side results in much longer search trajectories. During the initial three to five visits to a feeding site, downwind distance and length of the approach path are shortened notably, and the approach trajectory is straightened. Desert ants further exhibit considerable short-term flexibility in their approach. Experienced individuals are evidently able to decide upon leaving the nest which direction to choose toward the feeder, depending on current wind direction (that fluctuates slightly during the day). Notable changes in wind direction occur primarily overnight. For larger nest–feeder distances, the animals adjust their approach en route to the altered wind direction during their first foraging trip in the morning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The navigation feats of desert ants, genus Cataglyphis, have been studied in much detail (reviews in Wehner 1992, 1996, 2003). A major basis of this ant’s navigation performance is path integration. During extended foraging trips, path integration yields a homebound vector that always keeps the ant informed about direction and distance of the nest entrance. Thus, wherever a food item is encountered, the ant is able to return to the nest on a fairly straight trajectory, saving time and energy and minimizing heat stress and predation risk (Schmid-Hempel 1983; Wehner et al. 1983). Direction and distance of productive feeding sites are memorized by Cataglyphis ants and employed during repeated visits to such food sources (“food vectors” in Collett et al. 1999; see also Wolf and Wehner 2000), allowing for aimed approaches of the goal. Desert ants further use external (i.e., geocentric, as opposed to egocentric) orientation cues in their often flat and featureless habitat, whenever available. These include landmarks (e.g., Bisch-Knaden and Wehner 2003), surface structure (Seidl and Wehner 2006), wind direction, and olfactory cues emanating from food items.
Desert ants achieve remarkable accuracy in their navigation performance, finding a nest entrance of barely 2 cm in diameter from a distance exceeding 100 m or roughly 10,000 times their body lengths. Nevertheless, navigation errors occur, in both biological (Müller and Wehner 1988; Wehner and Wehner 1986) and artificial navigation systems. To deal with such errors, the animals are able to assess their own navigation uncertainty and to account for it when visiting a familiar feeding site (Sommer and Wehner 2004; Wolf and Wehner 2005). Homebound ants that miss their goal employ a systematic search strategy centered on the assumed nest position (Wehner and Srinivasan 1981; Merkle et al. 2006), while ants on their outbound journey perform a similar search for familiar feeding sites (Wolf and Wehner 2000), and they are attracted and guided by odor plumes emanating from the food (Wolf and Wehner 2005).
When a reliable wind is blowing, which is characteristic of the daytime in many desert habitats, the ants do not approach a familiar food source directly. Instead, they steer some distance downwind of the food source and pick up the odor filaments emanating from the food. The ants follow this odor trail upwind toward the goal. This approach strategy presumably prevents the animals from missing the goal on the upwind side, which might happen frequently when attempting a direct approach, depending on the extent of inaccuracies of their navigation system. This strategy appears particularly useful in the case of small food sources that might be missed easily, thus avoiding lengthy and expensive searches.
Cataglyphis ants are individual foragers that do not lay odor trails nor recruit nest mates (review in Wehner et al. 1983; Wehner 1987; Wehner 2003). In the rare cases that they encounter plentiful feeding sites, which merit repeated visits, for instance, larger carcasses or fruiting plants, foragers memorize feeding site location and visit the place reliably over long periods of time as long as they are rewarded. It has been noted previously that the approach trajectories of novice ants change and apparently shorten, in the course of the initial few visits to such a feeder (Wolf and Wehner 2000).
An adjustment of orientation toward food sources, often involving active exploratory mechanisms, has been reported for many animals and in particular insects (ants: Fresneau 1985; Harrison et al. 1989; Lehrer 1993; Nicholson et al. 1999; bees and wasps: Zeil et al. 1996; possible example in hamsters: Etienne et al. 1998). For instance, a strategy termed “turn-back-and-look” behavior (Lehrer 1993; Zeil et al. 1996) is employed by several groups of hymenopteran insects to sample landmark arrangements surrounding a new goal, in this case a feeding site (and on other occasions the nest). Memorizing the landmark array surrounding a particular feeding site probably allows for fast and safe visits in future foraging excursions. The actual gain in foraging efficiency and the mode of behavioral fine tuning of the approach have remained enigmatic in most cases, however, not least as they are often difficult to determine, particularly in flying insects.
In the present report, I examine how desert ants adjust and refine their approach to a familiar feeding site over time, with regard to parameters such as downwind distance and length and straightness of the approach path. Ants occasionally miss the feeder on the upwind side, resulting in lengthy searches. This may indicate that a trial-and-error strategy may contribute to these adjustments. A quantitative assessment further demonstrates that such searches represent a considerable extra effort, illustrating the ecological significance of path adjustment. Contrasting with the gradual adjustment of their approaches over three to five visits, the ants also exhibit as yet unknown short-term flexibility in their approaches, especially with regard to fluctuations in wind direction.
Materials and methods
Experiments were carried out just southeast of the Tunisian village Maharès (34°30′N, 19°29′E) during the months of June to September in the years 2002 to 2005 (in 2003 no experiments were conducted). Nests of Cataglyphis fortis (Forel 1902; Wehner 1983) were selected, the surroundings of which were devoid of vegetation and other landmarks for at least 20 m in all directions and for at least 40 m in the direction where the feeding site was established.
Training of Cataglyphis to feeding sites was according to standard procedures (e.g., Wolf and Wehner 2000). In short, feeders were established at distances of 5 to 15 m from the nest. The particular distance depended on the experiment and is stated in that context. Initially and to attract the ants to the feeding site, a trail of biscuit crumbs was laid out toward the feeder, although not closer to the feeder than 5 m. The feeder consisted of a Petri dish, 2.5 cm in diameter, and where appropriate, it was glued into the lid of a jar, 7 cm in diameter. This arrangement prevented food items from being blown out of the feeder and thus contaminating the desert surroundings. Furthermore, it allowed removal of the feeder without leaving an odor mark on the desert floor, for instance, in the context of test situations. The feeder was filled with biscuit crumbs of selected size (sieved to roughly 2 mm diameter). This promoted rapid and frequent visits to the feeder because the crumbs were small enough to be easily carried by foragers, and it reduced the number of (too small and lightweight) crumbs blown out of the feeder. The feeder was pressed into the substrate such that it presented itself as no visible landmark. For most experiments, ants were marked individually with a color code (small dots of automobile varnish applied with insect pins to mesosoma (alitrunk, “thorax”) and gaster (“abdomen”).
Concentric circles were drawn around the feeding site with 0.5 m spacing and divided into 15° sectors, or a 1-m grid of lines was drawn across the area surrounding nest and feeding site, using white wall paint. These markings aided in recording the ants’ approach trajectories (compare Fig. 1). I noted in particular the distance from the feeder at which the foraging ants changed their courses, often quite abruptly, from a roughly tangential approach downwind of the feeder to a slightly zigzagging course directed upwind toward the feeding site (presumably guided by odor filaments; compare approach trajectories depicted in Fig. 1). Along with this “downwind distance” (termed d) I usually recorded the approach trajectory on grid paper, the nest–feeder distance (termed D), the date and time of the day, the wind direction, the wind speed, and the animal identification in those cases where the ants had been marked individually.
Downwind approach of desert ants and changes in approach trajectory during the initial few visits. Approaches to a feeding site by an individual Cataglyphis forager in the course of a day are shown. It is evident that with the prevailing (eastern) wind directions, the ant did not approach the feeder directly but steered between 1.75 and 0.5 m downwind and turned against the wind upon encountering the odor trail emanating from the food. Final approach to the feeder was always against the wind (final approach paths thus reflecting fluctuations in current wind direction). The initial visit to the feeder was a chance encounter, the ant noticing the food odor from some 2.5 m downwind of the feeder; it was not recorded and noted as visit no. 0. The first goal-directed visit and four subsequent visits are shown and numbered; darker lines mark later visits and illustrate the successively closer downwind approach. Downwind distance, d, is indicated; the nest is located 11.3 m northeast of the feeder. In this and the subsequent figures that show approach path recordings, the following conventions are used: (1) the feeder is marked by the intersection of bold marker (grid) lines and surrounded by a 20 cm circle; (2) grid line spacing is 0.5 m, (3) average wind direction is indicated by a large open arrow, (4) a solid arrow (here, bottom left) indicates compass north
To evaluate the data shown in Fig. 3, the original protocols of the trajectories recorded on graph paper were digitized. This allowed determining approach path length and path curvature. Curvature was determined by noting every 6 cm (±0.4 cm, resolution of the above digitization) the deviation from a straight course of the two subsequent path segments that are defined by three digitized coordinates. The angles of these deviations were summated over the whole approach trajectory. According to this procedure, a perfectly straight approach has a curvature value of 0 and a full circle a value of 2π (360°).
Outbound directions of ants leaving the nest or final approach directions toward the feeder were determined by fitting a straight line through the initial or final 1 m, respectively, of the slightly zigzagging walking trajectories by eye.
For statistical analyses, the values obtained for any given individual were averaged, and the resulting mean values were used for further (second-order) statistical analyses. Hence, in all statistical treatments, each ant contributed just a single data point. Alternatively, the data obtained from single individuals in two different situations were compared (e.g., paired t tests to compare data in Fig. 4a,b).
Statistical analyses were performed according to Sachs (1992) and Sokal and Rohlf (1995). In the text below, N signifies the number of animals and n the number of measurements made (either per animal or in total). Exponential fit lines in Fig. 3 were calculated with the program package IgorPro (version 5.04b, WaveMetrics, Oregon, USA), including significance levels of regression and intercept. The exponential fit lines they take the form \(y = y_0 + A \times e^{\left( {{{ - n} \mathord{\left/ {\vphantom {{ - n} \tau }} \right. \kern-\nulldelimiterspace} \tau }} \right)} \), with y as the parameter plotted on the ordinate and n as the trip number on the abscissa; values for y 0, A, and τ are given in the figure, together with the number of animals, N, and probability, P (see legend Fig. 3). To test whether or not the data stabilize beyond a certain trip number (i.e., level of experience), the data of any given trip number in Fig. 3 were tested for significance against the pooled data of all subsequent trips (Helmhert contrast; U test used for calculation). The resulting significance levels are stated as significance of contrast, sc. Student’s t test for paired samples was used to compare the paired data sets shown in Figs. 2 and 4. Regression analysis was performed according to Sachs (1992). The use of circular statistics proved unnecessary because the distributions of angular values were extremely narrow in the present experiments (Batschelet 1981). Test results are termed significant at α < 0.05.
The downwind approach strategy normally exhibited by Cataglyphis ants reduces foraging path lengths. Foraging ants that missed a familiar feeder on the upwind side (a) needed an additional 9.7 m walking distance in their resulting search, on average, compared to downwind approaches by the same individuals (b) with the final trajectories guided by the odor plume emanating from the food. Twelve foraging runs of four ants (between two and five runs per ant) were selected where the animals had happened to miss a familiar feeder on the upwind side in a (probably because of little familiarity with the feeding site, ants missed the feeder mostly during the initial few visits; the initial eight visits were evaluated here). For comparison, the immediately preceding or following 12 runs—depending on which was closer in time—of the same animals leading into the downwind area were evaluated in b. Nest–feeder distance was 5.7 m, and the average path lengths were 17.0 (a) and 7.3 m (b)
Results
Shortening of the desert ants’ downwind approach over time
Cataglyphis ants forage individually, even if they encounter plentiful feeding sites that merit repeated visits. Foragers memorize such feeding sites and visit them regularly over long time periods. It has been noted previously that the approach trajectories of novice ants change and apparently shorten, in the course of the initial few visits to such feeders (Wolf and Wehner 2000).
This is illustrated in Fig. 1, which shows five approach trajectories of an individual Cataglyphis forager that followed its original chance encounter of the feeder (visit no. 0). With the prevailing eastern wind direction (indicated by open arrow), the ant did not approach the feeder directly but steered between 1.5 and 0.5 m downwind and turned against the wind upon encountering the odor trail emanating from the food (see also Wolf and Wehner 2000). The distance steered downwind of the food source decreased noticeably during the initial few visits. Comparison of the fourth and the seventh visits illustrates that after about four visits, there was not much further change. In fact, approach paths of some individuals were consistent to within a few cm (±5 cm, where determined) once the animals had visited the feeder for about 1 day. The reasons for remaining variations in approach trajectories appeared to be primarily changes in the foraging situation. Examples are, roughly in order of their frequency of occurrence, homebound nest mates carrying food items that distracted outbound ants by their odor, stray food items blown out of the feeder by wind gusts, wind gusts themselves that would occasionally even lift animals off the ground and carry them over a short distance, an occasional dead insect blown into the experimental area, and attacks by predators (mostly asilid flies).
Changes in approach trajectory, especially the distance steered downwind of the food source, did not just occur when establishing a new feeding site. They were also observed in experienced individuals when moving the feeder to a different location. That is, the term “novice” ant in the present context applies to the experience with regard to a given feeding site, not experience as a forager per se (all ants examined in this study were experienced foragers in the latter sense).
Large numbers of foraging trips were recorded from individually marked desert ants to assess the reduction in foraging costs associated with the above downwind approach and the gradual shortening of the ants’ approach trajectory. Two sets of evaluations were made from these data.
First, the ants indeed occasionally missed the feeding site, most often during their initial few visits to the feeder. This was true in particular for small feeders, sunk into the ground to avoid visual prominence (Petri dishes 2.5 cm in diameter and pressed into the substrate; see “Materials and methods”). When an ant walked past the feeder on the upwind side, it thus had no chance to notice the food, neither by odor nor by visual cues. In this situation, the animals started a search for the food after they had walked some 0.5 m or more past the feeding site. These searches were concentrated on the area downwind of the feeder and often exhibited walking trajectories oriented more or less perpendicular to the prevailing wind direction (Fig. 2a; see also Wolf and Wehner 2000). These searches accumulated up to 30.5 m walking distance before the food was encountered, as compared to 5.7 m nest–feeder distance. A more appropriate comparison, however, is that between the length of the approach path when the ant had missed the feeder and the path length covered by the same individual on its immediately preceding (or subsequent) successful downwind approach. Such a direct comparison is provided in Fig. 2a,b. With 5.7m nest–feeder distance, the ants traveled 17.0 m (SD ±6.2 m; n = 12, N = 4), on average, to reach the feeder on a search trajectory. A successful downwind approach, by contrast, took just 7.3 m (SD ±1.0 m; n = 12, N = 4). The difference between the two situations was significant (when evaluating mean values of the four individuals, paired t test, t = 3.75, N = 4, P = 0.009; when considering all runs separately, paired t test, t = 5.77, n = 12, P = 0.000075; details of statistic analysis in “Materials and methods”), and it amounts to an excess of 233% walking distance when missing the feeder on the upwind side.
The additional searching distance that became necessary when an animal passed the feeding site in the upwind direction, thus missing the odor cue, did not appear to differ with nest–feeder distance (9.7 m with 5.7 m nest feeder distance, above; 10.4 m with 11.3 m nest–feeder distance; n = 5, N = 3). This would make search effort relatively less expensive with increasing nest–feeder distance. Definitive statements are not yet possible, however, because Cataglyphis is usually quite accurate in locating a feeder, making the data shown in Fig. 2a the exception, rather than the rule.
In a second assessment, the reduction in foraging costs associated with the gradual shortening of the ants’ approach trajectory was determined. The outbound paths of 24 individuals were followed from their first encounter with the feeding site for at least eight further visits. To obtain a quantitative measure of the changes in approach trajectory, not only downwind distance d was determined for each approach but also the length and the curvature of the approach path (Fig. 3). As noted above, there was a marked change with experience, that is, number of feeder visits, with regard to all three parameters.
Changes in Cataglyphis’ downwind approach to a feeder during the initial visits. Data obtained from 24 marked individuals are shown, distance d steered downwind of the feeder in a, path length l of the approach in b, and curvature c of the approach trajectory in c; foraging trip number n on the abscissa. Stippled line in a marks 1.25 m, the downwind distance to be expected from previous reports. Path lengths in b were determined from a 4-m circle surrounding the feeder; that is, 4 m would have been the minimum distance possible (stippled line). In c, the angular deviations from a straight course were cumulated every 6 cm of the recorded approach trajectory (see “Materials and methods”); after trip no. 3, the curvature stabilizes around 12.3π, corresponding to slightly more than six full circles. Nest–feeder distance was 15 m. Data points represent means and standard deviations. Exponential fit lines are shown that were calculated from the original data set (d 0 set to 1.25 m in a; sample equation provided below graph to explain parameters). Parameter values for the fit lines are given in the top right corner of each graph, number of animals (n = 24) in the top line, significance level P of the fit in the second line, values for the exponential fit lines in the lines below: exponential fit line in a is described by the term \(d = d_0 + A \times e^{ - \left( {{n \mathord{\left/ {\vphantom {n \tau }} \right. \kern-\nulldelimiterspace} \tau }} \right)} \), corresponding terms in b and c. Note that not just the values decrease with the ants’ increasing familiarity with the feeding site but also the standard deviations, SD. The coefficients of variation cv (SD/mean) are provided for each trip no. to further illustrate this feature. Where significant, the significance levels of contrast sc (see “Materials and methods”) are provided for a given trip number, as contrasted against the pooled data of all subsequent trip numbers. The first visit to the feeder was always a chance encounter and thus is noted as number 0
Downwind distance d (Fig. 3a) stabilized from the fourth visit onward, to values around an average of 1.24 m. This value agrees closely with previous results; for the present nest–feeder distance of 15 m, a downwind distance of 1.25 m would have to be expected according to Wolf and Wehner (2005). The difference in d between subsequent visits stayed constant after about the fifth visit, at about 0.35 m.
Similar to downwind distance, the length of the approach path l (Fig. 3b) stayed more or less constant from the third visit onward. Path length was determined from a 4-m circle surrounding the feeder, where the measurements were started. The length of the approach path started with 21.0 m (SD ±13 m) for visit 0 (being a chance encounter). Visit no. 1 as the first aimed approach averaged 11.8 m (SD ±8.2 m), and path length stayed at 6.8 m (SD ±2.3 m) from the third visit onward. Adding to these values, the minimum path of 11 m required to reach the 4-m circle where the measurements were started, these data produce the realistic approach path lengths of 17.8 and 32.0 m, respectively. This corresponds to an average reduction in approach trajectory to 56% of its starting length or roughly halving of the foraging effort with experience. Nest–feeder distance was 15 m in these experiments, which means that the downwind approach strategy required an additional 2.8 m, on average, compared to a direct approach. Considering that the average downwind distance was 1.24 m (above), not more than some 1.6 m, or roughly 10%, appear to be attributable to the slightly sinusoidal path that is inevitably seen in the ants’ walking trajectories.
The curvature of the approach trajectory c (Fig. 3c) also decreased markedly, reaching a plateau from third visit onward. This result reflects the observation that experienced ants approached the downwind side of the feeder on a fairly straight course, while less experienced foragers, although clearly goal directed, usually followed more tortuous paths.
The stabilization of approach trajectories was also tested by statistical means, for all the parameters presented in Fig. 3a–c. The data for any given trip number were tested against the pooled data for all subsequent trip numbers to reveal possible contrasts (Helmhert contrasts; see “Materials and methods”). This yielded significant differences for the initial approaches, trip numbers 0 to 2 (Fig. 3b,c) or 3 (Fig. 3a; U test, statistical values stated in figure). Later approaches exhibited no significant differences anymore, supporting the view that the approach trajectories stabilize. This is of course also evident from the validity of the exponential fit lines drawn into the graphs of Fig. 3, as indicated by their significance levels of P < 0.001 (t test, t values 7.69 [a], 13.94 [b], 12.10 [c], n = 192 each).
Figure 3 further provides the coefficient of variation (cv = SD/mean), in addition to the standard deviation, for all the data points. This is done because in navigation performance, standard deviation often scales with the mean, resulting in a constant cv (details in Cheng et al. 1999; Merkle et al. 2006; see also “Discussion”). In agreement with the above observations, the cv is roughly constant from the third trip onward, except for downwind distance that exhibits a more or less constant cv right from the beginning.
Different strategies contribute to desert ants’ shortening of their approach path
The data and observations presented above demonstrate that Cataglyphis ants shorten their approach to a feeding site through learning, in terms of time and distance invested per food item. What remained open, however, were the orientation parameters the animals used to improve their approach trajectories.
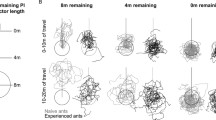
The most intriguing data set bearing on these questions was obtained in ants that had to travel almost with the wind, at least most of the time, to reach the feeding site. Even in this adverse situation, most ants stick to their idiosyncratic approach paths acquired through experience (Wolf and Wehner 2000). A few individuals proved more flexible, however (Fig. 4). In the present experiment, individually marked ants exploited a feeder located downwind of the nest, about 45° left of the prevailing wind direction. In this situation, most ants followed the downwind approach strategy described above; that is, they aimed into an area up to 1 m downwind of the feeder on straight paths (similar to the trajectories shown in Fig. 4b; in this particular situation that resulted in a counterclockwise turn of the animals from their initial approach trajectory to the final path against the wind). A few individuals chose indirect approaches of the downwind area, half-circling the feeder after an initial trajectory leading into the upwind area (similar to the trajectories shown in Fig. 4a; in the present situation, this resulted in an almost three-quarter clockwise turn around the feeder location). These ants were usually attracted to the feeder after they had previously exploited a sector of the nest surrounds located in this (upwind) range.
Comparison of different downwind approach trajectories, clockwise in a and counterclockwise in b. 47 pairs of foraging trips of six ants were selected, where clockwise and counterclockwise approach trajectories from a given ant were performed immediately after one another. Nest–feeder distance was 5.7 m. Details in text
Six animals (experienced because familiar with the feeding site for at least 4 days) switched between the clockwise and counterclockwise approaches. That is, their final approaches of the feeder either took the shape of a clockwise three-quarter circle (Fig. 4a) or, more frequently, that of a counterclockwise quarter circle (Fig. 4b). The foraging trips shown in Fig. 4a,b were selected such that they form pairs of clockwise and counterclockwise approaches performed immediately after one another by the same individual. Naturally, the walking distances differed between the two sets of approach strategies, averaging 8.6 and 6.7 m, respectively. More notably, the directions of the final, odor-guided approaches were also different. These directions closely reflect current wind directions (Wolf and Wehner 2000). The final approaches averaged 277° (SD ±18°) for the clockwise, and 285° (SD ±19°) for the counter-clockwise trajectories. These differences were significant when evaluating the pairs of approaches made by the two animals that yielded the largest data sets (paired t test, t = 3.161 and t = 3.039, n = 12 and n = 12, P = 0.009 and P = 0.013; the differences were also significant when pooling all available data; paired t test, t = 2.015, n = 47, P = 0.049). Finally and most importantly, the ants’ walking directions when leaving the nest also differed between the two situations. The outbound paths of all ants averaged 232° (SD ±4°) for the clockwise and 237° (SD ±5°) for the counterclockwise approaches. Differences were again significant when analyzing the two individuals yielding the largest data sets (paired t test, t = 4.646 and t = 4.289, n = 12 and n = 12, P = 0.0009 and P = 0.003).
For the counterclockwise approaches at least, the course on which an ant left the nest was correlated significantly to the direction in which its final approach to the feeder was directed, that is, to current wind direction (r 2 = 0.37; t test, t = 2.707, n = 47, P = 0.0053). Together with the above data, this indicates that the ants decided already upon leaving the nest whether to approach the feeder on a clockwise or a counterclockwise trajectory, depending on the prevailing wind direction during that particular foraging trip. When the wind was blowing from southeastern directions, the ants preferred a counterclockwise approach, while clockwise approaches predominated in situations where wind directions were from the east, and this relationship held for different ants as well as for different foraging trips of the same individual.
While the previous data set regards small fluctuations in current wind direction, an ability of desert ants to respond to changes in wind orientation cues was also evident when major changes in wind direction occurred. Such changes happen most reliably during the night. In the experimental area near Maharès, eastern wind directions prevail during the day, while in the early morning, there is often a soft northern wind. This natural change in wind direction was exploited to examine the first foraging trip in the morning of ants that had been familiar with a feeding site from the previous days. The feeder was located roughly to the southwest of the nest, and the animals had visited the feeder previously with the normal eastern wind blowing.
Figure 5 shows the respective approach trajectories of five ants. One sample trace is shown for each animal to illustrate the characteristic downwind approaches during the day of training (stippled line traces in Fig. 5), with their final trajectories pointing against current wind directions, showing south- to eastward trajectories. The first visits of the same ants in the following morning are shown for comparison, performed while a northern wind was blowing (solid line traces in Fig. 5). The ants initially followed their idiosyncratic outbound paths from the nest, located in the northern part of the nest surrounds, and directed toward the daytime downwind area with regard to the feeder. This is discernible in the figure by the fact that all initial path segments run north to the heavy black line that marks the nest–feeder axis. Individual ants are identified by symbols drawn next to the truncated path segments. This further illustrates that the animals tend to keep to their idiosyncratic paths close to the nest, for instance, the ant indicated by the open square following the northernmost trajectory and the animals marked by cross and open circle the southernmost paths. The ant marked by the triangle took an approach trajectory in between. Some 5 to 11 m from the nest, however, four ants gradually shifted their approach and crossed the nest–feeder axis into the actual downwind area of that morning. Because of the prevailing northern wind, the animals thus aimed into an area on the opposite side of the feeder than on the previous evening, and even the one ant that walked past the feeder on the northern side (filled circle, thinnest set of lines) took up its subsequent search immediately in an area appropriate for the current wind direction. It may be significant that this was the one ant that, although following a southerly trail close to the nest, had aimed into the northernmost area close to the feeder on the previous afternoon. It was thus the ant that had to change its approach trajectory the most. In summary, these observations demonstrate that the ants are able to adjust their approach to a memorized feeder location en route to the present wind condition. Similar pairs of trajectories were recorded in another three ants (not shown).
Ants remember the location of a feeder overnight and apparently account for nocturnal changes in wind direction. Five pairs of approach trajectories are shown, each set performed by a different ant; individuals are identified by symbols near the truncated path segments and by different line thickness. Feeder location is marked by 40-cm circle (grid line spacing 1 m), nest located on the heavy (vertical) marker line 15 m NE of the feeder. Approach trajectories drawn as stippled lines were performed in the late afternoon, with the wind blowing from eastern to southern directions; trajectories clipped 11 m from the nest. Approach paths drawn in black were the first foraging runs performed in the following morning (07:00–08:30 hours), when the wind was blowing softly from northern directions; trajectories clipped 6 m from the nest. Note gradual shift of the approach paths toward the downwind side of the feeder beyond about 7 m from the nest, except for one individual. Details in text
As noted above, ants usually rely strongly on their idiosyncratic paths close to the nest, notwithstanding gradual adaptations over time with changes in the foraging situation. This also means that experiments like the one shown in Fig. 5 were not possible with nest–feeder distances below about 10 m. In such situations, the ants tend to search for the food after covering more or less their normal—idiosyncratic—approach trajectory, without a discernible reorientation according to changed conditions. This is illustrated in Fig. 6, where ants were trained to a feeding station just 5 m south of the nest. The animals adopted their characteristic downwind approach, with downwind distance d ranging between 0.5 and 1 m. During brief time periods with virtually still air (Fig. 6b), the ants still followed their usual downwind strategy, instead of attempting a direct approach (see Wolf and Wehner 2000). However, their approach paths were slightly more tortuous. With their antennae immobilized and thus their main sense of wind perception eliminated (Fig. 6c), the ants did not attempt a direct approach either. They rather followed their normal outbound trajectories and started searching for food as soon as they entered the area where they would normally have encountered the odor plume. This behavior was apparently not dependent on the actual odor being present, however (i.e., with the food removed in test situations; see Wolf and Wehner 2000). This is even more evident in the three recorded trajectories of Fig. 6d, where the wind was blowing from south–southwest, rather than the normal south–southeast direction. This means that the ants found the feeder without encountering odor cues in this case, as those were blown away from the animals in the present situation.
In almost still air or with their main sense of wind direction eliminated, Cataglyphis ants appear to rely primarily on their knowledge of the nest surrounds when approaching a close-by feeding site (5 m nest–feeder distance). a Sample recordings of approaches of eight ants during a training period of several days (same individuals as in following figure parts). The characteristic downwind approach strategy is discernible, with the final approach against the wind. b Approach trajectories of the same ants in the late afternoon of a day with unusually low wind speeds, ranging below 0.1 m/s (light wind arrow) during the recordings shown. Before the trajectories shown in c and d were recorded, the bases of the animals’ antennae had been immobilized, eliminating the animals’ main sense for wind direction (Wolf and Wehner 2000). Wind direction was from SSE in c and from SSW in d. The searches clearly cover the area that was downwind of the feeder during the training period
Discussion
The present results illustrate that desert ants, C. fortis, exhibit as yet unknown aspects of flexibility and notable adjustment strategies when repeatedly visiting a plentiful food source. The animals usually do not follow a straight path from nest to feeder but rather steer a downwind course (Fig. 1). They pick up odor cues emanating from the food once they reach the actual downwind area and follow the odor trail upwind to the source. The location of the feeder is memorized during the first encounter (Wolf and Wehner 2000; see also Fig. 1) and employed during later visits. The approach trajectory to the feeder, however, is refined over time. In the course of the initial three to five visits, all examined parameters decreased toward equilibrium values that stayed fairly constant further on. These parameters are downwind distance, total path length, and curvature of the approach path (Fig. 3; compare also Fig. 1). At least three visits were needed to establish constant navigation performance and thus presumably route memory. This is in agreement with previous reports, including another ant species (Australian Melophorus bagoti; Cheng et al. 2006; Narendra et al. 2007; Wolf and Wehner 2000, 2005).
Despite the refined downwind approach strategy, individual desert ants usually do not follow a straight trajectory from the nest to the area downwind of the food, particularly close to the nest. Observation indicates that the animals rather follow idiosyncratic paths when leaving the nest, apparently guided by knowledge of the microstructure of the nest surrounds (see also Fig. 5; Seidl and Wehner 2006).
The approach trajectories reached after the abovementioned adjustments appear to serve good foraging efficiency, that is, represent a compromise between successful arrival at a small food source—without extended searching in the face of navigation uncertainties—and a short approach path length—saving time and energy and minimizing heat stress and predation risk. On the one hand, an appropriately scaled downwind approach is essential for effective foraging. With a direct approach, a small food source would often be missed because of navigation errors (in fact, with almost 50% probability where the feeder diameter is small compared to navigation uncertainty). This problem is illustrated clearly by the fact that the search that is necessitated when an ant misses the feeder on the upwind side is much longer, in fact, several times the detour needed by the downwind approach strategy (Fig. 2). It has been demonstrated previously that the downwind approach strategy employed by desert ants compensates for their navigation uncertainty (Wolf and Wehner 2005). The downwind distance assumed after extended familiarity with the food source appears to be just above the range of the ants’ navigation errors, thus leading the animals safely into the downwind area where they pick up the odor trail. On the other hand, the detour caused by the downwind approach should not exceed the range necessitated by the navigation error because any detour is a potential risk.
How is the approach path shortened, the appropriate downwind distance assumed? What is the error signal that the ants employ to judge inappropriate trajectories and adjust their outbound course accordingly? These questions cannot yet be answered conclusively, although there are several pieces of evidence bearing on the problem. First, the mere fact that the ants occasionally miss the feeder on the upwind side (Fig. 2) might suggest that they use these occasional major errors to adjust their approach paths. Second, the ants determine walking distance quite accurately in the context of navigation. This would enable them to not only measure walking distance to a food source but also evaluate it in the context of approach path shortening. The concomitant decrease in all parameters examined, namely downwind distance, approach path length, and trip curvature (Fig. 3), demonstrates that the animals do not just shorten downwind distance per se but instead improve the complete approach trajectory toward path minimization. This further suggests that during repeated visits to the feeder, ants improve their assessment of both feeder location and knowledge of the approach path. Reduction in downwind distance may thus also reflect a decrease in the ants’ navigation uncertainty and not just path shortening with regard to a pre-existing level of navigation performance. At present, this interesting possibility has to remain speculative, although learning of small features of the approach path—such as pebbles or substrate structure—appears as a distinct possibility.
An indication that points in this latter direction is the decrease in the cv with experience in Fig. 5, at least with regard to trajectory length (Fig. 3b) and trip curvature (Fig. 3c). In navigation performance, such as odometry, standard deviation often scales with mean values, yielding a constant cv (Cheng et al. 1999, 2006; Merkle et al. 2006; Wolf and Wehner 2005). This is immediately evident when assuming that in an odometer that is implemented as a stride counter (Wittlinger et al. 2006, 2007), for example, the error margin is 10% of each stride’s length. Such scaling of the error with the measurement mechanism will directly produce a constant cv, and the fact that the coefficient decreases and stabilizes along with the other characteristics of navigation performance indeed points toward an improved navigation accuracy, whatever the underlying mechanism.
In addition to the adjustments made in the course of several visits to a feeding site, desert ants are also able to adjust their approach to a well-known feeding site ad hoc, in response to changes of variable orientation cues namely, current wind direction. This is illustrated by minor changes in the ants’ outbound trajectories that are elicited by fluctuations in current wind direction during the day and that result in rather different final approaches of the feeding site (Fig. 4). It is also illustrated by the considerably altered approach trajectories that may be observed in the morning, in response to major changes in ambient wind direction and wind strength during the night (Fig. 5). It should be noted in this context that desert ant memory decays slowly and certainly outlasts one night. For instance, traces of the return path from feeder to nest persisted over at least 4 days in experiments where captured foragers were detained for variable time periods (Ziegler and Wehner 1997; see also Cheng et al. 2006).
The desert ants’ capacity for short-term flexibility appears to be employed mainly for nest–feeder distances beyond the immediate nest surrounds, where landmarks are not well known to the animals and thus not much heeded, at least in normal foraging situations (Fig. 6; Bisch-Knaden and Wehner 2003; see also Wehner et al. 2002). These examples further illustrate that—close to the nest—a route established in the context of certain wind conditions may later be followed independent of the current wind situation. Similar substitution and stabilization of orientation behavior by learned features has previously be termed route scaffolding (Collett et al. 2003).
The present results demonstrate a remarkable flexibility of the orientation behavior of desert ants toward food sources, including the appropriate integration of learned feeder positions and current wind conditions (e.g., Fig. 5). The data further demonstrate ecological significance of the experience-dependent changes in the approach to a feeding site. The final approach route enables fast and efficient foraging performance, achieved by surprisingly fine-grained behavioral adjustments (Fig. 3). The animals appear to strike a good compromise between safe arrival at the food source—which incurs the cost of additional path lengths necessitated by the downwind approach—and parsimonious investment in foraging time, heat stress, and predation risk (data in Fig. 2).
There are several examples of an adjustment of orientation toward food sources with experience, often involving active orientation mechanisms (see Introduction; also Collett et al. 2003). The turn-back-and-look behavior exhibited by several hymenopteran insects is a good example here. This behavior is used to sample landmark arrangements surrounding a new goal, either a feeding site or the nest (Lehrer 1993; Zeil et al. 1996). Memorizing the landmark array surrounding a particular goal probably allows for fast and safe visits in the sense outlined above, and the present account provides at least some initial data to support this assumption.
References
Batschelet E (1981) Circular statistics in biology. Academic, New York
Bisch-Knaden S, Wehner R (2003) Landmark memories are more robust when acquired at the nest site than en route: experiments in desert ants. Naturwissenschaften 90:127–130
Cheng K, Srinivasan MV, Zhang SW (1999) Error is proportional to distance measured by honey bees: Weber’s law in the odometer. Anim Cogn 2:11–16
Cheng K, Narendra A, Wehner R (2006) Behavioral ecology of odometric memories in desert ants: acquisition, retention, and integration. Behav Ecol 17:227–235
Collett M, Collett TS, Wehner R (1999) Calibration of vector navigation in desert ants. Curr Biol 9:1031–1034
Collett TS, Graham P, Durier V (2003) Route learning by insects. Curr Opin Neurobiol 13:718–725
Etienne AS, Maurer R, Berlie J, Reverdin B, Rowe T, Georgakopoulos J, Séguinot V (1998) Navigation through vector addition. Nature 396:161–164
Forel A (1902) Beispiele phylogenetischer Wirkungen und Rückwirkungen bei den Instinkten und dem Körperbau der Ameisen als Belege für die Evolutionslehre und die psychophysische Identitätslehre. J Psychol Neurol 1:99–110
Fresneau D (1985) Individual foraging and path fidelity in a ponerine ant. Ins Soc 32:109–116
Harrison JF, Fewell JH, Stiller TM, Breed MD (1989) Effects of experience on use of orientation cues in the giant tropical ant. Anim Behav 37:869–871
Lehrer M (1993) Why do bees turn back and look? J Comp Physiol A 172:549–563
Merkle T, Knaden M, Wehner R (2006) Uncertainty about nest position influences systematic search strategies in desert ants. J Exp Biol 209:3545–3549
Müller M, Wehner R (1988) Path integration in desert ants, Cataglyphis fortis. Proc Natl Acad Sci USA 85:5287–5290
Narendra A, Cheng K, Wehner R (2007) Acquiring, retaining and integrating memories of the outbound distance in the Australian desert ant Melophorus bagoti. J Exp Biol 210:570–577
Nicholson DJ, Judd SPD, Cartwright BA, Collett TS (1999) Learning walks and landmark guidance in wood ants (Formica rufa). J Exp Biol 202:1831–1838
Sachs L (1992) Angewandte Statistik. Springer, Berlin
Schmid-Hempel P (1983) Foraging ecology and colony structure of two sympatric species of desert ants, Cataglyphis bicolor and Cataglyphis albicans. Dissertation, Universität Zürich
Seidl T, Wehner R (2006) Visual and tactile learning of ground structures in desert ants. J Exp Biol 209:3336–3344
Sokal RR, Rohlf FJ (1995) Biometry: The principles and practice of statistics in biological research, 3rd edn. W. H. Freeman, New York
Sommer S, Wehner R (2004) The ant’s estimation of distance travelled: experiments with desert ants, Cataglyphis fortis. J Comp Physiol A 190:1–6
Wehner R (1983) Taxonomie, Funktionsmorphologie und Zoogeographie der saharischen Wüstenameise Cataglyphis fortis (Forel 1902) stat. nov. Senckenbergiana Biol 64:89–132
Wehner R (1987) Spatial organization of foraging behavior in individually searching desert ants, Cataglyphis (Sahara Desert) and Ocymyrmex (Namib Desert). In: Pasteels JM, Deneubourg JL (eds) From individual to collective behavior in social insects. Birkhäuser, Basel, pp 15–42
Wehner R (1992) Arthropods. In: Papi F (ed) Animal homing. Chapman and Hall, London, pp 45–144
Wehner R (1996) Middle scale navigation: the insect case. J Exp Biol 199:125–127
Wehner R (2003) Desert ant navigation: how miniature brains solve complex tasks. J Comp Physiol A 189:579–588
Wehner R, Srinivasan MV (1981) Searching behaviour of desert ants, genus Cataglyphis (Formicidae, Hymenoptera). J Comp Physiol 142:315–338
Wehner R, Wehner S (1986) Path integration in desert ants. Approaching a long-standing puzzle in insect navigation. Monit Zool Ital (NS) 20:309–331
Wehner R, Harkness RD, Schmid-Hempel P (1983) Foraging strategies in individually searching ants, Cataglyphis bicolor (Hymenoptera, Formicidae). Fischer, Stuttgart
Wehner R, Gallizi K, Frei C, Vesely M (2002) Calibration processes in desert ant navigation: vector courses and systematic search. J Comp Physiol A 188:683–693
Wittlinger M, Wehner R, Wolf H (2006) The ant odometer: stepping on stilts and stumps. Science 312:1965–1967
Wittlinger M, Wehner R, Wolf H (2007) The desert ant odometer: a stride integrator that accounts for stride length and walking speed. J Exp Biol 210:198–207
Wolf H, Wehner R (2000) Pinpointing food sources: olfactory and anemotactic orientation in desert ants, Cataglyphis fortis. J Exp Biol 203:857–868
Wolf H, Wehner R (2005) Desert ants compensate for navigation uncertainty. J Exp Biol 208:4223–4230
Zeil J, Kelber A, Voss R (1996) Structure and function of learning flights in ground-nesting bees and wasps. J Exp Biol 199:245–252
Ziegler PE, Wehner R (1997) Time-courses of memory decay in vector-based and landmark-based systems of navigation in desert ants Cataglyphis fortis. J Comp Physiol A 181:13–20
Acknowledgments
Kathrin Steck, Nadine Banerjee, and Selina Bucher assisted with data collection in the 2002 and 2005 field seasons. Ursula Seifert provided skilful help with collating the data and finishing the figures, and Wolfgang Mader and Uwe Rose were indispensable help for statistic analyses. Financial support was provided by the Volkswagen Stiftung (I/78 580) and the University of Ulm.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Beekman
Rights and permissions
About this article
Cite this article
Wolf, H. Desert ants adjust their approach to a foraging site according to experience. Behav Ecol Sociobiol 62, 415–425 (2008). https://doi.org/10.1007/s00265-007-0469-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-007-0469-0