Abstract
Pheromones may convey information about mate quality and social status. In the field cricket Gryllus integer, females mount the males for copulation, such that males cannot coerce females to mate. We examined whether virgin G. integer females preferred the scent of potentially dominant males to that of subordinate males. First, we collected pheromones by confining males on filter paper. Next, we offered filter paper from each of two size-matched males and control paper to females that had never been exposed to males, and measured the time spent by the female on each kind of paper. Finally, dominance status of the males in each size-matched pair was determined by pitting the two males against one another in agonistic contests. When offered filter paper from subsequently dominant versus subsequently subordinate males, females spent more time on the paper from the dominant male than the subordinate male, and much less time on control paper. Thus, pheromones may inform female G. integer about a male's potential to achieve dominant social status. Male pheromones were also associated with the female's tendency to mount a male. In contrast to cockroaches, where females prefer the scent of subordinate males (presumably to avoid risk of injury), female crickets prefer the scent of potentially dominant males and are more likely than males to wound their mating partners.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Competition between same-sex individuals dramatically affects reproductive output in many animal species (Andersson 1994), and therefore plays an important role in the evolution of mate selection and secondary sexual ornamentation (Qvarnstrom and Forsgren 1998; Candolin 1999). A male's ability to keep competitors away from potentially receptive females can increase his probability of achieving copulations. This has been shown, for example, in elephant seals (Haley 1994) and black grouse (Alatalo et al. 1991). High dominance rank of a male should increase his fertilization success, since rival males episodically disturb and interrupt copulation (Qvarnstrom and Forsgren 1998). However, it is still not clear whether females should always prefer dominant males as their mates (Qvarnstrom and Forsgren 1998; Kokko 2005). Although these males may offer better resources and/or sire dominant sons, they can also harm females. Nevertheless, male dominance status may provide a cue about male quality (direct and/or indirect (genetic) benefits) to selective females, making it equivalent to the sexual ornaments in some species (Cox and LeBoeuf 1977; Rantala and Kortet 2004).
Sex pheromones can convey information about mate quality and social status (Moore et al. 2001; Rantala et al. 2003a; Wyatt 2003). Pheromones are molecules that are released by an individual into the environment and affect the physiology or behavior of conspecifics (Karlson and Lüscher 1959). Pheromones are among the most commonly used social signals among organisms (Arnold and Houck 1982; Birch and Haynes 1982; Penn and Potts 1998; Rekwot et al. 2001; Wyatt 2003). For example, in cockroaches, females prefer the scent of subordinate males, presumably to avoid the risk of injury from aggressive dominant males. This has been interpreted as an example of evolutionary conflict between the sexes (Moore et al. 2001; Chapman et al. 2003).
In the field cricket Gryllus integer, females are larger than males, and mount the males for copulation, such that males cannot force females to mate. This may diminish the potential for male-dominated sexual conflict to evolve. However, male field crickets (Gryllus spp.) aggressively fight for and defend territorial shelters and females (Alexander 1961; Simmons 1986a; Hofmann and Schildberger 2001; Rantala and Kortet 2003). Female field crickets have strong individual preferences with respect to males, and mate choice is not random (e.g., Hedrick 1986; Hedrick and Weber 1998; Rantala and Kortet 2003, 2004). Females use both male calling and courtship songs as criteria in their mate choice (e.g., Hedrick 1986; Simmons 1986a; Hedrick and Weber 1998; Gray and Cade 1999; Wagner and Reiser 2000; Rantala and Kortet 2003). However, because the mating system in crickets also involves direct male competition with fierce fighting between males, success in competition can directly influence a male's mating success (Simmons 1986b; Rantala and Kortet 2004). Moreover, since success in contests may demand energetic resources, female field crickets may also use a male's position in the dominance hierarchy as a cue to select mates with good body condition. Interestingly, Wedell and Tregenza (1999) found that in G. bimaculatus, males who were successful fighters sired sons that were successful fighters.
Female field crickets can recognize males not only by their calls, but also by using male pheromones (Otte and Cade 1976; Loher and Dambach 1989; Simmons 1990). Moreover, sex pheromones of a Gryllid cricket species, G. bimaculatus, have been characterized using gas chromatography (Treganza and Wedell 1997). Gryllid crickets are able to detect and even memorise certain chemical cues and scents (Matsumoto and Mizunami 2000, 2002; Kortet and Hedrick 2004), suggesting that they often rely on olfaction. However, most previous studies of sexual selection in field crickets have underestimated the role of olfaction, focusing instead on acoustic signalling.
In the present study, we asked whether female field crickets (G. integer) could use pheromone cues to distinguish among males, and specifically, whether we could use pheromone preferences of females to predict the outcome of male–male competition. According to the theory of honest sexual signaling (Zahavi 1975; Grafen 1990), we hypothesized that the pheromone cues left by future winners of intra-sexual contests should be preferred by females over pheromones left by future losers. To test this hypothesis, we collected pheromones and/or other substances, including both volatile and non-volatile active compounds such as cuticular pheromones (hereafter, collectively referred to as “pheromones”) by confining males on filter paper. Then, we offered filter paper from the two size-matched males, plus a control paper (no male scent) to females that had never been exposed to these males, and recorded their time spent on each kind of paper. Finally, we determined dominance status of the males by staging contests between males. In addition, we also studied whether male pheromones are associated with a female's tendency to mount a male.
We also evaluated aggressiveness between the sexes and inter-sexual dominance of G. integer by recording the number of incidents in which male and female crickets wounded or presumably killed their mates (i.e., their mates were found dead) during five-day mating periods. These additional data were collected to confirm previous unpublished observations about the aggressiveness of females towards their male mating partners.
Methods
Crickets
The crickets used in this experiment were the first laboratory generation derived from wild-caught G. integer females (August 2003) collected from Davis, California. Wild-caught females had been inseminated in the field before capture. Laboratory crickets were maintained at 25±1°C with ad libitum food (Purina chick starter) and water, under a 12:12 h light/dark photoperiod. Experimental crickets were removed from bulk family boxes as nymphs (approximately 1/4 adult size) and reared individually (also with ad libitum food and water) in waxed cardboard cups. Individuals of both sexes were physically, but not acoustically, isolated from other individuals to ensure virginity. Both males and females were approximately three weeks past the final adult moult on the first day of the experiment. Before the experiments, we weighed the fresh body mass of the crickets to the nearest 0.0001 g. No cricket was used in more than one experiment. In previous studies with G. bimaculatus, a related species of G. integer, male weight affected fighting success (Hofmann and Schildberger 2001). A similar effect of body size on male fighting ability was found in house crickets, Acheta domesticus (Savage et al. 2005). Therefore, we matched male contestants by weight in our study.
Collection of pheromones
Two weight-matched males (at least 98.7% similarity in fresh weight, n=59 pairs of males) of about the same age (mean difference between males within pairs = 2.9±0.33 (SE) days) were chosen to form each experimental pair. To collect pheromones from the crickets, we placed each male in a small (60 mm diameter) petri dish containing a filter paper disc (58 mm diameter) for 24 h (method modified from pheromone collection by Rantala et al. 2002, 2003a). To test the female's preferences for the pheromone cues from males, we presented each female with three filter paper discs. Two contained pheromones from each of two males, and one was a control (clean) piece of filter paper. All of the filter discs were used within 2 h after the removal of the male from the disc, so that the age of the pheromone cue would not affect female preferences. The filter paper was continuously kept inside the petri dish to prevent any chemical cues from dispersing. The arena for female preference trials consisted of a 17 cm diameter plastic box (L17 cm × W17 cm, depth 10 cm). The paper discs were chosen haphazardly from the petri dishes (without reference to male identity), and then arranged in the center area of the arena, such that they were equidistant (each approximately 2 cm apart) and at least 2 cm from the sides of the arena. A female cricket was allowed to acclimate for 4 min under a plastic vial, and then released in the middle of the arena under dim red-light illumination (25-W red incandescent bulb at 60 cm) to mimic nocturnal conditions. Each trial lasted for 10 min, during which time the cricket's movements were observed and recorded using a software program for recording behavioral data (AV Bio-Statistics 4.4, available at http://www.cc.jyu.fi/~ansvain/avbs/). We used three different measurements from the resulting data to examine female preferences for male scents. First, we measured the total time that a female spent on each disc during the experiment (male A, male B, control), and used this time as a measure of her preference for that particular disc. Because the discs covered only approximately 27% of arena area and were positioned away from the sides of the arena where females tended to travel, the females did not necessarily spend the entire 10 minute trial inspecting discs. In fact, some females spent the majority of their time moving around the edges of the arena. Thus, the total time spent on all of the discs did not necessarily equal the total time of the trial.
Second, because some females remained immobile for a relatively long time after starting the experiment, we noted the time point when females first began moving in the arena. We then defined the female's “time spent active” as the time that elapsed between the time point when she first moved in the arena to the end of the trial. We then divided the female's total time spent on each disc by her total time spent active, to give a measure that controlled for the potential effect of a female's overall levels of behavioral activity on her disc preferences.
Finally, we also measured how long a female spent on a disc during individual visits to the three different kinds of filter paper, relative to her total time spent active. We then computed for each female a mean duration for visits to filter paper of future dominant versus future subordinate males. We regarded longer mean relative time durations on a particular disc as an indication of female preference.
Intra-sexual dominance test procedure
The day after pheromone collection, the size-matched experimental males (n=59 pairs) were marked on the pronotum with enamel paint to allow recognition in trials. Both males within a pair and one female (to trigger males to fight) were introduced into a sand-covered plastic arena under red-light illumination (L20 cm × W20 cm, depth 20 cm; method modified from Rantala and Kortet 2004). Females were used because our pilot trials suggested that at least some males needed a female present before they would become aggressive at all. Preceding the trial, crickets were placed for three minutes in the arena under plastic vials to calm them. After removal of the vials, the males usually started fighting immediately. Each trial lasted for 6 min, during which time the male-male contests were observed. The room temperature was kept at 26±1°C. To control for possible fluctuations of aggressiveness in a 24-h day, we conducted the experiments between 15:00 and 22:00 h. Dominance status of each male was determined by the number of times he won aggressive encounters, i.e., fights (defined as continuous physical contact that included wrestling or biting). This was easy to observe because after a fight, one of the crickets retreated (i.e., moved away from the other male) and showed avoidance behavior (i.e., withdrew whenever the other cricket approached). We regarded retreat and avoidance as signs of submission (losing a fight). The male that won more fights was scored as the dominant one within a pair. In one trial, males did not fight, so that trial was excluded from the data. Females (n=59) were different than the females used in the pheromone trials. Females were not allowed to mate; instead they were immediately separated from males using a long thin stick (30 cm length, 6 mm diameter) if they started mounting a male. However, we recorded the instances of mountings by females, and noted which males they mounted. Generally, females did not attempt to mount males until fighting had ceased, and they did not intervene in fights.
Inter-sexual dominance and aggression
To evaluate inter-sexual dominance and general aggressiveness between the sexes in G. integer, we recorded the number of times that either a female or male cricket ‘wounded’ or ‘presumably killed’ its mate (determined by the number of wounded or dead animals, respectively) during a five-day mating period (n=60 mating pairs). We used the extended five-day period to ensure that matings would occur, and to detect possible gender differences in aggressiveness. All of the crickets used in mating trials were virgins that had experienced similar conditions previous to the mating trials, and were non-siblings. They were about three weeks past the final adult moult on the first day of the experiment. The crickets were chosen randomly with respect to size, and represented natural variation in size within the species. Each cricket was used in only one mating experiment. Matings were conducted inside a plastic box (L19 cm × W12 cm, depth 5 cm), that contained a place to escape from agonistic interaction (crumpled paper), a food cup and a water vial. The crickets were fed ad libitum, since in G. bimaculatus the amount of food available affects the aggressiveness of females (Adamo and Hoy 1995). The crickets were checked and their condition noted both before and after mating. The injuries sustained during agonistic inter-sexual fights included missing pieces of wings, antennas, or in some cases, a leg. We did not observe crickets during the experiment, so we cannot be sure whether the dead crickets were actually killed by his/her mating partner. However, based on the fact that mortality rates for individually housed crickets in our lab were much lower than those in this experiment, and on the high incidence of injuries sustained by crickets in this experiment, we judge it to be likely that these crickets were killed by their mates.
Results
Intra-sexual dominance and female preference for the pheromones
In each trial, males engaged in one to 13 fights to establish their dominance. After the first five fights, one of the males usually won most of the further aggressive interactions, indicating that he achieved dominance status within a pair.
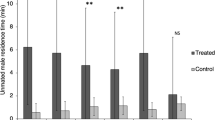
A Friedman test for related samples revealed that the females clearly discriminated among the three classes of filter paper (dominant male, subordinate male, control: χ2=64.877, df=2, N=58, p<0.001; see Fig. 1 for means). The median time females spent on the filter paper with male pheromones (all the males included) was 40.72 s while it was only 1.90 s for the control filter paper. Moreover, out of the 58 trials, 38 of 58 females spent more time on the filter paper from dominant males (the median time 52.37 s) than that from subordinates (the median time 21.83 s) (Wilcoxon signed rank test, one-tailed, Z=−2.164, N=58, p=0.015; Fig. 1). Since some females spent the beginning of the trial immobile, we also examined the time females spent on the filter paper as a percentage of their overall time spent active. The median percentage of their total time spent active on the filter paper with dominant male pheromones was 11.33%, while it was only 5.00% for the subordinate male's filter paper (Wilcoxon signed rank test, Z=2.450, N=58, p=0.008, one-tailed test; see Fig. 2 for means).
Finally, we measured the average duration of female visits to each kind of disc, in relation to each female's overall time spent active. This gave, for each female, an average duration for her individual visits to dominant and subordinate discs, each expressed in terms of a percentage of the female's total time spent active. Across all females, the median percent of a female's time spent active that she spent on individual visits to dominant discs was 3.17%, whereas this value for subordinate discs was 0.06% (Wilcoxon signed rank test, Z=−6.420, N=58, p<0.001, two-tailed test). Thus, females spent significantly more time per visit on paper from potentially dominant males.
Interestingly, despite the fact that we used weight-matched male pairs, dominant males were somewhat lighter than their subordinate partners (dominant median weight, 0.3570 g; subordinate median weight, 0.3585 g; Wilcoxon signed rank test, Z=2.497, N=58, p=0.013, two-tailed test), indicating that neither their success in fights, nor female preference for their pheromones was based on larger body mass. The latter conclusion was also confirmed by lack of correlation between male body mass and the time a female spent on his filter paper (Spearman r=−0.082, N=116, p=0.384) and between body mass and the time on the filter paper in relation to the female's overall activity (Spearman r=−0.049, N=116, p=0.598).
In our trials, 18 females out of the 58 preferentially mounted one male over the other (mounted him more often, a median of 2 times, than she mounted his rival, a median of 0 times). Paired t-tests revealed that the pheromones of preferentially mounted males were preferred over the pheromones of the male that was mounted less frequently (N=18, t=2.043, p=0.029, one-tailed test). Mean time on the filter paper for the preferentially mounted male was 79.89 s, while it was 46.35 s for the non-preferred male of the trial. We found a similar result regarding female preference to mount a male whose pheromones were preferred when we measured the time on the filter paper as a proportion of the females' overall time spent active (N=18, t=2.560, p=0.010, one-tailed test). Mounting generally did not occur until males had finished fighting, so we judge it unlikely that mounting influenced the outcome of male competition. However, our data on mounting is not independent of the data on male fights, and more subtle aspects of female behavior may have influenced male competition.
Inter-sexual dominance and aggression
Females wounded their male mating partners in 48 of 60 five-day mating trials, while males wounded their female partner in 11 trials out of 60. Moreover, males were found dead 14 times (likely killed by their female partners), whereas females were found dead only 5 times, likely killed by their male partners. These gender differences in wounding and ‘killing’ the opposite sex partner during the five-day mating period were statistically significant (wounding, χ2=23.203, df=1, p<0.001; killing, χ2=4.263, df=1, p=0.039).
Discussion
Female G. integer spent more time (in total) investigating the filter paper discs of males that became dominant than those of males that became subordinate. Females also spent a greater proportion of the time in which they were active in investigating the discs of males that became dominant. Finally, they spent longer durations on these discs before moving off them, relative to the time they spent on discs of males that became subordinate. Thus, females appeared to prefer the scents of males that subsequently became dominant. Additionally, females were more likely than males to injure their mating partners.
To our knowledge, this is the first study in insects indicating that females prefer the scent of males with high fighting ability and the potential for dominant status. Male status in dominance hierarchies, in addition to other sexual ornaments, is likely condition-dependent and can provide a cue to females about direct and/or indirect (genetic) benefits from mating selectively (Cox and LeBoeuf 1977; Andersson 1994; Qvarnstrom and Forsgren 1998; Kokko 2004). In some cases, male-male competition may contribute to the maintenance of honest sexual signalling (Berglund et al. 1996; Candolin 1999). If sexual advertising, such as pheromone production, is costly in intra-sexual contests, and the costs decrease with higher quality of the male, subordinate males may pay higher costs for sexual advertising. Positive relationships between male sexual advertising and male success in intra-sexual dominance have been observed previously in insects, birds, mammals and fish (e.g., Andersson 1994; Berglund et al. 1996; Kortet et al. 2004; Savage et al. 2005).
In vertebrates, social dominance and sexual advertising share the same causal factor. In these animals, androgens such as testosterone yield the commonly observed positive association between dominance and sexual advertising (e.g., Hillgarth and Wingfield 1997). Invertebrates do not have testosterone; instead, these animals adjust reproduction and sexual behavior by an assortment of juvenile hormones (e.g., Gullan and Cranston 2000; Teal et al. 2000; Rantala et al. 2003b). Therefore, concentrations of juvenile hormone or some similar substance may be involved in both male fighting ability and pheromone production. Certain juvenile hormones play an important determining role in dominance hierarchies among social insects (review in Hartfelder 2000), and juvenile hormone type III is associated with pheromone production in cockroaches (e.g., Sreng et al. 1999), fruit flies (Teal et al. 2000) and beetles (Rantala et al. 2003b). However, juvenile hormone type III does not affect male aggressiveness in two related species of field crickets, Gryllus bimaculatus and G. campestris (Adamo et al. 1994), so it is unlikely to be important in G. integer. Exploring the causal factors between social dominance and pheromone production in field crickets awaits further studies.
Gryllus integer females are more likely than males to wound their mating partners. Moreover, they mount the male before mating, such that males cannot force them to mate. Thus, G. integer females can presumably control male aggressiveness during mating, reducing the possibility of serious male-dominated intra-sexual conflict (cf. Chapman et al. 2003). In contrast, cockroach females (Nauphoeta cinerea) prefer the scent of subordinate males, presumably to avoid the risk of injury or manipulation by aggressive dominant males (Moore et al. 2001, 2003). Unlike N. cinerea cockroaches, G. integer females do not have to avoid the chemical cues left by a potentially dominant male. Rather, our results suggest that female field crickets preferentially investigate the scent of a potentially dominant male.
Our data also suggest that females preferentially mount those males whose pheromones are preferred by other females. However, our data on mounting is not independent of the data on male fights, and additional cues besides odor (such as auditory cues) were probably involved in a female's decision to mount a male. It is also possible that females can manipulate the outcome of male–male interactions by favouring or triggering the behavior of certain males over others. If this were the case in our trials, females might have been favouring males whose scent was preferred by other females. However, no female intervention in fights was observed and fights usually were over before a female attempted to mount a male. Our results are similar to recent findings on A. domesticus, where females found the dominant males more attractive (Savage et al. 2005).
Female G. integer may be able to remember the individual chemical cues of potential mates, since Gryllid crickets can detect and even memorise certain chemical cues that are associated with their water and food sources (Matsumoto and Mizunami 2000, 2002). This could be beneficial if chemical cues indicate the quality of the signaller, because selective females can copulate with multiple mates. So far, however, only a few studies have addressed whether chemical cues are important in sexual selection and mate choice in field crickets (Simmons 1990; Treganza and Wedell 1997).
In many species, female prefer dominant males as mates and females may incite competition between males to mate with the most dominant male (Cox and LeBoeuf 1977; Berglund et al. 1996). By preferring pheromones of potentially dominant males, female G. integer may preferentially mate with males that produce dominant sons. In the related cricket, Gryllus bimaculatus, success in dominance hierarchy fights is likely heritable (Wedell and Tregenza 1999).
By preferring the scent of potentially dominant males, female G. integer may prefer males that are not only good fighters, but are also in good nutritional condition and have an effective immune defence. Potentially, those males who were actively moving on the filter papers, were in better condition and correspondingly left more scent traces on a paper. In Gryllus bimaculatus, females prefer dominant males as mates and dominant males have both higher encapsulation rates and higher lytic activities than subordinate males, suggesting that they have had stronger immune systems (Rantala and Kortet 2004). Positive relationships between female preferences for male pheromones, male nutritional condition, and male encapsulation activity (a measure of immune function), have been found in the insect Tenebrio monitor (Rantala et al. 2002, 2003a, 2003b).
To conclude, pheromones may inform female field crickets about a male's potential to achieve high social status. Female G. integer prefer the scent of potentially dominant males and are more likely than males to wound their mating partners. Our results support the hypothesis of honest signalling in sexual selection (Zahavi 1975; Grafen 1990), and suggest that sexual selection in field crickets involves olfaction as well as acoustic signals. Quantifying the fitness consequences (both direct and indirect) of female preferences for potentially dominant males is a task for further study.
References
Adamo SA, Hoy RR (1995) Agonistic behavior in male and female field crickets, Gryllus bimaculatus, and how behavioral context influences its expression. Anim Behav 49:1491–1501
Adamo SA, Schildberger K, Loher W (1994) The role of the corpora allata in the adult male cricket (Gryllus campestris and Gryllus bimaculatus) in the development and expression of its agonistic behavior. J Insect Physiol 40:439–446
Alatalo RV, Höglund J, Lundberg A (1991) Lekking in the black grouse – a test of male viability. Nature 352:155–156
Alexander RD (1961) Aggressiveness, territoriality and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour 17:130–223
Andersson MB (1994) Sexual selection. Princeton University Press, Princeton
Arnold SJ, Houck LD (1982) Courtship pheromones: evolution by natural and sexual selection. In: Nitecki M (ed) Biochemical aspect of evolutionary biology. Chicago University Press, Chicago, pp 173–211
Berglund A, Bisazza A, Pilastro A (1996) Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol J Linnean Soc 58:385–399
Birch MC, Haynes KF (1982) Insect pheromones. Edward Arnold, London
Candolin U (1999) Male–male competition facilitates female choice in sticklebacks. Proc Roy Soc Lond B 266:785–789
Chapman T, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trends Ecol Evol 18:41–47
Cox CR, LeBoeuf BJ (1977) Female incitation of male competition: a mechanism in sexual selection. Am Nat 111:317–335
Grafen A (1990) Biological signals as handicaps. J Theor Biol 144:517–546
Gray DA, Cade WH (1999) Quantitative genetics of sexual selection in the field cricket, Gryllus integer. Evolution 53:848–854
Gullan PJ, Cranston PS (2000) The insects. An outline of entomology second edition. Blackwell Science, London
Haley MP (1994) Resource-holding power asymmetries, the prior residence effect, and reproductive payoffs in male northern elephant seal fights. Behav Ecol Sociobiol 34:427–434
Hartfelder K (2000) Insect juvenile hormone: from “status quo” to high society Braz J Med Biol Res 33:157–177
Hedrick AV (1986) Female preferences for male calling bout duration in a field cricket. Behav Ecol Sociobiol 19:73–77
Hedrick AV, Weber T (1998) Variance in female responses to the fine structure of male song in the field cricket, Gryllus integer. Behav Ecol 9:582–591
Hillgarth N, Wingfield JC (1997) Parasite-mediated sexual selection: endocrine aspects. In: Clayton D, Moore E (eds) Host–parasite evolution: general principles and avian models. Oxford University Press, Oxford, pp 78–104
Hofmann HA, Schildberger K (2001) Assessment of strength and willingness to fight during aggressive encounters in crickets. Anim Behav 62:337–348
Loher W, Dambach M (1989) Reproductive behaviour. In Huber F, Moore TE, Loher W (eds) Cricket behavior and neurobiology. Cornell University Press, Ithaca, pp 43–82
Karlson P, Lüscher M (1959) “Pheromones” a new term for a class of biologically active substances. Nature 183:55–56
Kokko H (2005) Treat ‘em mean, keep ‘em (sometimes) keen: Evolution of female preferences for dominant and coercive male. Evol Ecol (in press)
Kortet R, Hedrick A (2004) Detection of the spider predator, Hololena nedra by naïve juvenile field crickets (Gryllus integer) using indirect cues. Behaviour 141:1189–1196
Kortet R, Taskinen J, Vainikka A, Ylönen H (2004) Breeding tubercles, papillomatosis and dominance behaviour of male roach (Rutilus rutilus) during the spawning period. Ethology 110:591–602
Matsumoto Y, Mizunami M (2000) Olfactory learning in the cricket Gryllus bimaculatus. J Exp Biol 203:2581–2588
Matsumoto Y, Mizunami M (2002) Temporal determinants of olfactory long-term retention in the cricket Gryllus bimaculatus. J Exp Biol 205:1429–1437
Moore AJ, Gowaty PA, Wallin WG, Moore PJ (2001) Sexual conflict and the evolution of female mate choice and male social dominance. Proc Roy Soc Lond B 268:517–523
Moore AJ, Gowaty PA, Moore PJ (2003) Females avoid manipulative males and live longer. J Evol Biol 16:523–530
Otte D, Cade W (1976) On the role of olfaction in sexual and interspecies recognition in crickets (Acheta and Gryllus). Anim Behav 24:1–6
Qvarnstrom A, Forsgren E (1998) Should females prefer dominant males? Trends Ecol Evol 13:498–501
Penn D, Potts W (1998) Chemical signals and parasite-mediated sexual selection. Trends Ecol Evol 13:391–396
Rantala MJ, Kortet R (2003) Courtship song and immune function in the field cricket Gryllys bimaculatus. Biol J Linnean Soc 79:503–510
Rantala MJ, Kortet R (2004) Male dominance and immunocompetence in the field cricket (Gryllus bimaculatus). Behav Ecol 15:187–191
Rantala MJ, Jokinen I, Kortet R, Vainikka A, Suhonen J (2002) Do pheromones reveal male immunocompetence? Proc Roy Soc Lond B 269:1681–1685
Rantala MJ, Kortet R, Kotiaho JS, Vainikka A, Suhonen J (2003a) Condition dependence of pheromones and immune function in the grain beetle Tenebrio molitor. Funct Ecol 17:534–540
Rantala MJ, Kortet R, Vainikka A (2003b) The role of juvenile hormone in immune function and pheromone production trade-offs: a test of the immunocompetence handicap principle. Proc Roy Soc Lond B 270:2257–2261
Rekwot PI, Ogwu D, Oyedipe EO, Sekoni VO (2001) The role of pheromones and biostimulation in animal reproduction. Anim Reprod Sci 65:157–170
Savage KE, Hunt J, Jennions MD, Brooks R (2005) Male attractiveness covaries with fighting ability but not with prior fight outcome in house crickets. Behav Ecol 16: 196–200
Simmons LW (1986a) Inter-male competition and mating success in the field cricket, Gryllus bimaculatus (De Geer). Anim Behav 34:567–579
Simmons LW (1986b) Female choice in the field cricket, Gryllys bimaculatus (D Geer). Anim Behav 34:1463–1470
Simmons LW (1990) Pheromonal cues for the recognition of kin by female field crickets, Gryllus bimaculatus. Anim Behav 40:192–195
Sreng L, Leoncini I, Clement JL (1999) Regulation of sex pheromone production in the male Nauphoeta cinerea cockroach: role of brain extracts, corpora allata (CA), and juvenile hormone (JH). Arch Insect Biochem 40:165–172
Teal PEA, Gomez-Simuta Y, Proveaux AT (2000) Mating experience and juvenile hormone enhance sexual signaling and mating in male Caribbean fruit flies. Proc Natl Acad Sci USA 97:3708–3712
Wagner Jr. WE, Reiser MG (2000). The relative importance of calling song and courtship song in female mate choice in the variable field cricket. Anim Behav 59:1219–1226
Wyatt TD (2003) Pheromones and animal behaviour. University Press, Cambridge
Tregenza T, Wedell N (1997) Definitive evidence for cuticular pheromones in a cricket. Anim Behav 54:979–984
Wedell N, Tregenza T (1999) Successful fathers sire successful sons. Evolution 53:620–625
Zahavi A (1975) Mate selection – a selection for a handicap. J Theor Biol 53:205–214
Acknowledgements
Special thanks to M. Tam, R. Helkala, L. Berger and A. Leonard for their assistance in the laboratory. We thank A. Vainikka, H. Kokko and four anonymous reviewers for helpful comments on the manuscript. This study was supported by the Academy of Finland to RK (decision 204837) and by a grant from the National Science Foundation to AH (NSF IBN-0076484). The experiments reported here comply with the current laws of the USA
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Siva-Jothy
Rights and permissions
About this article
Cite this article
Kortet, R., Hedrick, A. The scent of dominance: female field crickets use odour to predict the outcome of male competition. Behav Ecol Sociobiol 59, 77–83 (2005). https://doi.org/10.1007/s00265-005-0011-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0011-1