Abstract
Social bees can deposit specialized glandular secretions, or signals, that allow foragers to revisit rewarding and to avoid unrewarding food sources. However, it is not known if bees can orient towards olfactory cues such as excreta deposited near food sources. We report that Melipona mandacaia foragers (stingless bees) deposit an odor cue, anal droplets, and a previously undescribed ventro-abdominal odor on food sources. Surprisingly, foragers deposited attractive odor marks on good food sources to which they recruited and on poor food sources to which they did not recruit. Foragers left the most anal droplets on dilute food sources to which they did not recruit (1.25-M sucrose solution), yet returning foragers were attracted to anal droplets obtained on poor food sources and presented in bioassays. Foragers were attracted to ventro-abdominal odors obtained on good food sources (2.5-M sucrose solution). Chemical extractions suggest that odor marks contain attractive polar compounds. We also provide the first detailed description of forager waggling and spinning behavior on poor and good food sources. Waggling may be a method of dispersing anal droplets and spinning may help foragers learn local landmarks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food-marking odors play an important role in social insect recruitment by assisting the orientation of foragers and recruited nestmates. Ants use a diverse array of glandular secretions in foraging communication (Hölldobler and Wilson 1990). Bees also deposit odor marks to improve their foraging efficiency: attractive marks for good food sources and repellant marks to avoid food sources that have been exhausted (Stout and Goulson 2001). In field tests, bumblebee-repellant odor marks were found to consist of tarsal gland compounds (Stout et al. 1998; Goulson et al. 2000). Honeybees deposit attractive odors produced by Nasanov and sting glands, and repellant odors secreted by mandibular glands (Free et al. 1982; Free and Williams 1983). Stingless bees secrete attractive odor marks from the mandibular and tarsal glands to mark rewarding food sources (Kerr et al. 1963; Hrncir et al. 2003; Schmidt et al. 2003). All of these odor marks are generally considered signals (von Frisch 1967; Kerr 1972; Kerr 1973; Jarau et al. 2002). Thus it is unclear if bees can also orient towards olfactory cues deposited by foragers on food sources.
A signal may be broadly defined as a stimulus, any association between a sensory channel and an environmental state. However, this definition gives relatively little weight to the role of natural selection (Dusenbery 1992), and thus a distinction is drawn between signals and cues. Seeley (1989, p 550) provides lucid definitions: "Signals are stimuli that convey information and have been molded by natural selection to do so; cues are stimuli that contain information but have not been shaped by natural selection specifically to contain information. Cues carry information only incidentally." This distinction is important because cues can provide valuable insights into signal evolution (Greenfield 2002). For example, Formicine ants use hindgut contents as trail pheromones, a procedure that may have evolved from the ritualization of defecation (Hölldobler and Wilson 1990).
We investigated whether stingless bee foragers, highly social bees (Hymenoptera, Apidae, Meliponini), can use olfactory signals and cues for food source orientation. In particular, we focused on the role of anal droplets, potential excreta consisting of a clear fluid that increases in volume and production rate at increasingly dilute sugar solutions in some species (Nieh 1998). There is debate about anal droplets as attractants (Aguilar and Sommeijer 1996, 2001; Nieh 1998). Melipona panamica foragers increased the rate of anal droplet production at poor food sources to which they did not recruit or to which they recruited weakly (Nieh 1998). Thus the putative attraction mark is paradoxically stronger for food sources that are less attractive. How can these observations be reconciled with the concept of an attractive recruitment odor?
Anal droplets may be odor cues. Nieh (1998) reported that M. panamica foragers produced larger anal droplets at a higher rate for poor food sources (1.0-M sucrose solution) than for rich food sources (2.5-M sucrose solution). However, M. panamica foragers recruited strongly for the 2.5-M sucrose solution and very weakly for the 1.0-M sucrose solution. Aguilar and Sommeijer (2001) found no statistically significant differences between anal droplet production at different honey concentrations. However, 35% of the anal droplets were produced by M. favosa foragers feeding at a 0.5-M honey solution, and 26% of the anal droplets were produced by foragers feeding at a 2.0-M honey solution. Thus increasing anal droplet production with decreasing sugar concentration is seen in M. panamica and may also occur in M. favosa.
The attractiveness of anal droplets is unclear. M. panamica foragers were not attracted to anal droplets in a paired feeder bioassay (Nieh 1998), yet Aguilar and Sommeijer (2001) reported that anal droplets attract M. favosa foragers. Species or methodological differences may account for these different results. We therefore decided to examine the odor-marking strategies of a third species, M. mandacaia, to increase our understanding of food-source odor marking in the genus Melipona. M. mandacaia Smith, 1863, is endemic to the Caatinga habitat, a semi-arid ecosystem in the southern portion of Brazil (Rizzini 1997) and is able to recruit nestmates to a specific distance and direction, but not to a specific height (Nieh et al. 2003). Our study had four objectives: determining (1) if M. mandacaia foragers odor mark food sources, (2) how they deposit odor marks, (3) the source of odor marks, and (4) whether anal droplets can serve as attractive odor cues.
Materials and methods
Study site and bee colonies
We collected our data during two field seasons, 8 August to 5 September 2000 and 3 July to 27 August 2001, at the Fazenda Aretuzina, in the state of São Paulo, Brazil (S21°26.385′, W047°34.880′). We used three colonies of M. mandacaia (300–400 workers) from the southern portion of Bahia state in Brazil. We housed colony 1 in an observation nest (plan in Nieh and Roubik 1995) inside a laboratory building and connected to the outside via a 25-cm-long, 1-cm-diameter vinyl tube. We housed colonies 2 and 3 outside in separate wooden nests (design in Nogueira-Neto 1997). Only foragers from the colony under study were allowed to forage. We blocked the entrances of non-subject colonies with mesh until the end of experiments each day. We used all colonies to investigate the role of feeder behaviors in odor mark deposition. We used colony 3 to investigate the existence of odor marks, their persistence time, their role in recruitment, and their source and composition.
Feeders, training, and marking
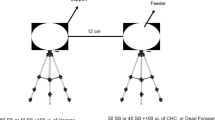
We fed the bees unscented sucrose solution (1.25 or 2.5 M) from a feeder consisting of a small glass bottle (5 cm diameter, 4.5 cm height, 65 ml) inverted over a flat base. Such a feeder models a rich inflorescence (Roubik 1980; Johnson 1981) or other densely arrayed food sources (such as raided stingless-bee honey pots, Rocha 1970; Roubik 1989). With colonies 1 and 2, the base consisted of a clear plastic, grooved circular plate (6.7 cm diameter, 40 grooves, von Frisch 1967). We placed this feeder on a 20-cm-diameter yellow plastic dish on a tripod. With colony 3, we used a rectangular glass plate (30 cm×10 cm). Between the bottle and the glass plate, we placed a circle of nylon mesh (5.5 cm diameter, 1-mm square mesh) to create a space for forager proboscises. We placed a 5-cm-diameter circle of pink paper underneath the base of each feeder to enhance its visual conspicuousness.
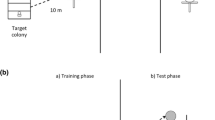
We trained foragers (method of von Frisch 1967) from colony 1 to a site 140 m southeast, foragers from colony 2 to a site 120 m southeast, and foragers from colony 3 to a site 20 m southwest of their respective nests. We used acrylic paints and paint pens to uniquely mark each bee visiting the feeder (von Frisch 1967) and verified that foragers came from the subject colony, not a wild colony, by watching the nest entrance for returning foragers. We maintained a constant number of 20 marked foragers visiting the feeder by censusing the foragers each 5 min and aspirating or releasing marked foragers as necessary (aspirator design in Nieh et al. 2003).
Measuring recruitment rate
We defined a poor food source as one to which the colony did not recruit. This decision depended upon several factors, including the availability of natural food sources, the status of colony food stores, and the demand for resources such as pollen and water (Roubik 1989; Biesmeijer and de Vries 2001). We chose 1.25 M because it was the lowest sucrose concentration that elicited reliable visitation, but no recruitment, whereas 2.5-M sucrose solution elicited substantial recruitment. We allowed 20 individually marked, experienced foragers to forage at either a 1.25 M (poor) or a 2.5 M (rich) feeder. For each sucrose concentration, we recorded the total number of newcomers arriving at the feeder within 1-h intervals (four trials at each sucrose concentration).
Bioassays
General methods
We performed feeder choice experiments using 100 foragers to assay forager attraction to potential odor marks. Each trial consisted of a collection phase followed by a test phase. In the collection phase, we placed a glass plate feeder in the center of a small wooden table (50 cm wide×40 cm long×100 cm high) located 20 m southwest from colony 3. At the end of the collection phase, we captured all visiting foragers. Immediately after the collection phase, we began the test phase, by removing the collection-phase feeder and placing the collection papers and clean control papers around two identical, clean feeders separated by 20 cm on a clean glass plate. The collection paper was filter paper onto which we collected putative odor marks. We centered the glass plate on the feeding table, with both feeders perpendicular to the nest−feeder direction. We exchanged control and experimental feeder locations each 5 min to control for potential site bias. We released one forager at a time, capturing her with a second aspirator once she landed, and recorded her feeder choice and arrival time.
During both phases, we used one pair of clean forceps to handle the collection paper and a separate pair for the clean paper. Once used, we washed all equipment in a strong detergent, rinsing thoroughly with hot water followed by two washes of 95% ethanol. We air-dried the glass for at least 3 h before reuse. Even without washing, this time interval is more than sufficient for odor marks to completely evaporate. Odor marks lose their attractiveness after 80 min (see Results). The experimenter wore disposable latex gloves during all feeder choice experiments. After each trial, we discarded the gloves and all paper and plastic items used in the trial.
All attractive odor marks
To collect all odor marks, we placed a ring of Whatman filter paper (5.5-cm inner diameter, 12-cm outer diameter) around a 2.5-M feeder for 15 min. A paper ring of this size was sufficient to insure that foragers positioned their entire bodies over the filter paper while feeding. To obtain the clean control paper, we placed an identical but unvisited feeder 5 m east of the collection feeder.
Ventro-abdominal odor marks
We used 2 mm×15 mm filter paper strips to collect odor marks potentially produced from the ventro-abdominal area of foragers (excluding anal droplets) foraging at 2.5 M. We targeted this area because of our forager departure observations. We held a filter paper strip under the abdomen of a forager, but not touching any part of the forager, during the entire time the forager was on the feeder (on average for 50 s). We discarded paper strips that touched any part of the forager's body or contacted an anal droplet. Upon the forager's departure, we sealed the paper strip inside a 1.5-ml centrifuge vial and immediately placed the vial inside a −23°C freezer. During the 30-min collection phase, we sampled five different foragers and collected five different experimental strips. We alternately collected five control strips by holding the control strip for 50 s with clean forceps 100 cm from the feeder. We placed the control strips in a separate vial in the −23°C freezer.
Attractive marks on poor food sources
We collected potential odor marks on paper rings in two sequential collection phases in which we fed bees either 1.25 M or 2.5 M. Foragers visited but did not recruit for a 1.25-M sucrose solution. During the test phase, we compared attraction between odor marks collected during the 1.25-M and 2.5-M phases.
In the collection phase, we collected odor marks from 38 forager visits to the 1.25-M feeder and from 38 visits to the 2.5-M feeder during 20 min at each feeder. We placed the paper ring from the first collection phase inside a sealed plastic bag, and stored it in a −23°C freezer during the second collection phase. We alternated the sucrose concentrations used in the first collection phase. The test phase lasted for 15 min. The 1.25-M ring contained anal droplets, but we immediately discarded the 2.5-M paper if foragers deposited any anal droplets on this ring (in order to have an experimental ring with anal droplets and a control ring with no anal droplets). Droplets were immediately visible on the dry white filter paper.
Anal droplets
We used a 1.25-M collection phase feeder. We held the tip of a 2 mm×15 mm filter paper strip posterior to the abdomen and in contact with each droplet as it was excreted but before it was deposited on the substrate, taking care not to touch the abdomen and to keep the paper away from the ventro-abdominal area. We discarded any strip that touched the bee. The paper strip was held near the forager for a fraction of a second during each droplet collection. We collected ten anal droplets per filter paper strip (five strips in total) before sealing the strip inside a clean vial and placing the vial inside the −23°C freezer. We alternately collected five control strips by using clean forceps to hold each strip for 50 s at a distance of 100 cm from the feeder.
Odor marks: polar or non-polar?
To evaluate odor mark composition, we extracted the collection paper ring with pentane or dichloromethane (Sigma-Aldrich). Pentane is non-polar (solvent polarity P'=0.0). Dichloromethane is polar (P'=3.1, Snyder and Kirkland 1979). For the bioassay, we used 2.5 M sucrose solution, and a 60-min collection phase followed by a 15-min test phase. We did not use any rings with anal droplets.
We used a solvent press to transfer compounds from the collection filter paper to a clean filter paper. We placed a clean ring on a glass plate and covered it with a circle of nylon mesh (13 cm diameter, 1-mm grid, 0.7 mm thick). We placed the collection paper on top of the mesh and rinsed the collection paper with 3 ml of solvent applied with a syringe. We then immediately covered the setup with another glass plate and applied firm, even pressure for 1 min. At no point did the collection paper directly contact the clean paper. We verified that the solvent reached the clean paper (the extract ring) by observing the bottom glass plate. To obtain the control ring, we used a clean glass press and mesh, and performed the same process with a clean paper ring in place of the collection ring. We used clean glass plates and new mesh and filter papers for each extraction.
Potential odor mark deposition behaviors
We used a Canon XL-1 digital video camera to film the behavior of 20 M. mandacaia foragers at 1.25 M- and at 2.5-M feeders. We used iMovie v2.1.1 and VideoPoint v2.0.3 software to extract and analyze the video data on a Macintosh iBook computer, respectively (30 fps, velocity values averaged within a 0.03-s window). The following behaviors were scored: anal droplet production, waggling, spinning, antennal grooming, tongue grooming, leg grooming, and thorax grooming.
Statistical analysis
In the feeder choice experiments, we calculated a two-tailed binomial probability (BP) based upon the null hypothesis that randomly orienting foragers will arrive equally at both feeders ( P =0.5). We performed a lag-sequential analysis to examine the behavioral sequence data, calculating Yule's Q and the odds ratio to discern common patterns (Bakeman and Gottman 1986). Statview v5.0.1 software was used to perform Mann-Whitney U - and χ 2-tests. We report all averages as mean ±1SD and use the abbreviations min. and max. to refer to minimum and maximum values respectively.
Results
Do foragers leave odor marks at the feeder?
In all six trials, a majority of foragers chose the 2.5-M collection paper with the putative odor marks over the control paper with no odor marks (BP, individual trials, P ≤0.018). We scored 174 choices for the experimental feeder and 52 choices for the control feeder (100 individuals, pooled data, BP, P <0.0001). Thus foragers deposited attractive odor marks on the 2.5-M food source.
How long do odor marks remain attractive?
Forager preference for the collection paper decreased from 89% to 58% in 60 min (Fig. 1, linear regression, P =0.004, R 2=0.164, n =49). Significantly more foragers chose the collection paper over the control paper in the first 70 min (seven 10-min intervals, BP, P ≤0.02 for each interval). However none of the subsequent time intervals show a significant preference for the collection paper. Thus odor marks remained attractive for 70 min.
How long do odor marks remain attractive? Each box plot consists of five horizontal lines that show the 10th, 25th, 50th, 75th, and 90th percentiles (combined data from six trials). Open circles above and below each plot indicate values that are respectively above the 90th percentile and below the 10th percentile. The dashed linear regression line is shown
Testing the attractiveness of ventro-abdominal marks
In all five trials, a majority of foragers chose the feeder with the ventro-abdominal odor marks over the control feeder (Table 1). A significant overall majority of foragers (65%) chose the experimental feeder (BP, P =0.01). Thus foragers were attracted to odors potentially released from the ventral side of foragers' abdomens at the 2.5-M feeder.
Attractive marks on poor food sources?
Foragers recruited for the 2.5-M sucrose solution, but not for the 1.25-M sucrose solution. During four 1-h trials at each concentration, 20 foragers recruited 36 newcomers to the 2.5-M feeder and no newcomers to the 1.25-M feeder. Thus recruitment to the two sucrose concentrations was significantly different ( χ 2=36, df= 1, P <0.0001). The recruitment rate for the 2.5-M feeder was 9.0±2.16 recruits/20 foragers/h.
However, foragers were equally attracted to odor marks deposited on the 1.25-M and the 2.5-M feeder. In total, 48 foragers chose the 2.5-M odor marks and 60 chose the 1.25-M odor marks (BP, P =0.290, Table 1). Thus foragers deposited attractive odor marks on food sources to which they recruited (2.5 M) and on food sources to which they did not recruit (1.25 M). Because of our methodology, the 1.25-M ring contained anal droplets, but the 2.5-M ring did not.
Are anal droplets attractive?
Departing foragers occasionally excreted a droplet of clear fluid (approximately 6 µl) from their anus before flying off. We filmed 23 anal droplet excretions and found that the average excretion lasted 0.19±0.07 s at 1.25 M. A majority of foragers chose the feeder with 1.25-M anal droplets over the clean control feeder in four out of five trials (Table 1). In total, a significant majority of foragers (62%) chose the experimental feeder over the control feeder (BP, P =0.032). Thus anal droplets attracted foragers despite being left at a feeder to which foragers did not recruit.
Are the 2.5-M odor marks polar or non-polar compounds?
In all trials with pentane extracts of 2.5-M odor marks (anal droplets excluded), foragers showed no significant preference for the experimental feeder over the control feeder (Table 1). In total, 47% of foragers chose the experimental feeder (BP, P =0.868). However, in four out of five dichloromethane trials, a majority of foragers chose the experimental feeder over the control feeder (Table 1). Overall, 73% percent of foragers chose the experimental feeder (BP, P =0.029). Thus the non-polar solvent (pentane) did not extract the attractive component or components of odor marks, whereas the polar solvent (dichloromethane) extracted an attractive component or components.
How do foragers deposit odor marks?
Just before foragers left the feeder, they performed a series of departure behaviors, some of which were similar to odor-deposition behaviors described in other stingless bees (Kerr 1972; Kerr 1973; Kerr and Rocha 1988) and in honeybees (Pflumm 1969, 1973, 1983). They groomed their antennae, produced an anal droplet, waggled their abdomen while dragging it in contact with the substrate, spun, groomed their tongue, groomed their legs, or groomed their thorax. These behaviors occurred singly or together. In our observations, all of these behaviors occurred after the foragers had finished feeding.
Waggling consists of the forager rhythmically shaking her body medio-laterally as she pivots around her thorax (Fig. 2). During waggling, the tip of her abdomen contacts the substrate, as if she were wiping herself clean. The abdominal tip moved at an average frequency of 4.9±1.2 Hz (max.=6.0 Hz, min.=3.2 Hz). The peak-to-peak displacement of the abdominal tip was 3.2±0.9 mm (within a 0.03-s time window, max.=4.4 mm, min.=1.7 mm). The average velocity of the abdominal tip was 39.6±16.7 mm/s (within a 0.03-s time window, max.=66.2 mm/s, min.=19.0 mm/s, n =10, all measurements taken from 2.5-M foragers).
Displacement and velocity of waggling behavior at the feeder (three typical examples). The solid trace corresponds to the solid scale bar and indicates abdominal tip displacement. The dashed trace corresponds to the dashed scale bar and indicates abdominal tip velocity. The position of the thorax and abdomen during the first waggling example is shown on the right (each 0.15 s). Open circles mark the center of the head and thorax. The filled circle marks the abdominal tip. The bee is 8.5 mm long
Spinning consists of the forager performing clockwise and counterclockwise spins around the center of her body, usually without her abdomen touching the substrate (Fig. 3). Spins occurred singly or in groups of up to four. We define a single spin as a movement in a clockwise or counterclockwise direction that terminates with no movement, directional reversal, or forager departure. There was often a change in angular velocity at the beginning and end of each spin (Fig. 3).
Three typical consecutive feeder spins at 2.5 M. Lines show the distance from the tip of the head ( unfilled circle) to the tip of the abdomen and correspond to a bee 8.7 mm long. Arrows indicate the direction of motion. We show the forager's position each 0.15 s and the angular spin velocity (calculated each 0.1 s) for each spin
How do departing foragers behave at different sucrose concentrations?
Departure sequence
Table 2 gives the transition probability matrices for feeder departure performances at 1.25 M and 2.5 M. There is no single most common sequence of all behaviors. However at both sucrose concentrations, waggling most frequently followed anal droplet production (98% at 1.25 M, 94% at 2.5 M). In addition, anal droplet production and waggling increased at the poor food source as compared to the good food source ( P =0.01, Table 3). The following sequence captures the essential features of departure behavior: (1) anal droplet production, (2) waggling, (3) spinning, (4) grooming, and (5) spinning. Grooming and spinning often alternated.
Grooming and spinning
At 1.25 M, Yule's Q =0.436 and the odds ratio is 2.5, indicating that the likelihood of spinning following grooming was 2.5 times that of spinning following any other behavior. A Yule's Q -value of +1 would indicate that spinning always followed grooming. At 2.5 M, Yule's Q =0.623 and odds ratio is 4.3, indicating that the likelihood of spinning following grooming was 4.3 times that of spinning following any other behavior. Thus spinning tended to occur after grooming at both sucrose concentrations, but Yule's Q and the odds ratio show that the effect was stronger at 2.5 M than at 1.25 M.
We found no significant differences ( P =0.082) between the number of spins per visits, the spin radius (the radius of a circle circumscribed by the tip of the forager's abdomen and thus a measure of whether a forager makes large radius or small radius spins), or the spin magnitude (how many degrees the bee spins) at 1.25 M versus 2.5 M (Table 4).
However, foragers spent 21% less time per spin at 2.5 M than at 1.25 M ( P =0.006, Table 4). Foragers spun 55% more rapidly (larger angular velocity, P <0.0001) and had a 360% higher average acceleration ( P <0.0001) at 2.5 M than at 1.25 M (Table 4, Fig. 4).
Tongue grooming was the only other feeder behavior to significantly change with sucrose concentration. Tongue grooming occurred more often at 2.5 M than at 1.25 M ( P =0.01, Table 3).
Discussion
M. mandacaia foragers can therefore deposit attractive odor marks from multiple sources, including potential cues (anal droplets) on good food sources to which they recruited and on poor food sources to which they did not recruit (Table 1). Densely arrayed food sources such as large inflorescences or stingless bee honey pots (exploited during raids) occur in nature and can provide rich or dilute sugar solutions (Johnson 1981; Roubik et al. 1995). We will consider mark deposition mechanisms and evidence for different mark types.
Feeder departure behaviors: odor deposition?
We began studying odor marking in M. mandacaia because of intriguing forager departure behaviors on food sources. We observed foragers landing on leaves, producing anal droplets, and performing waggling (Fig. 2) and spinning (Fig. 3) after feeding on natural nectar sources. Working with sucrose feeders, we observed the same behaviors. Lag-sequential analysis reveals that waggling followed over 94% of anal droplets. Spinning and grooming, often in alternation, then followed waggling. Tongue grooming occurred more often at 2.5 M than at 1.25 M, perhaps as result of the sticky residues left by a 2.5-M sucrose solution. This is in contrast to honeybees, which tongue-groom more frequently at higher sucrose concentration and in which grooming-departure behaviors on a feeder may result from the disinhibition of grooming motivation (Pflumm 1969, 1973, 1983). Aguilar and Sommeijer (2001) also observed abdominal "zigzagging" immediately after anal droplet production in M. favosa. Such waggling may help to disperse the anal droplet over the substrate and serve as a wiping behavior to clean the bee if anal droplets are excreta (Table 2).
We frequently observed departing foragers performing a looping, circular flight centered on the feeder, particularly just after it had been moved to a new location. Such looping departure flights help honeybee foragers learn landmark positions (Cartwright and Collett 1983; Lehrer 1991, 1993; Lehrer and Collett 1994; Zeil et al. 1996; Jander 1997; Capaldi and Dyer 1999), and Melipona feeder spinning may serve a similar function. Given the relatively limited resolution of bee eyes (Jander and Jander 2002), spinning may help foragers learn landmarks close to the food source.
Ventro-abdominal odor marks
Foragers feeding on a good food source produce an attractive odor that can be collected by holding a strip of paper underneath the forager's abdomen without touching any part of the forager or contacting an anal droplet. Although the odor source is unknown, it may volatilize from the ventro-abdominal area. Potential sources of such an odor include the Dufour's gland and the vestigial poison sac gland (Cruz-Landim 1967; Lello 1976; Cruz-López et al. 2001). It is unclear whether foragers only produce ventro-abdominal odor marks for good food sources. This hypothesis remains to be tested.
Are anal droplets cues or signals?
Foragers deposited twice as many anal droplets at 1.25 M as at 2.5 M (Table 3), and they did not recruit to 1.25 M. Nonetheless, foragers were attracted to anal droplets deposited at 1.25 M (Table 1.3). We collected droplets as they were excreted and before they touched the substrate. Thus we excluded potential contamination from tarsal gland marks and other contact-deposited marks. We also took care to keep the paper strips away from the ventro-abdominal area, bringing them close to, but not touching, the abdomen, for the fraction of a second necessary to collect the anal droplet. We discarded any strips that touched the forager. Nonetheless, it is possible that a very small quantity of ventro-abdominal odor may have been collected with the droplets, and that foragers oriented towards this small quantity of ventro-abdominal odor. Such sensitivity would be surprising because five paper strips held for 50 s each (not a fraction of second) underneath the ventral abdomen attracted only 65% of foragers as compared to the control.
More work is needed to determine whether anal droplets serve as cues. A detailed, chemical analysis of anal droplets obtained from bees foraging on attractive food sources and anal droplets collected on food sources that demonstrably repel foragers, or collected during waste-elimination flights, would be informative. In M. favosa, anal droplets contained small quantities of carbohydrates (12–16 µg/µl) and proteins (2.0–6.7 μg/µl) but it is unclear whether any of these compounds were specifically produced for the purpose of communication (Aguilar and Sommeijer 1996).
Are anal droplets simply excreta?
The social insect literature has focused on chemicals specifically produced for the purpose of communication (Lindauer and Kerr 1958; Kerr et al. 1963; von Frisch 1967; Hölldobler and Wilson 1990). However it is possible for foraging odor marks to consist of excreta without additional glandular products specifically produced for the purpose of communication. The use of excreta to odor mark has been described in many animals, where it often provides information to other individuals and serves a self-referential mark (Bradbury and Vehrencamp 1988). The desert isopod Hemilepistus reaumuri, a subsocial arthropod, builds walls of fecal matter that facilitate kin recognition (Linsenmair 1987). Solenopsis invicta ant larvae secrete milky anal excreta that are sought after and fed upon by workers and anal excreta consisting of clear droplets that workers gather and deposit at the edge of the nest (O'Neal and Markin 1973). Oecophylla ant workers also spread fecal pellets uniformly around their territory. These pellets contain colony-specific substances and enable workers to determine if they are in their own territory (Hölldobler and Wilson 1978)
There is a strong possibility that anal droplets are bee excreta. The production of anal fluid excretions increases when bees forage at increasingly dilute sugar solutions (honeybees, Apis mellifera , Pasedach-Poeverlein 1940, Rau 1970; bumblebees, Bombus lucorum L., Bertsch 1984; and carpenter bees, Xylocopa capitata , Nicolson 1990). For example, Bertsch (1984) showed that water from nectar consumption and metabolic water generated from sugar processing necessitates the excretion of water equal to the mass of a bumblebee (136 mg) each 24 h. Honeybees void similar anal droplets that are composed of almost pure water and increase the rate of anal droplet production at increasingly dilute sucrose solutions (Pasedach-Poeverlein 1940). In all stingless bee species in which the effect has been examined, anal droplet production increased at increasingly dilute sucrose solutions. The effect is only significant in M. mandacaia and M. panamica (Nieh 1998), not in M. favosa (Aguilar and Sommeijer 2001). M. favosa foragers left 35% more droplets at a 0.5-M honey solution than at a 2.0-M honey solution. The use of diluted honey instead of sucrose solution may account for the weak effect observed, because honey has a far more complex chemical composition than a pure sucrose solution (Qiu et al. 1999), and contains fructose, glucose, sucrose, maltose, free acids, lactone and trace amounts of amino acids that may influence excretion rates.
Aguilar and Sommeijer (2001) report increased anal droplet production with increased distance to the food source. As they suggest, increased anal droplet production could produce a larger olfactory mark to assist forager orientation towards more distant food sources. Foragers may also reduce the energetic cost of flight by excreting wastes and thus reducing excess weight, especially as the distance increases and the costs of excess weight increase. There is evidently variation in the function of anal droplets in different species. In M. panamica, foragers produced larger anal droplets at a higher rate at a low sucrose concentration than at a high sucrose concentration, but did not orient towards anal droplets in a feeder choice experiment (Nieh 1998).
References
Aguilar I, Sommeijer MJ (1996) Communication in stingless bees: are the anal substances deposited by Melipona favosa scent marks? Proc Sect Exp Appl Entomol Neth Entomol Soc 7:57−63
Aguilar I, Sommeijer M (2001) The deposition of anal excretions by Melipona favosa foragers (Apidae: Meliponinae): behavioural observations concerning the location of food sources. Apidologie 32:37–48
Bakeman R, Gottman JM (1986) Observing interaction: an introduction to sequential analysis. Cambridge University Press, New York
Bertsch A (1984) Foraging in male bumblebees ( Bombus lucorum L.): maximizing energy or minimizing water load? Oecologia 62:325–336
Biesmeijer JC, de Vries H (2001) Exploration and exploitation of food sources by social insect colonies: a revision of the scout−recruit concept. Behav Ecol Sociobiol 49:89–99
Bradbury J, Vehrencamp SL (1988) Principles of animal communication. Sinauer, Sunderland, Mass.
Capaldi EA, Dyer FC (1999) The role of orientation flights on homing performance in honeybees. J Exp Biol 202:1655–1666
Cartwright BA, Collett TS (1983) Landmark learning in bees. J Comp Physiol A 151:521–543
Cruz-Landim Dd (1967) Estudo comparativo de algumas glândulas das abelhas (Hymenoptera, Apoidea) e respectivas implicações evolutivas. Arq Zool (Sao Paulo) 15:177–290
Cruz-López L, Patricio EFLRA, Morgan DE (2001) Sections of stingless bees: the Dufour gland of Nannotrigona testaceicornis. J Chem Ecol 27:69–80
Dusenbery DB (1992) Sensory ecology: how organisms acquire and respond to information. Freeman, New York
Free JB, Williams IH (1983) Scent-marking of flowers by honeybees. J Apic Res 22:86–90
Free JB, Williams I, Pickett JA, Ferguson AW, Martin AP (1982) Attractiveness of (Z)-11-eicosen-1-ol to foraging honeybees. J Apic Res 21:151–156
Frisch K von (1967) The dance language and orientation of bees. Belknap, Cambridge, Mass.
Goulson D, Stout JC, Langley J, Hughes WOH (2000) Identity and function of scent marks deposited by foraging bumblebees. J Chem Ecol 26:2897–2911
Greenfield MD (2002) Signalers and receivers: mechanisms and evolution of arthropod communication. Oxford University Press, New York
Hölldobler B, Wilson EO (1978) The multiple recruitment systems of the African weaver ant Oecophylla longinoda (Latreille) (Hymenoptera: Formicidae). Behav Ecol Sociobiol 3:19–60
Hölldobler B, Wilson EO (1990) The ants. Belknap, Cambridge, Mass.
Hrncir M, Jarau S, Zucchi R, Barth FG (2003) On the origin and properties of scent marks deposited at the food source by a stingless bee, Melipona seminigra Friese 1903. Apidologie (in press)
Jander R (1997) Macroevolution of a fixed action pattern for learning: the exploration flights of bees and wasps. In: Greenberg G, Tobach E (eds) Comparative psychology of invertebrates: the field and laboratory study of insect behavior. Garland, New York, pp 79–99
Jander U, Jander R (2002) Allometry and resolution of bee eyes (Apoidea). Arthropod Struct Dev 30:179–193
Jarau S, Hrncir M, Zucchi R, Barth FG (2002) Footprint pheromones used to mark food sources by stingless bees. In: Billen J (ed) XIV International Congress of IUSSI: The golden jubilee proceedings. Hokkaido University Coop, Hokkaido University, Sapporo, Japan
Johnson LK (1981) Effect of flower clumping on defense of artificial flowers by aggressive stingless bees. Biotropica 13:151–157
Kerr WE (1972) Orientação pelo sol em Trigona spinipes. Cienc Cult [Suppl]:341–342
Kerr WE (1973) Sun compass orientation in the stingless bees, Trigona ( Trigona) spinipes (Fabricius, 1793) (Apidae). Anais Acad Bras Cien 45:301–308
Kerr WE, Rocha R (1988) Comunicação em Melipona rufiventris e Melipona compressipes. Cien Cult 40:1200–1203
Kerr W, Ferreira A, Simões de Mattos N (1963) Communication among stingless bees−additional data (Hymenoptera: Apidae). J NY Entomol Soc 71:80–90
Lehrer M (1991) Bees which turn back and look. Naturwissenschaften 78:274–276
Lehrer M (1993) Why do bees turn back and look? J Comp Physiol A 172:549–563
Lehrer M, Collett TS (1994) Approaching and departing bees learn different cues to the distance of a landmark. J Comp Physiol A 175:171–177
Lello E (1976) Adnexal glands of the sting apparatus in bees: anatomy and histology, V (Hymenoptera: Apidae). J Kans Entomol Soc 49:85–99
Lindauer M, Kerr WE (1958) Die gegenseitige Verständigung bei den stachellosen Bienen. Z Vergl Physiol 41:405–434
Linsenmair KE (1987) Kin recognition in subsocial arthropods, in particular in the desert isopod, Hemilepistus reaumuri. In: Fletcher DJC, Michener CD (eds) Kin recognition in animals. Wiley, New York, pp 121–208
Nicolson SW (1990) Osmoregulation in a nectar-feeding insect, the carpenter bee, Xylocopa capitata: water excess and ion conservation. Physiol Entomol 15:433–440
Nieh JC (1998) The role of a scent beacon in the communication of food location in the stingless bee, Melipona panamica. Behav Ecol Sociobiol 43:47–58
Nieh JC, Roubik DW (1995) A stingless bee ( Melipona panamica) indicates food location without using a scent trail. Behav Ecol Sociobiol 37:63–70
Nieh JC, Contrera FAL, Ramírez S, Imperatriz-Fonseca VL (2003) Variation in the ability to communicate 3-D resource location by stingless bees from different habitats. Anim Behav (in press)
Nogueira-Neto P (1997) Vida e criação de abelhas indígenas sem ferrão. Nogueirapis, Sao Paulo
O'Neal MCA, Markin JP (1973) Brood nutrition and parental relationships of the imported fire ant, Solenopsis invicta. J Ga Entomol Soc 8:294–303
Pasedach-Poeverlein K (1940) Über das "Spritzen" der Bienen und über die Konzentrationsänderung ihres Honigblaseninhalts. Z Vergl Physiol 28:197–210
Pflumm W (1969) Beziehungen zwischen Putzverhalten und Sammelbereitschaft bei der Honigbiene. Z Vergl Physiol 64:1–36
Pflumm W (1973) Zur Steuerung der Putzbewegungen der Honigbiene. Behaviour 45:104–122
Pflumm W (1983) Zum Sammel− und Putzverhalten der Honigbiene auf einer Abblühenden Zwergmispel ( Cotoneaster horizontalis). Behaviour 83:112–131
Qiu PY, Ding HB, Tang YK, Xu RJ (1999) Determination of chemical composition of commercial honey by near-infrared spectroscopy. J Agric Food Chem 47:2760–2765
Rau G (1970) Zur Steuerung der Honigmagenfüllung sammelnder Bienen an einer künstlichen Futterquelle. Z Vergl Physiol 66:1–21
Rizzini CT (1997) Tratado de fitogeografia do Brasil, 2nd edn. Âmbito Cultural, Rio de Janeiro
Rocha ESMO (1970) Raiding in Melipona rufiventris flavolineata. In: Ceccato S (ed) Proceedings of the 22nd annual meeting of the sociedade brasileira para o progresso da ciencia. Soc Bras Prog Cienc (Brazil)
Roubik DW (1980) Foraging behavior of competing Africanized honeybees and stingless bees. Ecology 61:836–845
Roubik DW (1989) Ecology and natural history of tropical bees. Cambridge University Press, New York
Roubik DW, Yanega D, Aluja SM, Buchmann SL, Inouye DW (1995) On optimal nectar foraging by some tropical bees (Hymenoptera: Apidae). Apidologie 26:197–211
Schmidt VM, Zucchi R, Barth FG (2003) A stingless bee marks the feeding site in addition to the scent path (Scaptotrigona aff. deplis). Apidologie 34:237–248
Seeley TD (1989) The honey bee colony as a superorganism. Am Sci 77:546–553
Snyder LR, Kirkland JJ (1979) Introduction to modern liquid chromatography, 2nd edn. Wiley, New York
Stout JC, Goulson D (2001) The use of conspecific and interspecific scent marks by foraging bumblebees and honeybees. Anim Behav 62:183–189
Stout JC, Goulson D, Allen JA (1998) Repellent scent-marking of flowers by a guild of foraging bumblebees ( Bombus spp.). Behav Ecol Sociobiol 43:317–326
Zeil J, Kelber A, Voss R (1996) Structure and function of learning flights in bees and wasps. J Exp Biol 199:245–252
Acknowledgements
We thank Zilá Luz Paulino Simões for generously providing supplies for our odor extraction experiments; Felipe A. L. Contrera and Vera L. Imperatriz-Fonseca for valuable assistance with the setup of the experiments; and Patrick Kelley for help with videotaping feeder behaviors. We would also like to thank caretakers Maridalva Arauso Dias and her husband Paulo Rovirsso Sousa Dias for their hospitality and hard work at the Fazenda Aretuzina. Melissa Thomas and four anonymous reviewers significantly enhanced the quality of this manuscript with their comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.F.A. Moritz
Rights and permissions
About this article
Cite this article
Nieh, J.C., Ramírez, S. & Nogueira-Neto, P. Multi-source odor-marking of food by a stingless bee, Melipona mandacaia . Behav Ecol Sociobiol 54, 578–586 (2003). https://doi.org/10.1007/s00265-003-0658-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0658-4