Abstract
Stingless bees foraging for food improve recruitment by depositing chemical cues on valuable food sites or pheromone marks on vegetation. Using gas chromatography/mass spectrometry and bioassays, we showed that Melipona solani foragers leave a mixture composed mostly of long chain hydrocarbons from their abdominal cuticle plus methyl oleate from the labial gland as a scent mark on rich food sites. The composition of hydrocarbons was highly variable among individuals and varied in proportions, depending on the body part. A wide ratio of compounds present in different body parts of the bees elicited electroantennogram responses from foragers and these responses were dose dependent. Generally, in bioassays, these bees prefer to visit previously visited feeders and feeders marked with extracts from any body part of conspecifics. The mean number of visits to a feeder was enhanced when synthetic methyl oleate was added. We propose that this could be a case of multi-source odor marking, in which hydrocarbons, found in large abundance, act as a signature mixture with attraction enhanced through deposition of methyl oleate, which may indicate a rich food source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tribe Meliponini (Hymenoptera, Apidae) are the most diverse group of all eusocial bees (Meléndez-Ramirez et al. 2013), comprised of a primarily tropical group of bees of several hundred species (~500) distributed through more than 36 genera (Michener 2013; Ascher and Pickering 2017). The term “stingless bee” is well established for this tribe because the parts of the sting are highly reduced and modified, relative to those of honeybees, and not functional for stinging (Michener 2007, 2013). Like honeybees, stingless bees are a group of bees that have developed an advanced eusocial colony organization. These bees show cooperation among adults in brood care and nest construction, as well as reproductive division of labor and overlapping of at least two generations. They live in perennial colonies in which the queen is replaced when needed (Amano et al. 2000; Michener 2007). The population of a stingless bee colony can range from a few tens to many thousands of adult workers, and is headed by a mated queen (Tóth et al. 2004; Michener 2007; Jarau 2009). For a colony to survive, foraging workers must collect enough important dietary components to nourish the entire population of the nest. Not all individuals within a colony leave the nest at the same time to forage for food (Jarau 2009). Young workers usually nurse the brood and clean the hive, while older workers are foragers. Stingless bee foragers are pollinators throughout the tropics and forage over a range of distances (Araújo et al. 2004; Greenleaf et al. 2007).
Stingless bees have evolved a variety of communication mechanisms for the transfer of information among workers, on both the nature and locality of a food source, to increase the overall foraging efficiency of the colony (Nieh 2004; Barth et al. 2008; Jarau 2009). Stingless bees display a variety of recruitment behaviors, which differ in both complexity and efficiency in exploiting food sources (Jarau et al. 2004b; Barth et al. 2008; Jarau 2009). These bees have evolved striking behavioral and ecological adaptations to cope with the challenges of living in the tropics, including the use of chemicals that can be self-produced, externally acquired or perceived from the environment (Leonhardt 2017). Foragers can deposit odors to assist orientation near a food source or they can deposit pheromone marks in the vegetation to guide nest mates to specific locations (Nieh 1998, 2004; Barth et al. 2008; Jarau et al. 2004a, 2010, 2011).
However, there are few studies about chemical communication involved in food source location by stingless bees. Some examples include, Trigona spinipes using a pheromone to mark rich food sites (Schorkopf et al. 2007), and Scaptotrigona pectoralis and some Trigona species using scent trails (Nieh 2004; Schorkopf et al. 2007; Jarau 2009; Reichle et al. 2011). It has been proposed that pheromones in the recruitment process of stingless bees are helpful but not obligatory (Schorkopf et al. 2011). Unlike other stingless bees, species in the genus Melipona do not use scent trails to guide recruits to a food source (Hrncir et al. 2004; Barth et al. 2008; Jarau 2009). Instead, they deposit scent marks near or at a food source to attract nest mates (Hrncir et al. 2004; Nieh 2004). In Melipona, the properties of such scent marks remain poorly understood, and sometimes controversial. Foragers of M. seminigra mark their food sources with pheromones produced by their claw retractor tendons (Jarau et al. 2004a), while M. favosa scent marks derive from anal droplets deposited by foragers at or near the food source (Aguilar and Sommeijer 2001). For M. panamica, foragers use a scent beacon at food sources, but the origin of the scent remains unclear (Nieh 1998). Recently, it was discovered that M. scutellaris foragers can associate footprint cues with food sources, but these cues were not analyzed chemically (Roselino et al. 2016). A deeper understanding of the recruitment processes of more Melipona species and their chemical significance is should help with future comparative studies on this genus (Jaffe et al. 2012).
In Mexico, stingless bees are ecologically, economically and culturally important (Ayala et al. 2013). One of these species is M. solani, a little studied stingless bee that is of special interest in the region due to its use in meliponiculture and crop pollination (Ayala et al. 2013). The objective of this study was to understand potential recruitment scent marks in the genus Melipona, using M. solani as a study species. The following questions were proposed: 1) Do M. solani foragers leave scent marks in rich food sites? 2) What body part(s) release(s) the compounds that foragers leave at the feeding sites? 3) Are forager’s antennae sensitive to chemical marks left at feeding sites? 4) How do chemicals influence the foraging behavior of M. solani?
Methods and Materials
Study Species and Training Sessions
Four colonies of M. solani (Hymenoptera, Apidae, Meliponini) were used for the experiments. The colonies were taken from a meliponary in Tuxtla Chico, Chiapas, Mexico, and housed in wooden boxes. The experiments were carried out at the campus of El Colegio de la Frontera Sur, in Tapachula, Chiapas, Mexico, from April–October 2014, February–July 2015 and February–November 2016. A beekeeper visually judged the colonies to be similar in vigor (with a functional physogastric queen, ca. 2500 adults, 8–10 brood cells, honey reserve ca. 1.5 l in 50 honey pots and 10 pots full of pollen) and overall health (free of fungi and parasites, and with a good resin accumulation).
For collection of scent marks and field bioassays, bees were trained to collect from a 2.5 M sucrose solution ad libitum at an artificial feeder consisting of a 10 cm diam. Petri dish with a cotton ball soaked with sucrose solution at the centre. The feeder was 20 m southwest of the hives. The experiments were carried out between 7:00–11:30 hr.
Collection of Scent Marks and Sample Preparation
Artificial Feeders
As for M. seminigra, at least 40 visits were necessary to scent mark a food source efficiently (Hrncir et al. 2004). Chemicals left by M. solani foragers on an artificial glass feeder after 40 visits were extracted by washing the feeders with 4 ml of n-hexane (HPLC grade, Aldrich, Toluca México). After training sessions, a feeder with 2.5 M sucrose solution was replaced by another one with a different sucrose concentration (0, 0.5, 1.0, 1.5, 2.0, 2.5 M). The extracts were concentrated to 400 μl using a gentle stream of dry N2 and stored in a freezer at −20 °C until analysis. In these experiments, the artificial feeder was a 20 × 20 cm piece of glass with a 2 cm diam. Hole in the center, where a cotton ball soaked with a sucrose solution was placed.
Volatiles
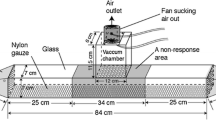
Volatiles were collected by solid phase micro extraction (SPME) with a poly-dimethylsiloxane fiber (SUPELCO, Deisenhofen, Germany). Workers were trained to forage on a sucrose solution in a volatile collection chamber made of glass (150 ml). This volatile collection chamber had three tunnels: one where the bees entered to collect a sugar solution at the bottom, with the two remaining tunnels used for the SPME fiber (Supplementary 1). A second identical feeder, from which workers were excluded by closing the entrance with foil, was used at the same time (ca. 5 m from two colonies), so as to determine the chemical background (emitted by the arena or in the surrounding air). An arena was visited by about 40 bees. Every bee visited an arena more than twice during a trial, with each trial lasting 1 hr. This experiment was conducted ten times.
Body Part Extracts
Bees were frozen at −20 °C before dissection and analysis. Body parts of foragers were dissected with forceps to yield head, thorax, abdomen, legs and wings. Extracts were prepared by macerating the respective body parts of four bees in 1 ml of hexane, concentrated to 400 μl using a gentle stream of dry N2 and stored at −20 °C until analysis.
Labial Gland Extracts
Gland extracts were prepared by dissecting the labial glands from the head of four foragers. The compounds were extracted with 1 ml of hexane, concentrated with dry N2 to 400 μl, and stored at −20 °C until analysis.
Chemical Analyses
Chemical Identification of Scent Marks
Compounds were identified by gas chromatography/mass spectrometry (GC/MS), using both electron impact (EI) and chemical Ionization (CI), as well as by Infrared spectroscopy (IR). Some compounds were confirmed by comparing retention indices and mass spectra with those of synthetic standards (Sigma-Aldrich, Toluca, Mexico). The relative amount of each compound was calculated from peak area, while the relative percentage of a component was calculated relative to the sum of all peak areas. A set of samples from one colony was treated with dimethyl disulfide (DMDS) (Shibahara et al. 2008) to identify the position of the double bond in the alkenes. An extract of 5 bees in 100 μl of hexane was treated with 100 μl DMDS and 20 μl iodine solution (60 mg iodine/1 ml diethyl ether) for 24 hr at 50 °C. Excess iodine was reduced with sodium thiosulfate solution (5% w/w in water). The organic phase was removed and excess DMDS evaporated. The residue was diluted with 100 μl hexane and analyzed by GC/MS.
To identify compounds, volatiles and extracts were analyzed on a Varian Star model CP-3800/Saturn 2200 (Palo Alto, CA, USA). A DB-5 fused silica capillary column (30 m × 0.25 mm ID) was temperature programmed from 50 °C (held for 2 min) to 280 °C at 15 °C min−1, and held at 280 °C for 10 min. The temperature of the injector was 250 °C. Ionization was by electron impact at 70 eV, 250 °C.
CI mass spectra were obtained using a GC/MS Triple-Quadrupole Mass Spectrometer TQ8040 with CI ion volume in Q1MS mode. The same DB-5 capillary column and GC conditions were used as in the electron impact experiments. Methane was used as CI reagent gas and conditions were: ion source pressure 2 Torr, ionization energy 70 eV, electron multiplier voltage 1500 V, emission current 200 μA; ionization temperature 150 °C, mass range m/z 50–600, scan time 1 sec.
Infrared spectra were obtained with a GC/IR spectrophotometer (DiscovIR-GC, Massachusetts, USA). The same 30 m DB-5 capillary column and GC conditions were used as in the GC/MS experiments.
Determination of Compound Proportions
Four replicates per extract were carried out. In the case of the feeder extracts, the compounds were extracted from four previously visited feeders.
Quantification of Methyl Oleate
The amount of methyl oleate in bees was calculated by the internal standard method, using a calibration curve made with three concentrations (10, 20 and 50 ng/μl) of methyl oleate, and using 20 ng/μl of decane as the internal standard. Three replicates were carried out per concentration. For this, the heads of two bees were macerated in hexane, the solution decanted and concentrated to 50 μl.
Electroantennography (EAG)
Thirty five foragers from 4 established colonies were sampled and their antennae carefully removed. The base of an antenna was inserted into a reference glass capillary electrode, previously filled with Ringer solution, while the distal end was inserted into the tip of the glass recording capillary electrode. The signals generated by the antenna were passed through a high impedance amplifier (NL 1200; Syntech, GmbH) and displayed by Syntech software for processing EAG signals. A stimulus flow controller (CS-05; Syntech) was used to generate a stimulus at 1 min intervals. Humidified pure air (0.7 l.min−1) was directed onto the antenna through a 10 mm diam. Glass tube (Malo et al. 2004).
Three amounts (0.1, 0.5 and 1 bee equivalent) of worker extracts (head, abdomen, thorax, legs and labial gland) were tested, with hexane as a control. Pieces of filter paper, 1.5 × 1.5 mm, were impregnated with the treatments, exposed to air for 20 sec to allow the solvent to evaporate, and placed in glass Pasteur pipettes for 20 sec before application. New pipettes with treatments were prepared for each antenna. To present a stimulus, the pipette tip containing the piece of filter paper was inserted into a hole at the end of the tube carrying the air stream. The treatment was puffed from the filter paper by a controlled air stream (0.5 l.min−1). The duration of stimulus was 1 sec. The continuous flow of air over the preparation ensured that odors were removed from the vicinity. Thirty five replicates per treatment were carried out. Due to the decline of excised antennae, only twelve replicates could be made per antenna.
Field Bioassays
Paired-Choice Bioassays
To assure that foragers left scent marks in the feeders, a paired-choice bioassay was performed, with bees choosing between two feeders: one marked (previously visited by foragers) and the other clean (not visited by foragers).
To test the behavioral responses of M. solani to body part extracts, another paired-choice experiment was carried out, with bees choosing between a marked feeder (baited with one bee equivalent of extract in 100 μl at the beginning of the experiment) and a clean feeder (same amount of hexane).
To test the influence of methyl oleate on foraging behavior of M. solani, a set of three paired-choice experiments was performed, with bees choosing between two marked feeders. In experiment 1, one feeder was marked with one bee equivalent of methyl oleate, and the feeder with hexane (control); in experiment 2, one feeder was marked with one bee equivalent of abdominal extract and the other with the same amount of methyl oleate; and in experiment 3, one feeder was marked with abdominal extract, and the other with a mixture of abdominal extract plus synthetic methyl oleate.
In all two-choice bioassays, the following conditions were used. The setup was mounted at the training site and, to stimulate foragers, a few drops of 2.5 M sucrose solution were injected into the nest entrances. Once bees started to visit a feeding site, the number that landed and extended their proboscides into the sugar solution on either a previously visited feeder or a clean feeder was recorded over 30 min. All bees were marked and captured on their first visit to one of the feeders (to avoid testing individual bees more than once). At the end of an experiment, bees were released. Only those bees captured during a given experiment and not already marked in preceding experiments were included in the analysis. Only bees landing while no other bees were at or near the feeders were counted in order to avoid the effects of visual or other unwanted signals provided by such bees. The distance between the two feeders was 30 cm. The positions of the two feeders were switched every 5 min to avoid side bias. To test whether bees visited two clean feeders equally, a control experiment was performed following the same procedure. The feeders used for the bioassays were the same as those used for training (see above). For each experiment, ten replicates were carried out.
Multiple-Choice Bioassays
Foragers were trained to collect a 2.5 M sucrose solution from a feeder located 10 m southwest of the colony (training sessions). To evaluate the choices of recruits to different bee extracts, a four-choice experiment was performed. The experiment evaluated the effect of the three most attractive feeders in the binary-choice experiments. A set of four feeders was mounted [control (hexane), head extract, leg extract, and a previously visited feeder (400 bee visits)]. Each feeder consisted of a 2 cm high × 6 cm diam. Petri dish, with cotton soaked with 2.5 M sucrose solution. The distance between feeders was 20 cm. Feeders were placed on a wooden table. One trial per day was conducted, each trial lasting 30 min. All bees were marked and captured on their first visit to one of the feeders. At the end of an experiment, bees were released. Only choices made by newcomers were registered (to avoid testing individual bees more than once). To avoid site bias, feeders were rotated every 5 min. Only bees landing while no other bees were at or near the feeders were counted in order to avoid the effects of visual or other unwanted signals provided by such bees.
Statistical Analyses
All data were analyzed in R software. Proportions of peak areas (i.e., relative amounts) of the compounds in each sample were compared via principal components analysis (PCA). To test the proportions of compounds found in feeders and those found in bee bodies, a one-way ANOVA followed by a Tukey test was performed. When necessary, data were Box-Cox transformed to reach normality and homoscedasticity (Box and Cox 1964; Sakia 1992).
The electroantennographic data were analyzed with a one-way ANOVA followed by a Tukey test to compare responses to extracts, and another one-way ANOVA-Tukey test was performed to compare responses to concentrations (0.1, 0.5 and 1 bee equivalent). Data from paired-choice bioassays were analyzed by Kruskal-Wallis tests (overall P < 0.05), to compare the number of bees that chose the marked feeder versus those that chose the clean feeder. In control experiments, the numbers of bees that chose each of the two control feeders were compared.
For the multiple-choice bioassay, a Kruskal-Wallis test and post hoc Dunn test were used to check significance of the differences in the percentage of bees at each of the four different feeders.
Results
Do M. solani Foragers Leave Scent Marks at Rich Food Sites?
GC/MS analysis of the feeder extracts showed that compounds left by foragers at the feeding site comprised mostly of long chain alkanes and alkenes and two esters, geranyl caproate and methyl oleate (Table 1). The most abundant compounds were 9-pentacosene, 7-pentacosene, n-pentacosane, 9-heptacosene, 7-heptacosene, 9-nonaeicosene. The amount of methyl oleate deposited was relatively small. Compounds deposited on the glass feeders were mostly the same as those in feeder headspace, except for the esters and some less abundant hydrocarbons found on glass feeders. Hydrocarbons were found at feeders for all tested sucrose solutions, while methyl oleate was not detected in feeders with low (<0.5 M) sucrose concentration (Fig. 1).
Chromatograms of extracts of feeders visited by Melipona solani foragers. Different sucrose solution were used in feeders (0–2.5 M). Three replicates per feeder were conducted. * Impurities. See Table 1 for identifies of numbered peaks
What Body Part(s) Release(s) the Compounds that Foragers Leave at Feeding Sites?
The compounds found in different body parts of bees were mostly alkanes, alkenes, esters and carboxylic acids (Supplementary 2). PCA revealed three groups: the first consisted of cephalic and labial gland extracts, the second of thorax and legs extracts and the third comprised wings, abdomen, volatiles and feeder extracts (Supplementary 3). Hydrocarbon composition found in feeders was qualitatively the same as that in abdomen extracts, and the hydrocarbon proportions were the same between abdomen and feeder extracts. Two principal components explain 49.71% of the variation. It should be noted that PCA was run with the compounds found in bee bodies that coincided with compounds found in the feeder extracts.
Methyl oleate was the most abundant compound in the labial gland (49%) and head extracts followed by minor amounts of 9-pentacosene, 7-pentacosene, n-pentacosane, 9-heptacosene, 7-heptacosene, 9-nonaeicosene. Methyl oleate was also found in a smaller proportion in the feeder extract. The amount of methyl oleate in one bee was highly variable, with mean (± S.E.) amount of 10.1 ± 2.81 μg.
There was no difference in the proportions of hydrocarbons found in bee-visited feeders and those in different parts of a bee’s body (Supplementary 4).
Are the Forager’s Antennae Sensitive to Chemical Marks Left at Feeding Sites?
The antennal responses of M. solani were different between low (0.1 bee equivalents) and high (1 bee equivalent) extract amounts. The middle amount (0.5 bee equivalent) was, in most cases, not different from the high amount. All treatments were different from the control (hexane). Therefore, extracts were electrophysiologically active and the response was dose dependent (Fig. 2).
Electroantennographic (EAG) responses of Melipona solani worker antennae to different extracts of bee body parts at three different concentrations. A one-way ANOVA followed by a Tukey test was done to compare treatments at one concentration (lower case letters) and to compare the control and different concentrations of extract (upper case letters). Different letters of the same case indicate differences in means. Boxcox λ = 0.141 for all treatments. Control: ANOVA (F = 0.45, df = 5, P = 0.81). 0.1 EQ (bee equivalents): ANOVA (F = 4.67, df = 5, P < 0.001). 0.5 EQ: ANOVA (F = 5.62, df = 5, P < 0.001). 1 EQ: ANOVA (F = 4.23, df = 5, P < 0.01). Labial: ANOVA (F = 52.73, df = 3, P < 0.001). Head: ANOVA (F = 61.05, df = 3, P < 0.001). Thorax: ANOVA (F = 35.85, df = 3, P < 0.001). Abdomen: ANOVA (F = 44.85, df = 3, P < 0.001). Legs: ANOVA (F = 36.42, df = 3, P < 0.001). Wings: ANOVA (F = 38.07, df = 3, P < 0.001). There were 35 replicates per treatment
How Do Chemicals Influence Foraging Behavior in M. solani?
Field bioassays showed that M. solani preferred a previously visited feeder to a clean feeder (Kruskal-Wallis: H = 14.76, df = 1, P < 0.001). Furthermore, the field bioassays showed that all extracts of body parts were attractive to bees in the context of foraging (Fig. 3). The multiple-choice experiment showed a difference between the number of visits to marked feeders and visits to the unmarked feeder (control) (Kruskal-Wallis: H = 18.00, df = 3, P < 0.001). A post hoc analysis showed that there was no difference between marked feeders (Supplementary 5). Foragers were attracted to feeders marked with compounds from any body part.
Paired-choice bioassays between feeders with various extracts and a solvent control (Clean). Wings extract (Kruskal-Wallis: H = 10.89, df = 1, P < 0.001). Leg extract (Kruskal-Wallis: H = 15.01, df = 1, P < 0.001). Abdominal extract (Kruskal-Wallis: H = 10.31, df = 1, P < 0.01). Thorax extract (Kruskal-Wallis: H = 9.55, df = 1, P < 0.01). LG (Labial gland) extract (Kruskal-Wallis: H = 14.76, df = 1, P < 0.001). Head extract (Kruskal-Wallis: H = 14.90, df = 1, P < 0.001). Previously Visited Feeder (PVF) extract (Kruskal-Wallis: H = 14.763, df = 1, P < 0.001). Clean experiment (Kruskal-Wallis: H = 0.058462, df = 1, P = 0.8089). Ten replicates per extract. (*** P < 0.001, ** P < 0.01. * P < 0.05, NS Non-significant)
The paired-choice bioassay with synthetic methyl oleate and control feeder showed that bees preferred to visit a feeder marked with the most abundant compound of the labial gland (Kruskal-Wallis: H = 9.69, df = 1, P < 0.01). In the paired-choice bioassays between abdominal extract and methyl oleate, there was no difference in bee preference (Kruskal-Wallis: H = 1.93, df = 1, P = 0.165). Moreover, the bioassay with abdominal extract plus synthetic methyl oleate showed that bees preferred to visit a feeder marked with bee hydrocarbons plus methyl oleate (Kruskal-Wallis: H = 7.51, df = 1, P < 0.01) (Fig. 4).
Paired-choice bioassays with synthetic methyl oleate (MO) and hydrocarbons from abdominal extract (ABD). MO vs CLEAN control, (Kruskal-Wallis: H = 9.6878, df = 1, P < 0.01). MO vs ABD, (Kruskal-Wallis: H = 1.9299, df = 1, P = 0.1648). ABD + MO vs ABD (Kruskal-Wallis: H = 7.5081, df = 1, P < 0.01). (** P < 0.01, NS Non-significant)
Discussion
In this paper, we report that M. solani marks rich food sites and these marks promote probing by conspecifics. Similar behavior has been observed in honeybees and bumblebees (Goulson et al. 2000; Stout and Goulson 2001), as well as in other Melipona species [e.g., M. panamica (Nieh 1998) and M. seminigra (Jarau et al. 2004a)]. Chemical analysis of the mark from a feeder visited by bees showed that M. solani deposited a mixture of compounds, predominantly hydrocarbons. The most abundant hydrocarbons, n-tricosane, 7 and 9- pentacosene, n-pentacosane, 7 and 9-heptacosene and 9-nonacosene, produced by M. solani have been also found in the pheromone from M. seminigra claw retractor tendon (Hrncir et al. 2004; Jarau et al. 2004a, 2005). Additionally, some of these hydrocarbons have been found as head space volatiles released by Apis mellifera (Schmitt et al. 2007) and as scent marks deposited by bumblebees (Goulson et al. 2000). Methyl oleate was also identified in visited feeders, and this compound originates from the M. solani labial gland secretion. This secretion is composed mainly of methyl oleate (49%) with minor amounts of 9-pentacosene, 7-pentacosene, n-pentacosane, 9-heptacosene, 7-heptacosene and 9-nonaeicosene. Other species of stingless bees use a trail pheromone produced in their labial gland (Jarau et al. 2010, 2011). Our study is the first to demonstrate that Melipona foragers deposit a labial gland secretion to mark rich feeding sites to attract other foragers.
Hydrocarbons left by M. solani in visited feeders were associated with compounds present in extracts of different parts of bees. The same major compounds present in leg, abdominal, cephalic and thoracic extracts were found at feeding sites. Although, the hydrocarbon composition did not vary qualitatively between the different body parts, the proportions of such compounds were different. Hydrocarbons are ubiquitous on the epicuticle of insects, explaining why they were found in the various extracts of bee parts. Previous studies on cuticular hydrocarbons in Melipona have been useful for comparing castes, colonies and individuals, but none of them reported variation among forager body parts (Ferreira-Caliman et al. 2010, 2012, 2013; Borges et al. 2012).
The most abundant compounds found in visited feeders were also largely found in the abdomen. In addition, it was observed that bees touched the surface of feeders with their abdomens while they were feeding on the sucrose solution (personal observation). Therefore, we propose that the M. solani hydrocarbons deposited on feeders originate from the abdomen. However, we do not rule out that bees might also leave marks when walking on the feeder or when grooming, like other eusocial bees, such as bumblebees, which mark food sites with their tarsi (Goulson et al. 2000) or M. seminigra, which leaves a pheromone from the claw retractor tendon (Jarau et al. 2004a), and M. scutellaris that leaves chemical footprints (Roselino et al. 2016).
Foragers of M. solani preferred to visit a feeder marked with any body part extract over a clean feeder (control). Thus, the mark was effective regardless of its body origin. Moreover, there was no difference in visits of stingless bees among marked feeders in multiple-choice bioassays. Although the chemical profile of hydrocarbons found on feeders was closely related to the chemical profile of the abdomen, the other body extracts (with different ratios of compounds) were also attractive to bees. The fact that bees preferred body part extracts suggests that this insect has a wide chemical acceptance window, which allows them to recognize the odor of conspecifics (Nehring et al. 2013); i.e., the mere presence of these compounds indicates valuable food sites. It may be that compounds left by M. solani act as a signature mixture rather than a signal.
We found broad variation in chemical profiles from individual to individual. Thus, it is unlikely that bee responses to ratios of compounds are innate. It is more likely that the chemical profile was learned by bees and that these compounds act as a signature mixture (Wyatt 2010, 2014). A similar phenomenon was observed in studies with M. scutellaris; the authors pointed out that footprints do not have an innate connotation for stingless bee foragers and that the bees are capable of establishing new associations between chemical cues and specific contexts (Roselino et al. 2016).
With other bee species, it has been reported that marks were cues rather than signals (Schmitt and Bertsch 1990; Saleh et al. 2007; Wilms and Eltz 2008). These marks could be interpreted as attractants or repellents by foragers, depending on the concentration of the mark, experience, associative learning, or status of reward (Goulson et al. 1998, 2001; Williams 1998; Saleh and Chittka 2006; Leadbeater and Chittka 2011). Additionally, a facultative use of scent-marks has been proposed, and the importance of other communication channels in foraging contexts has been highlighted (Saleh et al. 2006; Witjes and Eltz 2007). There are few studies on whether stingless bee scent marks act as signals or cues (Reichle et al. 2011; Schmidt et al. 2005) or whether response to the marks are innate or learned (Boogert et al. 2006; Reichle et al. 2013). As to concentration, some experiments have demonstrated that accumulation of a mark does not matter for attraction; a feeder with 40 visits is as attractive as a feeder with 200 visits (Hrncir et al. 2004).
Melipona solani stingless bees were able to detect compounds in the feeder, but they were not able to differentiate odors from different body extracts. That methyl oleate was found in feeders (on the sucrose-soaked cotton ball) suggests that M. solani leaves this compound from the labial gland to mark rich food sites while grooming. When we tested abdominal extract (a natural mixture of the most abundant hydrocarbons of M. solani) against abdominal extract plus synthetic methyl oleate, we found that methyl oleate enhanced a mark’s attractiveness. In addition, methyl oleate and abdominal extract were equally attractive, and methyl oleate was able to attract more bees than a clean feeder. Therefore, we propose that hydrocarbons left incidentally act as a signature mixture while their effect is enhanced by methyl oleate, which may indicate a rich food source. This could be a case of multi-source odor-marking, as reported for M. mandacaia (Nieh et al. 2003) and S. pectoralis (Reichle et al. 2011). We confirmed that M. solani leaves methyl oleate in rich food sites in the experiment with different sucrose concentration; methyl oleate was absent on feeders with low sucrose concentration but present on higher sucrose concentration feeders.
In summary, we showed that M. solani deposits a mixture of long chain hydrocarbons and methyl oleate at food sites and that it prefers to visit marked feeders over clean feeders. The origin of the hydrocarbons appears to be the cuticle of the abdomen, although other parts of the insect epicuticle cannot be ruled out, whereas the origin of methyl oleate is the labial gland. Methyl oleate appears to be primarily left at rich food sites and, when combined with hydrocarbons, it enhances a mark’s attractiveness. Future work is required to clarify the function of methyl oleate as a true signal or as a cue mediating the behavior of M. solani. These new findings are valuable for understanding the mechanisms that M. solani foragers use to find food sites and recruit nest mates.
References
Aguilar I, Sommeijer M (2001) The deposition of anal excretions by Melipona favosa foragers (Apidae: Meliponinae): behavioural observations concerning the location of food sources. Apidologie 32:37–48. https://doi.org/10.1051/apido:2001109
Amano K, Nemoto T, Heard T (2000) What are stingless bees and why and how to use in crop pollination? A review. Japan Agricultural Research Quarterly 34:183–190
Araújo E, Costa M, Chaud-Netto J, Fowler H (2004) Body size and flight distance in stingles bees (Hymenoptera: Meliponini): inference of flight range and possible ecological implicatons. Braz J Biol 64:563–568. https://doi.org/10.1590/S1519-69842004000400003
Ascher JS, Pickering J (2017) Discover life: bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila) http://www.discoverlife.org/mp/20q?guide=Apoidea_species&flags=HAS. Accessed 6 July 2017
Ayala R, Gonzalez V, Engel M (2013) Mexican stingless bees (Hymenoptera: Apidae): diversity, distribution, and indigenous knowledge. In: Vit P, Pedro S, Roubik D (eds) Pot-honey a legacy of stingless bees, 1st edn. Springer, New York, pp 135–152
Barth FG, Hrncir M, Jarau S (2008) Signals and cues in the recruitment behavior of stingless bees (Meliponini). J Comp Physiol A 194:313–327. https://doi.org/10.1007/s00359-008-0321-7
Boogert N, Hofstede F, Aguilar I (2006) The use of food source scent marks by the stingless bee Trigona corvina (Hymenoptera: Apidae): the importance of the depositor’s identity. Apidologie 37:366–375. https://doi.org/10.1051/apido:2006001
Borges A, Ferreira-Caliman M, Nascimento F, Campos L, Tavares M (2012) Characterization of cuticular hydrocarbons of diploid and haploid males, workers and queens of the stingless bee Melipona quadrifasciata. Insect Soc 59:479–486. https://doi.org/10.1007/s00040-012-0242-x
Box G, Cox D (1964) An analysis of transformations. J Roy Stat Soc B Met 26:211–252. https://doi.org/10.2307/2287791
Ferreira-Caliman M, Nascimento F, Turatti I, Mateus S, Lopes N, Zucchi R (2010) The cuticular hydrocarbons profiles in the stingless bee Melipona marginata reflect task-related differences. J Insect Physiol 56:800–804. https://doi.org/10.1016/j.jinsphys.2010.02.004
Ferreira-Caliman M, Zucchi R, Nascimento F (2012) Cuticular hydrocarbons discriminate distinct colonies of Melipona marginata (Hymenoptera, Apinae, Meliponini). Sociobiology 59:1–12. 10.13102/sociobiology.v59i3.881
Ferreira-Caliman M, Falcon T, Mateus S, Zucchi R, Nascimento F (2013) Chemical identity of recently emerged workers, males, and queens in the stingless bee Melipona marginata. Apidologie 44:657–665. https://doi.org/10.1007/s13592-013-0214-9
Goulson D, Hawson S, Stout J (1998) Foraging bumblebees avoid flowers already visited by conspecifics or by other bumblebee species. Anim Behav 55:199–206. https://doi.org/10.1006/anbe.1997.0570
Goulson D, Stout J, Langley J, Hughes W (2000) Identity and function of scent marks deposited by foraging bumblebees. J Chem Ecol 26:2897–2911. https://doi.org/10.1023/A:1026406330348
Goulson D, Chapman JW, Hughes WOH (2001) Discrimination of unrewarding flowers by bees; direct detection of rewards and use of repellent scent marks. J Insect Behav 14:669–678. https://doi.org/10.1023/A:1012231419067
Greenleaf S, Williams N, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153:589–596. https://doi.org/10.1007/s00442-007-0752-9
Hrncir M, Jarau S, Zucchi R, Barth F (2004) On the origin and properties of scent marks deposited at the food source by a stingless bee, Melipona seminigra. Apidologie 35:3–13. https://doi.org/10.1051/apido:2003069
Jaffe K, Issa S, Sainz-Borgo C (2012) Chemical recruitment for foraging in ants (Formicidae) and termites (Isoptera): a revealing comparison. Psyche 2012:1–11. https://doi.org/10.1155/2012/694910
Jarau S (2009) Chemical communication during food exploitation in stingless bees. In: Jarau S, Hrncir M (eds) Food exploitation by social insects: ecological, behavioral and theoretical approaches, 1st edn. CRC Press, Florida, pp 223–249
Jarau S, Hrncir M, Ayasse M, Schulz C, Francke W, Zucchi R, Barth F (2004a) A stingless bee (Melipona seminigra) marks food sources with a pheromone from its claw retractor tendons. J Chem Ecol 30:793–804. https://doi.org/10.1023/B:JOEC.0000028432.29759.ed
Jarau S, Hrncir M, Zucchi R, Barth FG (2004b) A stingless bee uses labial gland secretions for scent trail communication (Trigona recursa smith 1863). J Comp Physiol A 190:233–239. https://doi.org/10.1007/s00359-003-0489-9
Jarau S, Hrncir M, Zucchi R, Barth FG (2005) Morphology and structure of the tarsal glands of the stingless bee Melipona seminigra. Naturwissenschaften 92:147–150. https://doi.org/10.1007/s00114-004-0601-1
Jarau S, Dambacher J, Twele R, Aguila I, Francke W, Ayasse M (2010) The trail pheromone of a stingless bee, Trigona corvina (Hymenoptera, Apidae, Meliponini), varies between populations. Chem Senses 35:1–9. https://doi.org/10.1093/chemse/bjq057
Jarau S, Hemmeter K, Aguilar I, Ayasse M (2011) A scientific note on trail pheromone communication in a stingless bee, Scaptotrigona pectoralis (Hymenoptera, Apidae, Meliponini). Apidologie 42:708–710. https://doi.org/10.1007/s13592-011-0070-4
Leadbeater E, Chittka L (2011) Do inexperienced bumblebee foragers use scent marks as social information? Anim Cogn 14:915–919. https://doi.org/10.1007/s10071-011-0423-4
Leonhardt S (2017) Chemical ecology of stingless bees. J Chem Ecol 43:385–402. https://doi.org/10.1007/s10886-017-0837-9
Malo EA, Castrejón-Gómez V, Cruz-López L, Rojas JC (2004) Antennal sensilla and electrophysiological response of male and female Spodoptera frugiperda (Lepidoptera: Noctuidae) to conspecific sex pheromone and plant odors. Ann Entomol Soc Am 97:1273–1284. https://doi.org/10.1603/0013-8746(2004)097[1273:ASAERO]2.0.CO;2
Meléndez-Ramirez V, Meneses-Calvillo L, Kevan P (2013) Effects of human disturbance and habitat fragmentation on stingless bees. In: Vit P, Pedro S, Roubik D (eds) Pot honey: a legacy of stingless bees, first. Springer New York, New York, pp 269–282
Michener C (2007) The bees of the world, 2nd edn. The Johns Hoptkins University Press, Baltimore
Michener C (2013) The Meliponini. In: Vit P, Silva P, Roubik D (eds) Pot honey: a legacy of stingless bees, 1st edn. Springer-Verlag New York, New York, pp 3–17
Nehring V, Wyatt T, Ettorre P (2013) Noise in chemical communication. In: Brumm H (ed) Animal communication and noise. Springer, Berlin, pp 373–405
Nieh J (1998) The role of a scent beacon in the communication of food location by the stingless bee, Melipona panamica. Behav Ecol Sociobiol 43:47–58. https://doi.org/10.1007/s002650050465
Nieh J (2004) Recruitment communication in stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 35:159–182. https://doi.org/10.1051/apido:2004007
Nieh J, Ramírez S, Nogueira-Neto P (2003) Multi-source odor-marking of food by a stingless bee, Melipona mandacaia. Behav Ecol Sociobiol 53:578–586. https://doi.org/10.1007/s00265-003-0658-4
Reichle C, Aguilar I, Ayasse M, Jarau S (2011) Stingless bees (Scaptotrigona pectoralis) learn foreign trail pheromones and use them to find food. J Comp Physiol A 197:243–249. https://doi.org/10.1007/s00359-010-0605-6
Reichle C, Aguilar I, Ayasse M et al (2013) Learnt information in species-specific “trail pheromone” communication in stingless bees. Anim Behav 85:225–232. https://doi.org/10.1016/j.anbehav.2012.10.029
Roselino AC, Rodrigues AV, Hrncir M (2016) Stingless bees (Melipona scutellaris) learn to associate footprint cues at food sources with a specific reward context. J Comp Physiol A 202:657–666. https://doi.org/10.1007/s00359-016-1104-1
Sakia R (1992) The box-cox transformation technique: a review. Stat 41:169. https://doi.org/10.2307/2348250
Saleh N, Chittka L (2006) The importance of experience in the interpretation of conspecific chemical signals. Behav Ecol Sociobiol 61:215–220. https://doi.org/10.1007/s00265-006-0252-7
Saleh N, Ohashi K, Thomson J, Chittka L (2006) Facultative use of the repellent scent mark in foraging bumblebees: complex versus simple flowers. Anim Behav 71:847–854. https://doi.org/10.1016/j.anbehav.2005.06.014
Saleh N, Scott AG, Bryning GP, Chittka L (2007) Distinguishing signals and cues: bumblebees use general footprints to generate adaptive behaviour at flowers and nest. Arthropod Plant Interact 1:119–127. https://doi.org/10.1007/s11829-007-9011-6
Schmidt V, Zucchi R, Barth F (2005) Scent marks left by Nannotrigona testaceicornis at the feeding site: cues rather than signals. Apidologie 36:285–291. https://doi.org/10.1051/apido:2005002
Schmitt U, Bertsch A (1990) Do foraging bumblebees scent-mark food sources and does it matter? Oecologia 82:137–144. https://doi.org/10.1007/BF00318545
Schmitt T, Herzner G, Weckerle B et al (2007) Volatiles of foraging honeybees Apis mellifera (Hymenoptera: Apidae) and their potential role as semiochemicals. Apidologie 38:164–170. https://doi.org/10.1051/apido:2006067
Schorkopf D, Jarau S, Francke W et al (2007) Spitting out information: Trigona bees deposit saliva to signal resource locations. Proc R Soc B 274:895–898. https://doi.org/10.1098/rspb.2006.3766
Schorkopf D, Morawetx L, Bento J et al (2011) Pheromone paths attached to the substrate in meliponine bees: helpful but not obligatory for recruitment success. J Comp Physiol A 197:755–764. https://doi.org/10.1007/s00359-011-0638-5
Shibahara A, Yamamoto K, Kinoshita A, Anderson BL (2008) An improved method for preparing dimethyl disulfide adducts for GC/MS analysis. J Am Oil Chem Soc 85:93–94. https://doi.org/10.1007/s11746-007-1169-7
Stout J, Goulson D (2001) The use of conspecific and interspecific scent marks by foraging bumblebees and honeybees. Anim Behav 62:183–189. https://doi.org/10.1006/anbe.2001.1729
Tóth E, Queller D, Dollin A, Strassmann J (2004) Conflict over male parentage in stingless bees. Insect Soc 51:1–11. https://doi.org/10.1007/s00040-003-0707-z
Williams C (1998) The identity of the previous visitor influences flower rejection by nectar-collecting bees. Anim Behav 56:673–681. https://doi.org/10.1006/anbe.1998.0794
Wilms J, Eltz T (2008) Foraging scent marks of bumblebees: footprint cues rather than pheromone signals. Naturwissenchaften 95:149–153. https://doi.org/10.1007/s00114-007-0298-z
Witjes S, Eltz T (2007) Influence of scent deposits on flower choice: experiments in an artificial flower array with bumblebees. Apidologie 38:12–18. https://doi.org/10.1051/apido:2006048
Wyatt T (2010) Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J Comp Physiol 196:685–700. https://doi.org/10.1007/s00359-010-0564-y
Wyatt T (2014) Introduction to chemical signaling in vertebrates and invertebrates. In: Mucignat-Caretta C (ed) Neurobiology of chemical communication, first. Taylor & Francis, Boca Raton, pp 1–22
Acknowledgments
Thanks are given to the National Council of Science and Technology CONACYT for scholarship to D. A. R. (CV/grant number: 387462/255265). We also thank Dr. Mauricio Maldonado (UNAM) for his comments on an earlier version of this manuscript. This study was supported by CONACYT INFR-2014-01(224846).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alavez-Rosas, D., Malo, E.A., Guzmán, M.A. et al. The Stingless Bee Melipona solani Deposits a Signature Mixture and Methyl Oleate to Mark Valuable Food Sources. J Chem Ecol 43, 945–954 (2017). https://doi.org/10.1007/s10886-017-0886-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0886-0