Abstract
Purpose
Because of significant complications related to the use of autologous bone grafts in spinal fusion surgery, bone substitutes and growth factors such as bone morphogenetic protein (BMP) have been developed. One of them, recombinant human (rh) BMP-2, has been approved by the Food and Drug Administration (FDA) for use under precise conditions. However, rhBMP-2-related side effects have been reported, used in FDA-approved procedures, but also in off-label use.A systematic review of clinical data was conducted to analyse the rhBMP-2-related adverse events (AEs), in order to assess their prevalence and the associated surgery practices.
Methods
Medline search with keywords “bone morphogenetic protein 2”, “lumbar spine”, “anterolateral interbody fusion” (ALIF) and the filter “clinical trial”. FDA published reports were also included. Study assessment was made by authors (experienced spine surgeons), based on quality of study designs and level of evidence.
Results
Extensive review of randomised controlled trials (RCTs) and controlled series published up to the present point, reveal no evidence of a significant increase of AEs related to rhBMP-2 use during ALIF surgeries, provided that it is used following FDA guidelines. Two additional RCTs performed with rhBMP-2 in combination with allogenic bone dowels reported increased bone remodelling in BMP-treated patients. This AE was transient and had no consequence on the clinical outcome of the patients. No other BMP-related AEs were reported in these studies.
Conclusions
This literature review confirms that the use of rhBMP-2 following FDA-approved recommendations (i.e. one-level ALIF surgery with an LT-cage) is safe. The rate of complications is low and the AEs had been identified by the FDA during the pre-marketing clinical trials. The clinical efficiency of rhBMP-2 is equal or superior to that of allogenic or autologous bone graft in respect to fusion rate, low back pain disability, patient satisfaction and rate of re-operations. For all other off-label use, the safety and effectiveness of rhBMP-2 have not been established, and further RCTs with high level of evidence are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone morphogenetic proteins (BMPs) include an extensive group of growth factors, of which over 20 types have been identified to date and have been proved to be indispensible in fractured bone healing [1–4]. The first reports on the use of BMP in bone surgery came from Urist and co-workers [5, 6], who purified the protein and used it in clinical applications, with encouraging results. As the extraction from cadaver bone and purification of human BMP provided small amounts, the production was limited. Therefore, human recombinant gene technology was used to develop BMPs (rhBMP) and focused on those with the greatest bone induction properties. In the following years, BMP products were developed to increase the rate of solid bone fusion in lumbar surgeries, and therefore to reduce the need for subsequent revision surgery and avoid potential side effects of iliac crest bone harvesting. Eventually, recombinant human BMP-2 (rhBMP-2) was approved by the FDA in 2002 for anterior lumbar interbody fusion (ALIF) surgeries in indications including one-level degenerative disc disease and grade I spondylolisthesis, between L4 and S1 [7]. During surgery, BMP soaked collagen sponge (InFUSE®; Medtronic Sofamor Danek, Memphis, TN) is inserted into an LT-cage (Lumbar Tapered Fusion Device; Medtronic Sofamor Danek, Memphis, TN). All data were published on the FDA website [7] and in level I publications, including randomised controlled trials (RCTs) [8–10].
Soon after its approval in the United States, the use of BMP dramatically increased, up to nearly 25 % of all spine fusion procedures in 2006 [11]. A high proportion of surgeries performed with BMP deviated from the original FDA approved indications (e.g. use with a different fusion device, surgery methods or medical indications, or at a different concentration than recommended). Thus, while BMP was used for ALIF in only 16.6 % of the reported cases in the United States, it was mainly used off-label in first-time posterior lumbar interbody fusion (PLIF) or transforaminal interbody fusion (TLIF) (30.0 % for both), first-time posterolateral spine fusion (20.4 %), cervical fusions (13.6 %) and first-time thoracolumbar fusions (3.9 %) [12]. Since, for most of these indications (except posterolateral lumbar fusion), very few RCTs on safety and efficacy have been published, the risks and benefits of these off-label use of BMP remain under-evaluated. Off-label use of BMP has also been reported in general orthopaedic surgery and trauma, and recommendations have been issued in a recent literature review [13].
In a systematic literature review, Mroz et al. [14] concluded that the overall strength of evidence regarding BMP-related safety was “low” for cervical spine surgery and “very low” for thoracic spine surgery. This conclusion is in line with the FDA notification of 2008: “Since the safety and effectiveness of rhBMP for treatment of cervical spine conditions has not been demonstrated, and in light of the serious adverse events described, FDA recommends that practitioners either use approved alternative treatments, or consider enrolling as investigators in approved clinical studies” [15]. Therefore, the objective of this review is to analyse articles published since 2002, listing the different uses of rhBMP-2 in lumbar spine surgery, in approved and off-label indications, excluding cervical and thoracic surgery, and give precise up-to-date recommendations.
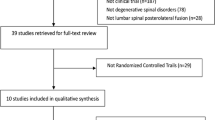
Methods
We searched the PubMed database with the following keywords: bone morphogenetic protein 2, lumbar spine, anterolateral lumbar fusion. The search was limited to the period from 1 January 2002 to 15 December 2012 and identified a total of 231 articles. We applied the Oxford level of evidence scale to select final articles for the review. As a final choice, we included two original RCTs [8, 9], the FDA approval document [7], four subsequent RCTs [10, 16–18], seven controlled series [19–25], all of which were of level of evidence 1 to 3 (Table 1). Seven additional reports on clinical use of BMP in ALIF surgery [20, 26–31] are also discussed because of their detailed clinical complications reports. Other relevant publications were also included to argument discussion [14, 32].
Results
The results are summarised in Tables 2 and 3.
Bone remodelling and subsidence (see Table 2)
Subsidence (i.e. reduction in disk space between two adjacent vertebral bodies) is commonly observed in stand-alone ALIF surgeries [33] and results from osteolysis and remodelling of the allogenic bone graft and/or the endplate of the adjacent vertebrae. The pilot RCTs mentioned no case of subsidence greater than 1 mm [7–9]. Overall, the subsidence rates reported by the FDA were 2.4 % in the BMP group (276 patients) and 1.4 % in the control group (136 patients), respectively [7]. No subsidence was observed in two other studies with follow-ups ranging from six months to six years [10, 20].
In another RCT performed by Burkus et al. [16], rhBMP-2 was used in conjunction with allograft bone dowels in 24 ALIF patients, compared to 22 control patients who received bone dowels and autograft. Of note, this technique using allograft is not currently approved by the FDA, but is quite close to the approved indication. No subsidence was reported at 24 months of follow-up. However, in a subsequent comparable multi-centre RCT that included 79 patients in the investigational group and 52 in the control group, the same authors found “localised areas of bone remodelling in the vertebral body adjacent to an allograft dowel” in 18 % of BMP patients, which appeared as lucent areas on patient’s radiographs. However, this bone remodelling was transient and had resolved by 24 months after surgery [17, 18]. This could explain why, examining figures of the first study [16], Smoljanovic and Pecina [34] found clues of bone remodelling and resorption of vertebral bodies occurring six months after surgery.
An early premarketing prospective study was conducted by Kleeman et al. [26], who performed laparoscopic ALIF using NOVUS LT tapered cages (Sofamor Danek, Memphis, TN) and rhBMP-2 soaked sponges on 22 consecutive patients. No AEs were reported and the clinical outcomes were very satisfactory, with all patients showing solid fusion at six months according to computed tomography scan read by a radiologist. Post-marketing data were reported later on. In a controlled cohort study published by Vaidya et al. [23], BMP was applied together with allogenic spacers (plus posterior fixation) in ALIF surgery. Data analysis found significantly increased rates of subsidence in the BMP than in the control group. As previously observed, radiolucency and subsidence mainly developed in the early postoperative period [17]. An off-label use and concentration differences can explain this observation (see “Discussion”) [35].
Other studies on rhBMP-2 used in ALIF surgeries, reported a high rate of bone resorption and subsidence, but again, these were off-label use [25, 27].
Infection (see Table 2)
The first investigational device exemption RCTs for the use of rhBMP-2 in ALIF with tapered interbody cages did not report any infection in the investigational group, including the six year follow-up [8, 9, 20]. However, the FDA approval document that gathered data from all known patients implanted with InFUSE™ Bone Graft/LT-CAGE™ (including the above-mentioned published cases) mentioned rather high infection rates [7]. This discrepancy has been recently underlined by Carragee et al. [36], who criticised the lack of documentation of infection rates in the original publication of these studies. However, as the incidence of infection was similar in the investigational than in the control groups, the FDA considered this as not related to rhBMP-2 [7].
None of the subsequent RCTs conducted by Burkus and co-workers did report infections during the follow-up period [10, 16, 17, 37]. Also, no specific concerns were reported in prospective or retrospective series where rhBMP-2 was used in ALIF procedures [23–27].
A recent retrospective review by Williams et al. [32] examined the role of BMP in peri-operative complications of spine fusion cases from the US Scoliosis Research Society (SRS) database. Despite this report gathering a wide range of indications and surgical techniques, it remains valuable as it compiles data from 5374 thoracolumbar interbody fusions, including 2,049 with adjunction of BMP. The analysis revealed a higher rate of deep wound infections in patients undergoing anterior/posterior thoracolumbar surgery with BMP, compared with patients in which BMP was not used (1.1 % versus 0.2 %; p < 0.001). However, regarding anterior-only thoracolumbar fusion, the authors found no significant differences in the rates of superficial or deep wound infection whether BMP was used or not (1.1 % versus 0.9 %, p = 0.5). Accordingly, univariate analysis of 328,468 American patients who underwent spinal fusion procedures (regardless of indication and surgical approach) revealed that lumbar fusions were not associated with a higher rate of wound-related complications, whereas cervical fusions were [11].
Thus, there is no strong evidence that BMP increases the rate of early or delayed infections in ALIF surgery.
Retrograde ejaculation (see Table 2)
Retrograde ejaculation (RE) is a rare but serious AE of ALIF surgery. Rates reported in the literature vary widely (0.42-5.9 %) [38].
In the original ALIF with BMP/LT-cage RCT, RE was observed in six of the 146 male patients (4.1 %) [9]. Instead of reporting RE rates in BMP and control groups separately as has been done for other AEs, the rate of RE was reported for the total patient population. In a later response to a commentary by Smoljanovic et al. [39], Burkus et al. stated that the RE rate in the original RCT was 6.4 % in the BMP group and 1.5 % in the control group, a statistically non-significant difference (p = 0.216). Why RE rates were not separately reported for BMP and control groups in the corresponding publication remains unclear. Burkus et al. [9] chose to compare RE AEs whether patients underwent a transperitoneal or a retroperitoneal approach (13.3 % and 1.8 %, respectively, p = 0.017). The subsequent FDA approval document contained additional data on BMP patients where the fusion device was implanted through a laparoscopic approach. Here, the RE rate in the BMP group was higher than in the control group (7.9 % versus 1.4 %) [7]. The laparoscopic surgical technique in itself has been shown to increase the risk for RE, so that it cannot be directly blamed on BMP [38, 40, 41].
No other cases of RE have been reported in subsequent RCTs and follow-up publications from Burkus et al. [10, 16–18, 20]. A retrospective analysis of RE complications extracted from five RCTs (i.e. 508 patients: 207 with rhBMP-2, 301 without) concluded that the incidence of RE was increased in the rhBMP-2 group, but the difference was not statistically significant (3.4 % versus 1.7 % in the control group, p = 0.242) [31]. The other prospective and retrospective series of ALIF did not mention rhBMP-2 as a risk factor for RE [23–27] except that of Carregee et al. In this publication, the authors performed a retrospective cohort-controlled study on rhBMP-2 versus demineralised bone matrix, in conjunction with allogenic femoral rings and posterior instrumentation in ALIF surgery (1-2 levels). They observed RE in 7.2 % of the 69 rhBMP-2 patients as opposed to 0.6 % of the 174 control patients (p = 0.0025). All patients underwent a transrectus or anterior-lateral retroperitoneal approach with a similar incidence between groups, so that the approach could not be incriminated. This significant difference in RE rates seems, therefore, attributable to rhBMP-2 [21]. Similar results have been published by the same authors more recently [22]. However, it is noticeable that in both of these non-randomised studies, the use of rhBMP-2 did not follow FDA-approved guidelines. The subjects of these studies underwent surgery at separated periods (2002-2003, then 2010-2011 without rhBMP-2; 2003-2010 with rhBMP-2). As noticed by the authors, “there was a trend to a decrease in RE rate in the 4th quartile of patients” (i.e. those having surgery later than mid-2008) [22], suggesting that a careful handling of rhBMP-2 may improve its safety. One more recent review article reported higher RE rates in ALIF with BMP, but it was not statistically significant (7.3 % versus 2.3 %; p = 0.03) [42].
Other urogenital events (see Table 2)
The pilot RCT published by Boden et al. [8] reported one event of urinary retention, which occurred in a control patient. In the original publication of the pivotal RCT, Burkus et al. [9] did not mention urogenital events as BMP-related AEs. However, in the extended FDA approval document, urogenital events were reported and were more frequent in the rhBMP-2 group (11.5 %) compared with the control group (7.2 %) [7]. This difference was not statistically tested and did not lead to specific concern in the safety report. No other case of urogenital event has been reported in the subsequent RCTs and follow-up publications from Burkus et al. [10, 16–18, 20], nor in the prospective and retrospective series of rhBMP-2 associated-ALIF [23–27]. The review article of Carragee et al. [36] reported higher rates (7.9 % in the BMP group versus 3.6 % in the control group, p = 0.04), but it only referred to the zero to nine weeks period after surgery. Between ten weeks and 30 months, the rate of urogenital events was similar in BMP and control patients (4.9 % and 4.3 %, respectively). Urogenital AEs appear very variable, and the correlation to BMP cannot be established.
Clinical outcome (see Table 3)
Clinical outcome—measured by fusion rate, low back pain disability (Oswestry questionnaire), patient satisfaction and rate of reinterventions—has been reported in several clinical studies comparing ALIF surgeries [8, 9, 17–19]. In all studies, fusion with BMP was equally or more efficient than allogenic or autologous bone graft alone. Low back pain disability was equal or lower in BMP treated patients, and patient satisfaction was equal or higher. In addition, the reintervention rate was lower in the BMP group in all studies [8, 9, 17–19]. Conversely to the assertion of deliberate omission [36] in the pivotal RCT report, 11 (7.0 %) and 14 (10.3 %) patients were reported to have undergone a second surgery before the 24-month follow-up in the investigational and control groups, respectively [9]. Reintervention rates were also listed in the FDA safety and effectiveness data summary (10.4 % and 13.7 % in the investigational and control groups, respectively) [7]. The six year follow-up report also mentioned 18 second surgeries for failures before 24 months [20]. In a prospective cohort where rhBMP-2-treated patients were compared with historical retrospective controls without rhBMP-2, Pradhan et al. [25], however, found a trend “toward a higher nonunion rate with rhBMP-2, although this was not significant with the numbers available”. This is the only study that reported lower fusion rates with rhBMP-2 than without. Note that the overall rate of treatment failure was rather high in this short series, regardless of the grafting material. Several other non-RCT studies showed results in favour of a benefit for the patient, with an improvement of the fusion rate and a higher degree of patient satisfaction [23, 24, 27].
Recently, Lykissas et al. [43] analysed nerve injury and recovery after lateral lumbar interbody fusion through a retroperitoneal approach with and without BMP2 in a cohort-controlled study. At the last follow-up, there was a significantly higher number of patients in the BMP group who complained of persistent anterior thigh or groin pain than the control group (8 versus 0 patients) (OR 16.470; 90 % CI, 1.477-183.700; p = 0.006). The author suggested a potential direct deleterious effect of rhBMP-2 on the lumbosacral plexus. In this study the confidence intervals and the statistical evidence were very weak, making it very difficult to draw conclusions.
Carcinogenicity (see Table 2)
Carcinogenicity of rhBMP-2 has been suspected, but not argued by preclinical or clinical data. In the FDA approval document, one pancreatic cancer was reported at the 12-month visit [7]. This case seems to be random, however, as the large retrospective cohort study performed in elderly patients failed to show any increased risk of pancreatic cancer linked to rhBMP-2 exposure [29]. Carragee et al. [44] reported the results extracted from an RCT for spine fusion including 239 BMP patients and 224 patients in the control group. At 24 months, the cancer risk was increased with rhBMP-2 (risk ratio, 3.45; 95 % CI, 1.98–6.00), but event rates were low and cancer was heterogeneous and 37 % of patients were lost at five years of follow-up, which decreased significantly the power of statistical analysis. More recently, Kelly et al. [45] reported a retrospective series analysing the incidence of cancer in 467,916 Medicare patients undergoing spinal arthrodesis from 2005 to 2010. The relative risk of developing cancer after BMP exposure was 0.938 (95 % CI, 0.913–0.964), which was significantly low. Cancer rates were similar in BMP and control groups (5.9 % versus 6.5 %). The conclusion was that use of BMP was not associated with an increased risk of developing cancer within a mean 2.9-year time window [45].
Other complications (see Table 2)
Based on the FDA’s safety and effectiveness data summary, some AEs, such as back and leg pain, neurological, gastrointestinal and cardiovascular events, were frequently reported during patient follow-up. However, their frequency was similar in the rhBMP-2 group and control group [7].
Ectopic bone formation has been described as a side effect of inappropriate BMP usage, particularly in PLIF, TLIF and anterior cervical discectomy and fusion (ACDF) [46], but not ALIF. Burkus et al. [9, 10, 16, 17 did not observe any case of ectopic bone formation in their published RCTs]. Neither did other prospective and retrospective series [23–27]. Similarly, painful seroma or epidural haematoma was never mentioned as a side effect of ALIF, although some cases were reported in TLIF surgeries, posterolateral lumbar fusions and anterior cervical fusions [46, 47]. Latzman et al. [30] raised a potential concern about transient renal impairment associated with rhBMP-2 in a small retrospective cohort of 24 patients, controlled with 105 patients who underwent lumbar or lumbosacral fusion [48]. A case report was also published on similar effects. This AE could be related to a high dose of rhBMP-2 or to an allergic reaction. However, the lack of surgical approach specification in those cases prevents the drawing of any definitive conclusion.
Discussion
Whereas the FDA-approved indication of rhBMP-2 (i.e. surgical treatment of degenerative disc disease by ALIF) is the only one to be well supported by a wide body of data [14], off-label use in other pathologies and surgical approaches has dramatically increased since 2002 [11, 12]. RCTs have been conducted to compare rhBMP-2-soaked collagen sponges to iliac crest autograft posterolateral lumbar arthrodesis [8, 37, 49–52]. But to our knowledge, only one RCT reporting the use of BMP has been published for PLIF [53], one for ACDF [54], and none for TLIF. In the posterolateral approach, Papakostidis et al. [55] conducted a meta-analysis of RCTs to evaluate the effectiveness of BMPs (rhBMP-2 or rhBMP-7) and concluded to highly significant superiority of rhBMP-2 compared with iliac crest bone graft in promoting fusion, particularly when additional instrumentation reinforced the construct. Based on a wide multisurgeon database, Williams et al. [32] realised univariate and multivariate analyses of the incidence of complications in spine fusion procedures, stratified according to the addition or not of rhBMP-2, the spine area and the surgical approach. Overall, rhBMP-2 use did not emerge as a risk factor for complications in anterior-only thoracolumbar fusion procedures, although patients who received the product were older and had more frequently undergone revision surgery. Conversely, considering anterior cervical fusion procedures, rhBMP-2 was a predictor of higher complication rates, particularly for wound infection. Similar results were reported by Cahill et al. [11].
Subsidence may result from osteoclast stimulation and bone resorption, a phenomenon that has been described in case of high rhBMP-2 concentration [35], progressively followed and replaced by a reactivation of osteoblasts and new bone formation. Hinsenkamp and Collard [56] recently reported the importance of concentration of rhBMP that may introduce some interactions with the effectiveness. The difference in concentration between DBM (demineralised bone matrix) and BMPs can vary to a magnitude order of 106, and this may explain the variability in efficiency and the adverse effects.
The unsatisfactory radiological outcomes reported in several series are likely to be attributable to the substitution of titanium cages with femoral ring [25] or bone dowel allografts [17, 18, 27]. The resorptive effect of rhBMP-2 on femoral ring allografts has also been mentioned in a case report [28]. Strengthening the construct with posterior pedicle screws during the time of bone remodelling may be a favourable option, as shown by Slosar et al. [24] in a prospective series of 75 patients (165 surgical levels). However, the majority of studies found no correlation between subsidence rates and lower clinical outcome in ALIFs [33, 57, 58]. Smoljanovic and Pecina [34] also commented on the transient period of bone remodelling observed by Burkus et al. [17] that “a clinical significance in this case seems to be negligible”. Other studies concluded the same [23, 27].
AEs associated with BMP include: ectopic bone formation, bone resorption or remodelling, haematoma, neck swelling and painful seroma [14, 46, 59]. Extradiscal, ectopic or heterotopic bone formation have been mainly reported for posterolateral fusions, TLIF or PLIF procedures [14]. A pilot RCT on the use of BMP in PLIF was stopped by the FDA due to several cases of intracanal bone formation [60]. The risk of foraminal bone formation and subsequent spinal cord compression may be increased when placing rhBMP-2-soaked sponges close to the dura mater; thus it may be decreased by inserting anterior fusion cages [61]. However, in most cases, those observations of unintended bone formation were not related to any lower clinical outcome neither did they require additional surgery or treatment [14, 62].
As might have been expected, dysphagia, neck swelling or respiratory difficulties occur more frequently after cervical surgery with rhBMP-2. These AEs remain scarce in lumbar fusion and with similar incidence whether rhBMP-2 is applied or not, no matter the surgical approach [11]. As a consequence, the United States’ authorities issued a public health notification in order to warn surgeons from inconsiderate usage of rhBMP-2 in cervical spine fusion, before any safe technique had been characterised and validated [15]. Based on animal studies, noxious effects of the exogenous growth factor on the nervous system have been assumed [63]. Neurological troubles, such as radiculitis, have not been reported for ALIF, and they remain a minor issue for patients after TLIF or PLIF [14, 47], even in case of intra-operative dural tear repair [47]. It should be emphasised that in the first RCTs, the surgeons preserved the posterior annulus and the posterior longitudinal ligament. This procedure decreased the risk for posterior rhBMP-2 leakage.
The application of rhBMP-2 in FDA-approved ALIF surgeries has received criticism for its potentially high rate of related AEs, which have been under-reported in the original publications on RCTs [36]. Thus, Carragee et al. claimed that some specific complications, notably subsidence, infections, RE and other urogenital events, arose more frequently in patients who received rhBMP-2 during ALIF surgery compared with those receiving autologous iliac crest bone graft without BMP. The controversy raised by Carragee et al. is questionable, as data from industry-sponsored pre-marketing studies were transmitted to the health authorities [7]. Carragee et al. [36] also present infection rates with bias. For instance, the authors pointed out high infection rates in BMP patients, but did not mention that the rates were similar in control patients. The authors also noted that early infection complications (<6 weeks after surgery) were similar in BMP and control patients according to FDA documents, while delayed infections (within 6-12 months after surgery) were more common in the BMP group (4.2 %) than in the control group (1.4 %). Again, the information is biased, as the authors did not mention that, if not only the six to 12 months but also the 12-24 months observation period is included in the calculation, the infection rate rises to 2.9 % in the control group. If only the 12-24 months period is considered, infection rates are 0 % and 1.4 % in the BMP and control groups, respectively [7]. Thus, depending on the observation period, fluctuations in the infection rates occur within both patient groups, whereas the overall rate of infections is similar in BMP and control patients (12.2 % versus 11.5 %).
Nevertheless, Carragee et al. also highlighted relevant safety concerns. When examining follow-up studies reported by Burkus et al. together with data published by the FDA, it becomes evident that rates of subsidence, RE and other urogenital events were higher in the BMP group [7, 21]. Additional reports on RE rates in ALIF surgeries performed with and without BMP have been published. A low incidence of RE (0.4 % and 1.3 %) was reported in two studies with over 600 patients receiving open ALIF surgeries, but no BMP [64, 65]. In another study, the RE rate in over 200 patients treated with ALIF surgery was 6.4 %. However, the authors did not report the type of graft applied in those patients, nor the presence of rhBMP-2 [66], so that the conclusion of Carragee et al. [36], that this relatively high RE rate was due to the application of BMP seems unfounded. The more recent review by Singh et al. [42] is nevertheless in favour of a higher rate of RE, particularly if high doses of BMP are used which can be considered as off-label usage.
The laparoscopic approach has been assumed to be a risk factor for some AEs (RE, subsidence, device loosening/displacement) in the FDA report [7], although a later publication did not confirm a higher frequency of subsidence and device-related events in the laparoscopic group [20]. The potential role of the laparoscopic surgical approach with the use of BMP in the occurrence of AEs was not evaluated, as the approach was left at the surgeon’s discretion, to avoid the need for learning a new technique. However, the original RCTs did not include any laparoscopic patient, whereas RE and subsidence rates were higher in the BMP group. Thus, although these increased rates of AEs may not have been statistically significant, the omission of a more detailed report on potentially BMP-related AEs gave rise to questions and mistrust amongst spinal surgeons [36]. The multivariate analysis of combined RCT data confirmed that RE incidence was significantly different whether ALIF was performed through a retroperitoneal or a transperitoneal approach (p = 0.029) [31]. As shown by Sasso et al. [38], the transperitoneal approach is a known cause of RE. The retroperitoneal approach appears much safer, with a limited rate of AEs, especially of RE (10 times less). Further studies are needed to clearly assess these safety concerns in the FDA-approved rhBMP-2 usage, i.e. ALIF procedure with tapered fusion cages. Supposed carcinogenicity of rhBMP-2 that was suspected in 2011 [36] is definitively not supported by the largest retrospective study reported by Kelly et al. [45] on half a million patients treated for lumbar fusion with and without BMP. As shown by Albilia et al. [67], the serum level of BMP is highly correlated with degenerative joint diseases and therefore it is impossible to associate the use of one dose of BMP-2 that disappeared completely of the body after 1 week to be responsible of cancer. This is confirmed by Kelly et al.’s study [45].
Conclusions and recommendations
The purpose of this review was to examine the safety profile of rhBMP-2 when used in ALIF surgery according to FDA approval [7]. After extensive review of RCTs and controlled series published up to the present point, we found no strong evidence of a significant increase of AEs related to rhBMP-2 during ALIF surgeries if its application follows FDA guidelines (level 1 evidence, two RCTs, one single-arm study, a total of 288 patients treated with BMP) [9, 10, 20]. Two additional RCTs performed with rhBMP-2 in combination with allogenic bone dowels reported increased bone remodelling in BMP patients. This AE was transient, and it had no consequence on the clinical outcome in those patients [16, 17]. No other BMP-related AEs were reported in these studies (level 1 evidence, two RCTs, a total of 79 patients treated with BMP).
Significantly increased rates of subsidence [23] and RE [21] were reported in case of an ALIF with rhBMP-2 (two cohort-controlled studies, level 2-3 evidence). Although these studies suggest that BMP was associated with an increased risk for those AEs, neither of them applied rhBMP-2 as approved by the FDA (indication, surgical method and implant, BMP dose). Notably, the following paragraph can be found in the respective FDA approval documents: “The safety and effectiveness of the BMP Bone Graft component with other spinal implants, implanted at locations other than the lower lumbar spine, or used in surgical techniques other than anterior open or anterior laparoscopic approaches have not been established” [7]. The clinical efficiency of rhBMP-2 is equal or superior to that of allogenic or autologous bone graft with respect to fusion rate, low back pain disability, patient satisfaction and rate of re-operations, provided that the treatment protocol closely adheres to FDA guidelines for the use of BMP/LT-cages in ALIF surgeries. In contrast, off-label application of rhBMP-2 in lumbar fusion surgery may lead to increased subsidence and RE, and possibly other unanticipated AEs.
References
Lavery K, Swain P, Falb D, Alaoui-Ismaili MH (2008) BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem 283(30):20948–20958. doi:10.1074/jbc.M800850200
Kawabata M, Imamura T, Miyazono K (1998) Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev 9(1):49–61
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A (2011) Bone morphogenetic proteins: a critical review. Cell Signal 23(4):609–620. doi:10.1016/j.cellsig.2010.10.003
Schwabe P, Simon P, Kronbach Z, Schmidmaier G, Wildemann B (2014) A pilot study investigating the histology and growth factor content of human non-union tissue. Int Orthop 38(12):2623–2629. doi:10.1007/s00264-014-2496-6
Johnson EE, Urist MR, Finerman GA (1988) Bone morphogenetic protein augmentation grafting of resistant femoral nonunions. A preliminary report. Clin Orthop Relat Res 230:257–265
Urist MR (1965) Bone: formation by autoinduction. Science 150(3698):893–899
FDA (2002) InFUSE™ Bone Graft/LT-CAGE™ Lumbar Tapered Fusion Devices—P000058. United States Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cftopic/pma/pma.cfm?num=P000058. Accessed 05 Sept 2012
Boden SD, Zdeblick TA, Sandhu HS, Heim SE (2000) The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine 25(3):376–381
Burkus JK, Gornet MF, Dickman CA, Zdeblick TA (2002) Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech 15(5):337–349
Burkus JK, Dorchak JD, Sanders DL (2003) Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine (Phila Pa 1976) 28(4):372–377. doi:10.1097/01.BRS.0000048469.45035.B9
Cahill KS, Chi JH, Day A, Claus EB (2009) Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 302(1):58–66. doi:10.1001/jama.2009.956
Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD (2010) Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine 35(19):1794–1800. doi:10.1097/BRS.0b013e3181ecf6e4
Courvoisier A, Sailhan F, Laffenetre O, Obert L, French Study Group of BMPiOS (2014) Bone morphogenetic protein and orthopaedic surgery: can we legitimate its off-label use? Int Orthop 38(12):2601–2605. doi:10.1007/s00264-014-2534-4
Mroz TE, Wang JC, Hashimoto R, Norvell DC (2010) Complications related to osteobiologics use in spine surgery: a systematic review. Spine 35(9 Suppl):S86–S104. doi:10.1097/BRS.0b013e3181d81ef2
FDA (2008) FDA public health notification: life-threatening complications associated with recombinant human bone morphogenetic protein in cervical spine fusion. US FDA. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm062000.htm
Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA (2002) Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine 27(21):2396–2408. doi:10.1097/01.BRS.0000030193.26290.DD
Burkus JK, Sandhu HS, Gornet MF, Longley MC (2005) Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am 87(6):1205–1212. doi:10.2106/JBJS.D.02532
Burkus JK, Sandhu HS, Gornet MF (2006) Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine (Phila Pa 1976) 31(7):775–781. doi:10.1097/01.brs.0000206357.88287.5a
Burkus JK, Heim SE, Gornet MF, Zdeblick TA (2003) Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech 16(2):113–122
Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA (2009) Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am 91(5):1181–1189. doi:10.2106/JBJS.G.01485
Carragee EJ, Mitsunaga KA, Hurwitz EL, Scuderi GJ (2011) Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J 11(6):511–516. doi:10.1016/j.spinee.2011.02.013
Comer GC, Smith MW, Hurwitz EL, Mitsunaga KA, Kessler R, Carragee EJ (2012) Retrograde ejaculation after anterior lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: a 10-year cohort controlled study. Spine J 12(10):881–890. doi:10.1016/j.spinee.2012.09.040
Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD (2007) Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg (Br) 89(3):342–345. doi:10.1302/0301-620X.89B3.18270
Slosar PJ, Josey R, Reynolds J (2007) Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J 7(3):301–307. doi:10.1016/j.spinee.2006.10.015
Pradhan BB, Bae HW, Dawson EG, Patel VV, Delamarter RB (2006) Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 31(10):E277–E284. doi:10.1097/01.brs.0000216442.12092.01
Kleeman TJ, Ahn UM, Talbot-Kleeman A (2001) Laparoscopic anterior lumbar interbody fusion with rhBMP-2: a prospective study of clinical and radiographic outcomes. Spine (Phila Pa 1976) 26(24):2751–2756
Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG (2008) Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech 21(8):557–562. doi:10.1097/BSD.0b013e31815ea897
Hansen SM, Sasso RC (2006) Resorptive response of rhBMP2 simulating infection in an anterior lumbar interbody fusion with a femoral ring. J Spinal Disord Tech 19(2):130–134. doi:10.1097/01.bsd.0000168512.61351.3a
Mines D, Gu Y, Kou TD, Cooper GS (2011) Recombinant human bone morphogenetic protein-2 and pancreatic cancer: a retrospective cohort study. Pharmacoepidemiol Drug Saf 20(2):111–118. doi:10.1002/pds.2057
Moshel YA, Hernandez EI, Kong L, Liu C, Samadani U (2008) Acute renal insufficiency, supraventricular tachycardia, and confusion after recombinant human bone morphogenetic protein-2 implantation for lumbosacral spine fusion. J Neurosurg Spine 8(6):589–593. doi:10.3171/SPI/2008/8/6/589
Burkus JK, Dryer RF, Peloza JH (2012) Retrograde ejaculation following single-level anterior lumbar surgery with or without recombinant human bone morphogenetic protein-2 in 5 randomized controlled trials. J Neurosurg Spine. doi:10.3171/2012.10.SPINE11908
Williams BJ, Smith JS, Fu KM, Hamilton DK, Polly DW Jr, Ames CP, Berven SH, Perra JH, Knapp DR Jr, McCarthy RE, Shaffrey CI (2011) Does BMP increase the incidence of perioperative complications in spinal fusion? A comparison of 55,862 cases of spinal fusion with and without BMP. Spine. doi:10.1097/BRS.0b013e318216d825
Zhang JD, Poffyn B, Sys G, Uyttendaele D (2012) Are stand-alone cages sufficient for anterior lumbar interbody fusion? Orthop Surg 4(1):11–14. doi:10.1111/j.1757-7861.2011.00164.x
Smoljanovic T, Pecina M (2008) RE: complications attributable to the use of rhBMP-2 inside the femoral ring allograft during anterior lumbar interbody fusion. Spine J 8(2):413–414. doi:10.1016/j.spinee.2007.11.004, author reply 414
Kanatani M, Sugimoto T, Kaji H, Kobayashi T, Nishiyama K, Fukase M, Kumegawa M, Chihara K (1995) Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J Bone Miner Res 10(11):1681–1690. doi:10.1002/jbmr.5650101110
Carragee EJ, Hurwitz EL, Weiner BK (2011) A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 11(6):471–491. doi:10.1016/j.spinee.2011.04.023
Dimar JR, Glassman SD, Burkus KJ, Carreon LY (2006) Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine (Phila Pa 1976) 31(22):2534–2539. doi:10.1097/01.brs.0000240715.78657.81, discussion 2540
Sasso RC, Burkus JK, LeHuec JC (2003) Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine (Phila Pa 1976) 28(10):1023–1026. doi:10.1097/01.BRS.0000062965.47779.EB
Smoljanovic T, Siric F, Bojanic I (2010) Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am 92(15):2614–2615, author reply 2615-2616
Than KD, Wang AC, Rahman SU, Wilson TJ, Valdivia JM, Park P, La Marca F (2011) Complication avoidance and management in anterior lumbar interbody fusion. Neurosurg Focus 31(4):E6. doi:10.3171/2011.7.FOCUS11141
Inamasu J, Guiot BH (2005) Laparoscopic anterior lumbar interbody fusion: a review of outcome studies. Minim Invasive Neurosurg: MIN 48(6):340–347. doi:10.1055/s-2005-915634
Singh K, Ahmadinia K, Park DK, Nandyala SV, Marquez-Lara A, Patel AA, Fineberg SJ (2014) Complications of spinal fusion with utilization of bone morphogenetic protein: a systematic review of the literature. Spine (Phila Pa 1976) 39(1):91–101. doi:10.1097/brs.0000000000000004
Lykissas MG, Aichmair A, Sama AA, Hughes AP, Lebl DR, Cammisa FP, Girardi FP (2014) Nerve injury and recovery after lateral lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: a cohort-controlled study. Spine J 14(2):217–224. doi:10.1016/j.spinee.2013.06.109
Carragee EJ, Chu G, Rohatgi R, Hurwitz EL, Weiner BK, Yoon ST, Comer G, Kopjar B (2013) Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am 95(17):1537–1545. doi:10.2106/jbjs.l.01483
Kelly MP, Savage JW, Bentzen SM, Hsu WK, Ellison SA, Anderson PA (2014) Cancer risk from bone morphogenetic protein exposure in spinal arthrodesis. J Bone Joint Surg Am 96(17):1417–1422. doi:10.2106/jbjs.m.01190
Benglis D, Wang MY, Levi AD (2008) A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery 62(5 Suppl 2):ONS423–ONS431. doi:10.1227/01.neu.0000326030.24220.d8, discussion ONS431
Glassman SD, Gum JL, Crawford CH 3rd, Shields CB, Carreon LY (2011) Complications with recombinant human bone morphogenetic protein-2 in posterolateral spine fusion associated with a dural tear. Spine J 11(6):522–526. doi:10.1016/j.spinee.2010.05.016
Latzman JM, Kong L, Liu C, Samadani U (2010) Administration of human recombinant bone morphogenetic protein-2 for spine fusion may be associated with transient postoperative renal insufficiency. Spine (Phila Pa 1976) 35(7):E231–E237. doi:10.1097/BRS.0b013e3181c71447
Glassman SD, Dimar JR, Carreon LY, Campbell MJ, Puno RM, Johnson JR (2005) Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine 30(15):1694–1698
Glassman SD, Carreon LY, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, Dimar JR (2008) RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: a randomized, controlled trial in patients over sixty years of age. Spine 33(26):2843–2849. doi:10.1097/BRS.0b013e318190705d
Dawson E, Bae HW, Burkus JK, Stambough JL, Glassman SD (2009) Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation. A prospective randomized trial. J Bone Joint Surg Am 91(7):1604–1613. doi:10.2106/JBJS.G.01157
Dimar JR 2nd, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY (2009) Clinical and radiographic analysis of an optimized rhBMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am 91(6):1377–1386. doi:10.2106/JBJS.H.00200
Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK (2004) Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J 4(5):527–538. doi:10.1016/j.spinee.2004.03.025, discussion 538-529
Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA (2003) A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine 28(12):1219–1224. doi:10.1097/01.BRS.0000065486.22141.CA, discussion 1225
Papakostidis C, Kontakis G, Bhandari M, Giannoudis PV (2008) Efficacy of autologous iliac crest bone graft and bone morphogenetic proteins for posterolateral fusion of lumbar spine: a meta-analysis of the results. Spine (Phila Pa 1976) 33(19):E680–E692. doi:10.1097/BRS.0b013e3181844eca
Hinsenkamp M, Collard JF (2015) Growth factors in orthopaedic surgery: demineralized bone matrix versus recombinant bone morphogenetic proteins. Int Orthop 39(1):137–147. doi:10.1007/s00264-014-2562-0
Choi JY, Sung KH (2006) Subsidence after anterior lumbar interbody fusion using paired stand-alone rectangular cages. Eur Spine J 15(1):16–22. doi:10.1007/s00586-004-0817-y
Beutler WJ, Peppelman WC Jr (2003) Anterior lumbar fusion with paired BAK standard and paired BAK Proximity cages: subsidence incidence, subsidence factors, and clinical outcome. Spine J 3(4):289–293
Winkler S, Niedermair T, Fuchtmeier B, Grifka J, Grassel S, Anders S, Heers G, Wagner F (2015) The impact of hypoxia on mesenchymal progenitor cells of human skeletal tissue in the pathogenesis of heterotopic ossification. Int Orthop 39(12):2495–2501. doi:10.1007/s00264-015-2995-0
McKay B, Sandhu HS (2002) Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine (Phila Pa 1976) 27(16 Suppl 1):S66–S85
Mummaneni PV, Pan J, Haid RW, Rodts GE (2004) Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the joint section meeting on disorders of the spine and peripheral nerves, March 2004. J Neurosurg Spine 1(1):19–23. doi:10.3171/spi.2004.1.1.0019
Joseph V, Rampersaud YR (2007) Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine (Phila Pa 1976) 32(25):2885–2890. doi:10.1097/BRS.0b013e31815b7596
Dmitriev AE, Lehman RA, Symes AJ (2011) Bone morphogenetic protein-2 and spinal arthrodesis: the basic science perspective on protein interaction with the nervous system. Spine J 11(6):500–505
Sasso RC, Best NM, Mummaneni PV, Reilly TM, Hussain SM (2005) Analysis of operative complications in a series of 471 anterior lumbar interbody fusion procedures. Spine (Phila Pa 1976) 30(6):670–674
Sasso RC, Kitchel SH, Dawson EG (2004) A prospective, randomized controlled clinical trial of anterior lumbar interbody fusion using a titanium cylindrical threaded fusion device. Spine (Phila Pa 1976) 29(2):113–122. doi:10.1097/01.BRS.0000107007.31714.77, discussion 121-112
Jarrett CD, Heller JG, Tsai L (2009) Anterior exposure of the lumbar spine with and without an “access surgeon”: morbidity analysis of 265 consecutive cases. J Spinal Disord Tech 22(8):559–564. doi:10.1097/BSD.0b013e318192e326
Albilia JB, Tenenbaum HC, Clokie CM, Walt DR, Baker GI, Psutka DJ, Backstein D, Peel SA (2013) Serum levels of BMP-2, 4, 7 and AHSG in patients with degenerative joint disease requiring total arthroplasty of the hip and temporomandibular joints. J Orthop Res 31(1):44–52. doi:10.1002/jor.22182
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of competing interests
Nothing to disclose for the authors.
Rights and permissions
About this article
Cite this article
Faundez, A., Tournier, C., Garcia, M. et al. Bone morphogenetic protein use in spine surgery—complications and outcomes: a systematic review. International Orthopaedics (SICOT) 40, 1309–1319 (2016). https://doi.org/10.1007/s00264-016-3149-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-016-3149-8