Abstract
Purpose
The reason for the formation of an atrophic non-union is not clear and an altered vascularization as well as a deregulation of endogenous growth factors is hypothesized. To obtain more information, we analysed human non-union tissue regarding the histology and quantity of several growth factors.
Methods
Tissue from patients with an atrophic non-union (n = 44) or with a healed fracture (n = 13) was analysed. Using histological and immunohistochemical methods the tissue composition was investigated. On the protein level the amount of several growth factors important for bone healing was analysed.
Results
The tissue composition was very inhomogeneous containing fibrous, cartilaginous and bony tissue. Vessels were present in all investigated samples without a difference between the tissue from non-union and control patients. The growth factor BMP-2 was below the detection limit in all samples, whereas IL-6 and IGF-I were measured only in a few samples of both groups. TGF-ß1, VEGF-A and BMP-4 were detectable in the majority of the samples of both groups with a high variability in the amount but no difference between the groups. The quantity of both growth factors, BMP-7 and PDGF-AB, was significantly lower in the non-union tissue compared to the healed controls.
Conclusion
The reduced quantity of BMP-7 and PDGF-AB might be responsible for the impaired healing. Further studies analysing material from more patients and investigating the early healing phases, however, are necessary to obtain further information and consequently improve healing strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the optimal case, a fracture heals within an appropriate period of time resulting in the reconstitution of cortical union and the mechanical properties, which ensure the function and stability of the bone. Several factors influence the healing process, for example, the stability of the fixation, the defect size, bone quality, cellular composition, vascularization and other biological factors [1]. An alteration of one or more of these factors can lead to a delayed healing or, even worse, to the formation of a non-union. It is assumed that an inappropriate mechanical stabilization is mainly responsible for the formation of a hypertrophic non-union which is characterized by the formation of a large soft tissue callus, which does not mineralize. Atrophic non-unions can also occur without previous hypertrophy, whereupon the reasons are still not known. The process of bone healing recapitulates partially the phases of embryonic tissue formation starting with the condensation of mesenchymal stem cells and the creation of a cartilaginous callus that is mineralized over time [2]. This bone formation process is tightly regulated by the interplay of several cell types and the action of specific factors. It has been shown in previous studies that factors important for healing and cellular processes are expressed differentially during different phases of bone healing [3]. In contrast to embryonic bone formation fracture healing starts with the phase of inflammation and cytokines such as IL-1, IL-6 and TNF-a are expressed. IL-1 and TNF-a are also associated with bone remodeling at the end of the healing [4]. During cartilage formation, resorption and bone formation members of the TGF-ß superfamily are important factors [3]. Their activity is regulated by several receptors, agonists and antagonists [5].
Angiogenesis is a crucial process for bone formation and a disturbance might cause a healing impairment. This process is regulated by Angiopoitin and VEGF, but also by BMPs [6].
To gain more knowledge on local factors that might be altered in the case of impaired healing, human tissue was harvested during surgical intervention for a non-union treatment and from patients during implant removal (control). The tissue and growth factor composition was analysed in both group.
Material and methods
Human tissue specimens
A total of 57 patients were included in the study (Table 1). Patients were either treated for the condition of a manifest atrophic non-union or received an implant removal and served as the control group. Before surgery all patients signed informed consent. Inclusion criteria for the non-union group were a manifest atrophic non-union and a time span from the initial operation until the revision surgery of a least six months. For the implant removal group the former fracture or rather the fracture callus area had to be easily accessible through the same approach as the planned implant removal. Therefore only patients who were initially treated with a plate osteosynthesis were included in the study. All patients had to be at least 18 years of age and had to be in a good general state of health.
All surgical procedures were performed under general anaesthesia. Patients with atrophic non-unions received a complete exposure of the non-union area, followed by a profound debridement of the former fracture gap by resection of atrophic tissue and re-stabilisation. In patients who received an implant removal a specimen was obtained from the callus area around the former fracture gap after osteosynthetic material was removed and the bone was exposed. The harvested tissue was prepared for further histological and protein/growth factor analysis immediately.
The study was approved by the institutional review board (Ethikkommission der Charité-Universitätmedizin Berlin, Nr.: EA1/031/09).
Tissue preparation
Tissue samples were harvested during surgery and, if possible, the samples were split for histological analysis and protein quantification. For histological processing the tissue sample was placed in 4 % formaldehyde and fixed for 48 hours. After fixation the sample was split again and processed for undecalcified plastic embedding in Technovit 9100 neu (Heraeus Kulzer, Germany) and decalcified paraffin embedding.
The other half of the samples were stored immediately in PBS plus Proteinase inhibitor (Complete, Roche, Penzberg, Germany) at −80 °C. For protein analysis the proteins were isolated by the principle of dialysis. For this purpose the samples were pulverized using a cooled mortar and pestle (Retsch, Haan, Germany) and finally homogenized with an ultra turrax (IKA-Labortechnik, Staufen, Germany). The homogenized bony material was then diluted in a 4 M solution of guanidine hydrochloride and dialyzed against aqua dest. for 24 hours (for further details see [7]).
Histology and immunohistology
The following histological stainings were performed: hematoxylin/eosin (general overview), Masson Goldner (fibrous tissue components), Movat-Pentachrome (differentiation mineralized and non-mineralized cartilage), Alcian blue (cartilage) for paraffin embedded tissue and van Kossa/safranin orange (mineralized tissue), methyl green/safranin orange (mineralized cartilage) for PMMA embedded tissue.
For the immunohistological staining, anti-alpha smooth muscle antibody (Dako, German; monoclonal mouse, Clone 1A4) was used in a dilution of 1:100 to visualize the vessels, and anti-noggin (Abcam, Cambridge; rabbit polyclonal, clone ab16054) in a dilution of 1:600 and anti-sclerostin (Santa Cruz Technology, USA; rabbit polyclonal, clone sc-130258) in a dilution of 1:100 to visualize bone morphogenic protein antagonists. As secondary antibody a biotin labeled antibody raised against the primary antibody host species was used. After incubation with a complex of avidin and biotin/alkaline phosphatase (Vector Laboratories, USA; Vectastain Alkaline Phosphatase Standard, AK-5000) visualization was done with chromogenic substrate (Vector Laboratories, USA; Alkaline Phosphatase Substrate Kit I).
Protein and growth factor quantification
Total protein concentration was quantified with Coomassie Plus Protein Assay (Pierce, Perbio Science GmbH, Germany). The ELISA-method was used to quantify the following proteins: IGF-I, BMP-2, BMP-4, PDGF-AB, VEGF-A (human ELISA kits obtained from R&D-Systems, Wiesbaden, Germany), IL-6 and TGF-β1 (human ELISA kits obtained from IBL, Hamburg, Germany). The analyses were performed as instructed by the manufacturer. If necessary, samples were pretreated as directed in the manufacturer’s instructions.
Statistical analysis
The values are reported as median with the 25 and 75 % quartile. For comparison of data a non-parametric Mann–Whitney U Test was used. Statistical differences were defined at a 95 % confidence level. PASW 18 (SPSS Inc., Chicago, USA) software supported statistical evaluation.
Results
Human tissue specimens
A total of 57 patients were included in the study. Forty-four patients underwent surgery for the treatment of an atrophic non-union and 13 patients received an implant removal (control group). The mean age of all patients was 49 years (range 20–74). The age distribution was comparable between the groups (non-union group, 50 years; control group, 44 years). The gender distribution in the non-union group was even (22/22), the control group showed a difference to favour the male population (m/f: 9/4). The main location of the non-unions was femur (16), followed by tibia (12), clavicle (nine), ulna (four) and humerus (three). In the control group implants were removed from tibia (four), ulna (four), femur (two), radius (one) and metacarpus (one).
After collection of the tissue samples they were prepared for either histological investigation or growth factor quantification. In the non-union group 25 samples were used for histology and growth factors were quantified in 19 samples. The samples harvested during implant removal were divided in five for histology and eight for growth factor analysis.
Histology
The tissue was a very heterogeneous mixture of fragments of lamellar bone, immature and hypertrophic cartilage, unorganized fibrous tissue and newly formed woven bone (Fig. 1). Vessels and blood cells were also abundant. Independent of the group, bone apposition and resorption was seen in the tissue samples. Differences between the groups were not obvious.
Histological staining of the tissue from control (left) or non-union (right) patients. Paraffin embedded tissue, stained in a, g, shows hematoxylin/eosin, in b, h with Alcian blue, in c, i with Masson Goldner and in d, j with Movat-Pentachrome. The plastic sections are stained with van Kossa/safranin orange (e, k), and methyl green/safranin orange (f, l). The different stainings shows the heterogeneity of control tissue as well as in nonunion tissue. The heterogeneity caused by different quantity of cartilage (b+h; f+l), mineralized bone (e+k), no mineralized bone (d+j) and fibrous tissue (c+i). Scale bars: 200 μm
Immunohistology
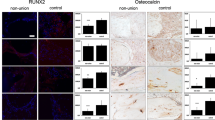
Vessels were visualized with the antibody against alpha smooth muscle actin. Bone morphogenic protein antagonists were visualized with the antibody against sclerostin and noggin.
All investigated tissue samples showed well vascularized but also unvascularized areas and no difference between the non-union and the control tissue was found (Fig. 2a, and d). Bone morphogenic antagonists were demonstrated in non-union and control tissue (Fig. 2b–f).
Total protein and growth factor quantification
After weighing the material the protein was extracted. Total protein and the quantity of different growth factors were analyzed. Differences were seen in the amount of protein in the samples. The values range from 1.07 up to 12.28 μg/mg. This reflects the inhomogeneous tissue composition as seen in the histology. To consider this variation, the measured quantity of the different growth factors was either normalized to the extracted total protein or to the weight of the sample tissue (Table 2).
The values for BMP-2 were under the detection limit in all samples.
IL-6 or IGF-I were detectable in 9/19 or 7/19 non-union samples and 1/8 (same sample in both factors) control samples, respectively. The other factors were detectable in the majority of the samples. TGF-ß1 showed the highest median values with 3,353 pg/mg protein (17 pg/mg tissue) and BMP-4 the lowest median values with 13 pg/mg protein (0.04 pg/mg tissue). The variability of the measured values was high for all quantified growth factors (Table 2). The amount of BMP-7 quantified in the non-union tissue was significantly lower (p = 0.031) compared to the healed control (Fig. 3a). A significantly lower (p = 0.047) PDGF amount was also detected in the non-union samples (Fig. 3b).
Discussion
Non-unions are a feared complication in trauma surgery. Animal models and clinical studies focus on the elucidation of the reason for this healing impairment [8–10]. This study focuses on the identification of factors, which are altered resulting in the non-union formation. The results show a significant reduction of the growths factors BMP-7 and PDGF on the protein level in the human non-union tissue compared to tissue from healed fractures. This is the first study quantifying factor alterations in human non-union tissue on a protein level. Despite the rather small number of patients and the heterogeneous patient and non-union collection in this study, the results are supported by previous studies investigating RNA expression patterns and western blots [11]. The expression of BMP-7 and BMP-8 was significantly down regulated in the non-union tissue; BMP-2 expression was not altered, whereas BMP-4 and BMP-5 were significantly up regulated. In the present study BMP-2 was under the detection level of the ELISA and BMP-4 was similar between the groups. The comparability of the results is from special interest, because they investigated hypertrophic non-union tissue and the present study used tissue from atrophic non-unions. Kloen et al. recently published a study showing a co-localization of BMPs, phosphorylated Smads and the co-receptors in non-union tissue with a different pattern compared to fracture callus tissue [10]. Focusing on cartilage tissue, a reduction of the BMP-2 and BMP-14 staining was seen in the non-unions compared to healing tissue [12]. No difference was detectable in the staining for the antagonist noggin, which was also observed in the present study. Taken together, the investigation of human material harvested from non-unions highlights the importance of the BMP signaling during healing. The major drawbacks of the analysis of human tissue are the high variability of the obtained material and that mainly end-point analyses are possible. An additional limitation of the presented study is the rather low number of patients embracing the fact of high variability of collected tissue. Also, adequate controls for human non-union tissue are difficult to obtain. In the present study control tissue from healed fractures at the time of implant removal was harvested. Other studies used no control tissue [13] or biopsies from malunions, fixation failures or fresh fractures [12]. These tissues, however, do not represent the optimal controls. Therefore, animal studies are an advantage, because the fracture localization is standardized, different healing time points can be investigated, and controls are available. The major challenge in the animal studies, however, is the induction of a non-union similar to the clinical situation. Induction of a non-union by cauterization of the periosteum is a reliable method [9], but it does not mirror completely the clinical situation. Using this model a global gene profiling was done at several time points to analyse the expression of the BMP signaling pathway [14]. BMP-2/4/6/7 were significantly lower expressed in the atrophic non-union tissue starting after day 10 post surgery. The inhibitors noggin, drm (gremlin), sclerostin and BAMBI were also significantly reduced, mainly at the later time points. This comparison of the different time points shows that changes in the BMP signaling occur primarily in the middle and late healing phase and support the results from the human tissue studies.
A significantly lower amount of the PDGF-AB protein was measured in the human non-union tissue in the present study. PDGF is released by platelets and detectable in fracture tissue [15]. No studies investigating the expression of PDGF during impaired bone healing were found, but reduction of PDGF was seen during impaired wound healing [16]. Investigating polymorphism in patients suffering from non-unions, a significant association of a PDGF haplotype with non-unions was observed [17]. These results highlight the importance of PDGF during healing.
Using different histological stainings the tissue composition was investigated. Due to the high heterogeneity of the obtained tissue it was very difficult to compare the samples and no quantification of tissue types was possible and also there was no quantification of the vessels within the tissues. Independent of the healing type, fibrous tissue, cartilage, mineralized and well vascularized areas were seen, however, no differences were obvious.
Surprising was that BMP-2 was not detectable in the samples. Using the same extraction method we previously quantified both factors in demineralized bone matrix (DBM) [7]. Interestingly, BMP-4 was under the detection limit in the DBM samples. The extraction of proteins from the tissue might influence the detectable amount of different growth factors. Therefore, we compared in a previous study three different extraction methods and found the best extraction efficacy for BMP-2 using the guanidine HCl method [18].
A just recently published study reviewed the biological effect and clinical use of BMP-2 and BMP-7. In this context the authors presented the preclinical data of BMP-6 locally applied from a whole blood device [19]. Unfortunately, we have not quantified BMP-6 in our samples but, based on the data presented in the review, BMP-6 is also an important factor during bone healing.
Conclusion
This study demonstrates a significantly reduced amount of BMP-7 and PDGF-AB protein in tissue harvested from atrophic non-unions when compared to healed fracture tissue, even using a limited number of patient materials. The tissue composition and the quantity of the analysed factors were very heterogeneous, but it was still possible to detect significant differences. Due to the fact that human tissue cannot easily be harvested at different time points of the healing process, reliable animal models are necessary to investigate in detail the pathology of impaired bone healing. The combination of the results from human tissue examinations and animal models will provide new insights into the bone healing process and will allow the development of new treatment strategies.
References
Giannoudis PV, Einhorn TA, Schmidmaier G, Marsh D (2008) The diamond concept—open questions. Injury 39(Suppl 2):S5–S8
Ferguson C, Alpern E, Miclau T, Helms JA (1999) Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev 87:57–66
Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA (2008) Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res 87:107–118
Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, Graves D et al (2001) Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res 16:1004–1014
Ruschke K, Hiepen C, Becker J, Knaus P (2012) BMPs are mediators in tissue crosstalk of the regenerating musculoskeletal system. Cell Tissue Res 347:521–544
David L, Feige JJ, Bailly S (2009) Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev 20:203–212
Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G (2007) Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res 81:437–442
Garcia P, Histing T, Holstein JH, Klein M, Laschke MW, Matthys R et al (2013) Rodent animal models of delayed bone healing and non-union formation: a comprehensive review. Eur Cell Mater 26:1–12
Kokubu T, Hak DJ, Hazelwood SJ, Reddi AH (2003) Development of an atrophic nonunion model and comparison to a closed healing fracture in rat femur. J Orthop Res 21:503–510
Kloen P, Lauzier D, Hamdy RC (2012) Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone 51:59–68
Fajardo M, Liu CJ, Egol K (2009) Levels of expression for BMP-7 and several BMP antagonists may play an integral role in a fracture nonunion: a pilot study. Clin Orthop Relat Res 467:3071–3078
Kwong FN, Hoyland JA, Freemont AJ, Evans CH (2009) Altered relative expression of BMPs and BMP inhibitors in cartilaginous areas of human fractures progressing towards nonunion. J Orthop Res 27:752–757
Kloen P, Doty SB, Gordon E, Rubel IF, Goumans MJ, Helfet DL (2002) Expression and activation of the BMP-signaling components in human fracture nonunions. J Bone Joint Surg Am 84-A:1909–1918
Niikura T, Hak DJ, Reddi AH (2006) Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. J Orthop Res 24:1463–1471
Andrew J, Hoyland J, Freemont A, Marsh D (1995) Platelet-derived growth factor expression in normally healing human fractures. Bone 16:455–460
Beer HD, Longaker MT, Werner S (1997) Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol 109:132–138
Zeckey C, Hildebrand F, Glaubitz LM, Jurgens S, Ludwig T, Andruszkow H et al (2011) Are polymorphisms of molecules involved in bone healing correlated to aseptic femoral and tibial shaft non-unions? J Orthop Res 29:1724–1731
Wildemann B, Kadow-Romacker A, Pruss A, Haas NP, Schmidmaier G (2007) Quantification of growth factors in allogenic bone grafts extracted with three different methods. Cell Tissue Bank 8:107–114
Vukicevic S, Oppermann H, Verbanac D, Jankolija M, Popek I, Curak J et al (2014) The clinical use of bone morphogenetic proteins revisited: a novel biocompatible carrier device OSTEOGROW for bone healing. Int Orthop 38:635–647
Acknowledgments
The study was supported by a grant from Synthes, USA.
Conflict of interest
The authors have no conflict of interest related to the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwabe, P., Simon, P., Kronbach, Z. et al. A pilot study investigating the histology and growth factor content of human non-union tissue. International Orthopaedics (SICOT) 38, 2623–2629 (2014). https://doi.org/10.1007/s00264-014-2496-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2496-6