Abstract
Human glycoprotein of cartilage (YKL-40) synthesises chondrocytes and synovial cells in inflammatory conditions or remodels the outer cell matrix in osteoarthritis. The aim of this study was to conduct a parallel analysis between thickness of cartilage and length of osteophytes, ultrasound indicators of joint destruction, with levels of YKL-40 in serum in patients with primary osteoarthritis. Ultrasound findings and concentration of YKL-40(ng/ml) were examined in 88 patients. The average value cartilage thickness measured on medial condyles of the femur was 1.30 ± 0.23 mm and on lateral was 1.39 ± 0.27 mm. Median YKL-40 in patients with shorter osteophytes was 62.0 (44.5–90) ng/ml, and with longer osteophytes was 119 (range 80–171) ng/ml (p = 0.000). YKL-40 can be a marker for the appearance of longer osteophytes (sensitivity = 79.1%; specificity = 61.9%;cut off = 75.0 ng/ml). The duration of illness is very much connected to values of YKL-40 (r = 0.651, p = 0.000). After an illness duration of five years, the concentration of YKL-40 was 83.68 ± 33.65 ng/ml, after ten years it was 138.22 ± 48.88 ng/ml, and after 15 and 20 years it was 209.30 ± 79.36 ng/ml and 218.50 ± 106.51 ng/ml, respectively. Higher concentrations of YKL-40 indicate the level of cartilage destruction and can be used for assessment of destruction.
Résumé
La glycoprotéine YKL-40 est synthétisée par les chondrocytes des cellules synoviales lorsqu’il existe une d’inflammation et un remodelage dans l’arthrose du genou. Le but de cette étude est de faire, en parallèle, une analyse entre l’épaisseur du cartilage et la dimension des ostéophytes, les lésions constatées par échographie ainsi que le taux sérique de YKL-40 dans l’arthrose primaire du genou. L’échographie et la concentration de YKL-40 (ng/ml) ont été examinées chez 88 patients. L’épaisseur moyenne du cartilage a été mesurée au niveau du condyle interne du fémur. Elle était de 1,30 +/– 0,233 mm, au niveau du condyle externe elle était de 1,39 +/– 0,27 mm. Le taux moyen de YKL-40 des patients porteurs d’ostéophytes relativement brefs était de 62,0 (44,5–90)ng/ml, pour des ostéophytes plus longs de 119 (80–171)ng/ml (p = 0,000). Le taux sérique de YKL-40 peut donc être un marqueur de la dimension des ostéophytes avec une sensitivité de 79,1% , une spécificité de 61,9%, et un taux limite de 75,0 ng/ml. La durée de l’évolution de la pathologie est également en relation directe avec les valeurs de YKL-40 (r = 0,651 p = 0,000). Après une durée d’évaluation des lésions de moins de 5 ans la concentration d’YKL-40 est de 83,68 +/– 33,65 ng/ml, après 10 ans de 138,22 +/– 48,88 ng/ml et après 15 et 20 ans de 209,30 +/– 79,36 ng/ml et 218,50 +/– 106,51 ng/ml. Une concentration élevée de YKL-40 est donc un indicateur permettant d’évaluer le niveau de destruction du cartilage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a group of chronic degenerative joint diseases of the synovial joint characterised by focal deterioration, abrasion and loss of articular cartilage, with sclerosis, cystic formations and destruction of subchondral bone, in addition to hypertrophy of bone tissue on the edges of joint and formation of osteophytes on the joint surface [14]. Knee osteoarthritis is one of the most common osteoarthroses of peripheral joints.
Ultrasound examination (US) of the joints, arthrosonography, is much more sensitive than clinical examination and is highly recommended in knee diseases [11]. The radiological characteristics of joint osteoarthritis, narrowing of joint space, sclerosis of subchondral bone and osteophytes on the joint edges can usually be noticed after several years of continuing disease. In comparison to standard radiography, ultrasound is able to display the cartilage, soft tissue structures and joint effusion [17].
With most patients, there is permanent or intermittent inflammation of the synovial lining giving rise to joint effusion and increased discomfort. The development of proliferative synovitis worsens chondropathy and damages joint structures [11]. The application whole-genome analysis techniques has also contributed to a better understanding of physiological and pathological processes involved in homeostasis of bone and cartilage tissues [1]. The disturbance of the physiological balance between decomposition and regeneration of the cartilage outer cell matrix leads to progressive loss of cartilage tissue and bone damage. During this process fragments of connective tissue matrix are released. These molecules specifically show the quantitative and dynamic changes in remodelling of the outer cell matrix, i.e. a process of decomposition and reparation [13]. They are detected by biological fluids and their concentrations reflect the metabolic changes of bone, cartilage and synovial tissue. Human glycoprotein of cartilage (YKL-40), known as human cartilage glycoprotein 39 (HCgp-39; GP-39) is a 38-40-kDa glycoprotein that is homologous to bacterial chitinases and may function as a lectin and play a role in tissue remodelling, including articular cartilage. YKL-40 is a major secretory product of chondrocytes and synovial cells, but is also produced by activated macrophages, liver fibrocytes, cancer cells of the colon, breasts, lungs, ovaries and prostate [6, 18], as well as osteosarcoma cells (MG-63) [9]. YKL-40 mRNA is expressed by cartilage from patients with rheumatoid arthritis, but is not detectable in normal human cartilage [21].
In chondrocyte monolayer cultures, interleukin-1beta (IL-1beta) and transforming growth factor beta (TGFbeta) decreased the levels of secreted YKL-40, and this was associated with a reduction in YKL-40 messenger RNA levels. Immunofluorescence microscopy showed YKL-40 staining in the Golgi system of the chondrocytes, but YKL-40 could not be detected in the extracellular matrix [10].
YKL-40 levels are low in normal human cartilage but are increased in both inflammatory and degenerative joint disease; therefore, YKL-40 may be a biomarker of cartilage turnover and synovitis [7].
YKL-40 in synovial fluid is derived from cells in the inflamed synovium, chondrocytes and synovial fluid neutrophils and reflect local disease activity [3, 20]. Increased serum levels of YKL- 40 in both RA and OA were significantly correlated with increased levels of YKL-40 in synovial fluid (10–15 higher than in serum), suggesting that the increased serum levels derived from arthritic joints [8].
Our findings, together with previous observations, suggest that YKL-40 may be of importance in cartilage remodelling/degradation of osteoarthritic joints [21] and synovitis [7].
Aim of the study
The aim of this study was a parallel analysis and determination of the degree of connectivity between ultrasound indicators of joint destruction and the thickness of cartilage and length of osteophytes with levels of YKL-40 in the serum with patients with primary OA.
Materials and methods
The analysis included 88 patients diagnosed with primary knee osteoarthrosis according to the criteria of the ACR (American College of Rheumatology), where the disease was present at least six months prior to the start of the study.
Criteria for exclusion from the research included: patients with primary OA in the fourth degree of functional score according to Steinbrocker; patients with secondary osteoarthrosis, knee injuries in the last six months or arthroscopy of the knee joint in the year prior to the research; and patients that had received intra-articular corticosteroids or chondroprotective four weeks or radionuclide three months prior to the research. Patients experiencing a phase of active rheumatoid arthritis, or having inflammatory bowel diseases (ulcerative colitis or Crohn’s disease), bacterial infections, liver fibrosis, malignant diseases (breast, lung, colorectal, ovarian carcinoma), diabetes mellitus types 1 and 2, asthma, systemic sclerosis, multiple myeloma, lymphoprolipherative disorders, alcoholic hepatitis and/or cirrhosis were also excluded.
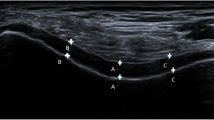
Ultrasound of both knees in B mode was produced by two rheumatologists on the apparatus SDU-1200 using a linear probe of 7,5–10 megahertz. The thickness of the cartilage (expressed in mm) was measured with a front transversal approach over the medial and lateral femoral condyle with the knee in 90 degrees of flexion. The existence and size of the osteophytes (marked as shorter or ≤2 mm and longer or ≥2 mm) was determined by a medial and lateral approach to the medial and lateral femoral and tibial condyles.
Blood samples were obtained from each subject on the day of the ultrasound. Serum was collected using standard venepuncture technique. Specimens were collected without anticoagulants and processed to avoid haemolysis, allowing the blood to clot before separating the serum by centrifugation at 2000 g for ten minutes at room temperature. The serum was immediately frozen and stored in aliquots at −35°C. Serum YKL-40 was assayed on the same day by a sandwich enzyme immunoassay in a microtitre stripwell format (Metra-YKL-40 EIA kit, Quidel Corp. San Diego, USA) according to manufacturer’s instructions. The Fab fragment of a monoclonal anti-YKL-40 antibody conjugated to biotin binds to streptavidin on the strip and captures YKL-40. A conjugated polyclonal anti-YKL-40 antibody conjugated with alkaline phosphatase binds to the captured YKL-40. Bound enzyme activity is detected with p-nitrophenyl phosphate as the substrate. The intra-assay CV was less than 7%. All YKL-40 assays were performed in duplicate with a minimum detection limit of 20 ng/ml.
Statistical data interpretation
During the procedure the following descriptive statistics were used: arithmetic mean, standard deviation, median, quartiles. For the examination of normal dispersion, Kolmogorov-Smirnov and Shapiro-Wilk’s tests were used. Comparison of middle values of two populations was done using a t test and Mann-Whitney test. The correlation between the category variables was examined by using the chi-square test. Correlation between the categories of constant variables was examined using the Spearman coefficient of correlation. Linear regression was used for examining the dependence of constant variables and other variables. The backward method was also used with regression. Confidence level in all applied methods had a limit of 0.05. For the examination of marker quality, the ROC curve was used and a cut-off was determined. Also, the sensitivity and specificity of the test obtained in such a way was calculated.
Results
Arthrosonography was performed with 88 patients, 20 (22.7%) male and 68 (77.3%) female, with primary knee OA. Average age of the subjects was 69.97 ± 9.37 years, duration of the disease was 6.46 ± 6.73 years, and duration of present discomfort was 44.10 ± 47.89 days (Table 1).
Mean value of cartilage thickness measured at the medial femur condyles was 1.30 ± 0.23 mm, while at the lateral femur condyles it was 1.39 ± 0.27 mm.
All patients had osteophytes at the femoral and tibial condyles as indicated by ultrasound examination. Shorter osteophytes at the tibia and femur condyles were present in 23.9% (21/88) of the subjects, and longer osteophytes in 76.1% (67/88).
There was a significant difference between the median of biomarker YKL-40 between patients with shorter in comparison to patients with longer osteophytes at the medial and lateral condyles of the tibia and femur (p = 0.000) (Table 2). The median of biomarker YKL-40 in patients with shorter osteophytes was 62.0 (44.5–90) ng/ml and with longer osteophytes was 119 (80–171) ng/ml (p = 0.000).
There was a significant connection in negative direction between the concentration of YKL-40 with the thickness of cartilage on medial (r = −0.249; p = 0.019) but not in the lateral condyle of the femur (r = −0.080; p = 0.460).
Sensitivity of biomarker YKL-40 for appearance of longer osteophytes on condyles of the tibia and femur was 79.1%, and its specificity was 61.9 % (cut-off = 75.0; area 0.806; p = 0.000; confidence interval 0.706–0.906) (Fig. 1).
Multivariate binary logic regression shows that the duration of the disease is very much connected to the values of biomarker YKL-40 obtained in serum (r = 0.651, p = 0.000) (Fig. 2).
Comparing the median of the concentration of biomarker YKL-40 after five, ten, 15 and 20 years of the disease, a significant difference was found (p = 0.000). The median of biomarker YKL-40 increases as the disease remains. After a duration of five years it was 83.68 ± 33,65 ng/ml, after ten years it was 138.22 ± 48.88 ng/ml, after 15 years it was 209.30 ± 79.36 ng/ml, and after 20 years it was 218.50 ± 106.51 ng/ml (Fig. 3).
Discussion
With the increasing life span of the elderly population, the prevalence of osteoarthritis (OA) is higher. More than 80% of people over 56 years of age have radiographic signs of OA out of which only 25% have clinical symptoms of the disease, which means that over 50% of OA at that age is asymptomatic.
At the locations bearing the most weight in the osteoarthritic joint, the loss of cartilage leads to focal necrosis and cystic changes of exposed subchondral bone and the genesis of cystic changes. In the late stage, the surface of the damaged joint may be covered with regenerated cartilage, thus evenly distributing the pressure in the joint, and the cysts are often reduced or even disappear. In the process of reparation, of the cartilage and subchondral bone, osteophytes are produced—fibrous, cartilaginous and osseous prominences that are usually developed on the joint’s periphery. Osteophytes are palpable, can be sensitive and in all joints can lessen the range of movement and contribute to the increase in pain during motion.
Arthrosonography can uncover the outset of the destructive changes and surface erosion on bones in rheumatoid arthritis and osteoarthritis [15] and is therefore useful for the detection of early signs of joint structure damage, especially cartilage and bone in patients with knee OA.
In this study 88 patients were diagnosed with OA. The ultrasound of a painful knee was performed and a specific concentration of biological marker YKL-40 in the serum of the patient was determined. The average age of the subjects was 69.97 ± 9.37, duration of the disease was 6.46 ± 6.73 years, and duration of present discomfort was 44.10 ± 47.89 days.
Arthrosonographic measurements and procedure techniques were harmonised with the recommendations of the EULAR report, part 1, released in 2005 [4].
Several studies examined the connection between the structural changes and biochemical markers in OA joints [2, 5]. Several biochemical markers such as CTX-II, Glc-Gal-PYD, PIIINP [19] and YKL-40 [16] reflect the metabolism of bone, cartilage and synovial tissue and are used to diagnose patients with high risk for rapid joint destruction in OA. A study by Kawasaki gives different data for osteoarthritis of the hip and states that the level of YKL-40 better reflects the degree of synovial inflammation than the cartilage metabolism and can be a useful inflammatory marker for the hip joint [12]. A longitudinal Conrozier study suggested that serum YKL-40 was significantly increased in patients with hip OA and may reflect both cartilage damage and synovitis [3].
Also, our research determined that there is a significant difference between the median of biomarker YKL-40 concentration between patients with shorter versus patients with longer osteophytes (p = 0.000). The median of biomarker YKL-40 in patients with shorter osteophytes was 62.0 (44.5–90) ng/ml and with longer osteophytes was 119 (80–171) ng/ml. Osteophytes appear as a consequence of damage and loss of cartilage tissue together with destruction of the cartilage and indirectly reflect the degree of damage. The increased concentration of biomarker YKL-40 can indicate the degree of the destructive changes in knee osteoarthritis. This reflects the possibility for the use of this marker for the assessment of joint destruction.
Our research agrees with that of Johansen et al. [8] which showed that patients in later stages of knee osteoarthritis have increased levels of YKL-40 in the serum, but a study by Garnero et al. [5] could not confirm a high level of YKL-40 in patients in late osteoarthritis.
In our research we found significant connection in the negative direction between the concentration of YKL-40 with the thickness of cartilage on the medial (r = – 0.249; p = 0.019) but not in the lateral condyle of the femur (r = – 0.080; p = 0.460).
Results have shown that biomarker YKL-40 can be the marker (indicator) for the appearance of longer osteophytes on condyles of the tibia and femur, with very high sensitivity (sensitivity = 79.1 %, specificy = 61.9%). It has been found that the cut-off is 75.0 ng/ml, which means that all patients with osteoarthritis that have a level of biomarker YKL-40 below 75.0 ng/ml have a milder degree of joint destruction and that patients with osteoarthritis with a level of biomarker YKL 40 above 75.0 ng/ml have a more distinct degree of destruction and presence of longer osteophytes.
Our results are also in accordance with the research of Morgante et al. [16] that concluded that YKL-40 is a local forecasting marker for joint destruction. In this study high levels of YKL-40 in serum were mentioned in patients with erosive osteoarthritis or coxitis (440 ng/ml) while it was significantly lower in patients with rheumatoid arthritis (190 ng/ml). A study by Johansen et al. notes higher levels of this biomarker in serum of patients with progressive joint destruction in RA [10].
Based on the presented data it can be concluded that the concentration of biomarker YKL-40 in serum indicates the appearance of osteophytes in osteoarthrotic joints and thus shows the degree of joint destruction.
Multivariate binary logic regression shows that the duration of the disease is very much connected to the values of biomarker YKL-40 obtained in serum (r = 0.651, p = 0.000). This points out the fact that with prolonged disease the concentration of biomarker YKL-40 in serum constantly increases.
Comparing the median of the concentration of biomarker YKL-40 after five, ten, 15 and 20 years of the disease, a significant difference was found (p = 0.000). The median of biomarker YKL-40 increases as the disease remains. After a duration of five years, it was 83.68 ± 33.65 ng/ml, after ten years it was 138.22 ± 48.88 ng/ml, after 15 years it was 209.30 ± 79.36 ng/ml, and after 20 years it was 218.50 ± 106.51 ng/ml.
Based on the presented data it can be concluded that the concentration of biomarker YKL-40 in serum with prolonged disease is constantly increasing. Higher concentration of YKL-40 indicates the appearance of osteophytes in osteoarthritic joints and, in that way, indirectly shows the degree of joint destruction. Obtained data point to the possibility of using human cartilage glycoprotein 39 (YKL-40) for the estimation of joint destruction.
References
Pecina-Slaus N, Vukicevic S (2007) Biological mechanisms of bone and cartilage remodeling—genomic perspective. Int Orthop 31:799–805
Bruyère O, Collette JH, Ethgen O, Rovati LC, Giacovelli G, Henrotin YE et al (2003) Biochemical markers of bone and cartilage remodelling for the prediction of long-term progression of knee osteoarthritis. J Rheumatol 30:1043–1050
Conrozier T, Carlier MC, Mathieu P, Colson F, Debard AL, Richard S et al (2000) Serum levels of YKL-40 and C reactive protein in patients with hip osteoarthritis and healthy subjects: a cross sectional study. Ann Rheum Dis 59:828–831
D’Agostino MA, Conaghan P, Le Bars M, Baron G, Grassi W, Martin-Mola E et al (2005) EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Ann Rheum Dis 64(12):1703–1709
Garnero P, Pipernoc M, Gineytsa E, Christgaud S, Delmasa PD, Vignonc E (2001) Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis 60:619–626
Hakala BE et al (1993) Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem 268:25803–25810
Harvey S, Michael W, James O’D, Tonya S, Mindy K, Cathy S (1998) Chondrex: new marker of joint disease. Clin Chem 44:509–516
Johansen JS, Hvolris J, Hansen M, Backer V, Lorenzen I, Price PA (1996) Serum YKL-40 levels in healthy children and adults. Comparison with serum and synovial fluid levels of YKL-40 in patients with osteoarthritis or trauma of the knee joint. Br J Rheumatol 35(6):553–559
Johansen JS, Jensen HS, Price PA (1993) A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol 32(11):949–955
Johansen JS, Olee T, Price PA, Hashimoto S, Ochs RL, Lotz M (2001) Regulation of YKL-40 production by human articular chondrocytes. Arthritis Rheum 44(4):826–837
Kane D, Balint PV, Sturrock RD (2003) Ultrasonography is superior to clinical examination in the detection and localization of knee joint effusion in rheumatoid arthritis. J Rheumatol 30(5):966–971
Kawasaki M, Hasegawa Y, Kondo S, Iwata H (2001) Concentration and localization of YKL-40 in hip joint diseases. J Rheumatol 28(2):341–345
Kraus VB (2005) Biomarkers in osteoarthritis. Curr Opin Rheumatol 17:641–646
Martel-Pelletier J (1999) Pathophysiology of osteoarthritis. Osteoarthr Cartil 7:371–373
Mc Gonagle D, Gibbon W, O’Connor P, Blythe D, Wakefield R, Green M et al (1999) Preliminary study of ultrasound aspiration of bone erosion in early rheumatoid arthritis. Rheumatology 38(4):329–331
Morgante M, Metelli MR, Morgante D (2001) Observations on the increased serum levels of YKL-40 in patients with rheumatoid arthritis and osteoarthritis. Minerva Med 92(3):151–153
Naredo E, Cabero F, Palop MJ, Collado P, Cruz A, Crespo M (2005) Ultrasonographic findings in knee osteoarthritis: a comparative study with clinical and radiographic assessment. Osteoarthr Cartil 13(7):568–574
Register T, Carlson C, Adams M (2001) Serum YKL-40 is associated with osteoarthritis and atherosclerosis in nonhuman primates. Clin Chem 47:2159–2161
Vignon E, Garnero P, Avouac B, Bettica P, Boers M, Delmas P et al (2001) Recommendations for the registration of drugs used in the treatment of osteoarthritis: an update on biochemical markers. Osteoarthr Cartil 9:289–293
Volck B, Johansen JS, Stoltenberg M, Garbarsch C, Price PA, Ostergaard M et al (2001) Studies on YKL-40 in knee joints of patients with rheumatoid arthritis and osteoarthritis. Involvement of YKL-40 in the joint pathology. Osteoarthr Cartil 9(3):203–214
Volck B, Ostergaard K, Johansen JS, Garbarsch C, Price PA (1999) The distribution of YKL-40 in osteoarthritic and normal human articular cartilage. Scand J Rheumatol 1999 28(3):171–179
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Živanović, S., Rackov, L.P., Vojvodić, D. et al. Human cartilage glycoprotein 39—biomarker of joint damage in knee osteoarthritis. International Orthopaedics (SICOT) 33, 1165–1170 (2009). https://doi.org/10.1007/s00264-009-0747-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-009-0747-8