Abstract
Mouse CD8+ T cells conditioned with interleukin (IL)-12 ex vivo mediate the potent regression of established melanoma when transferred into lymphodepleted mice. However, the quantitative and qualitative changes induced by IL-12 in the responding mouse CD8+ T cells have not been well defined. Moreover, the mechanisms by which IL-12-conditioning impacts human CD8+ T cells, and how such cells might be expanded prior to infusion into patients is not known. We found that ex vivo IL-12-conditioning of mouse CD8+ T cells led to a tenfold–100-fold increase in persistence and anti-tumor efficacy upon adoptive transfer into lymphodepleted mice. The enhancing effect of IL-12 was associated with maintenance of functional avidity. Importantly, in the context of ongoing ACT clinical trials, human CD8+ T cells genetically modified with a tyrosinase-specific T cell receptor (TCR) exhibited significantly enhanced functional activity when conditioned with IL-12 as indicated by heightened granzyme B expression and elevated peptide-specific CD107a degranulation. This effect was sustainable despite the 20 days of in vitro cellular expansion required to expand cells over 1,000-fold allowing adequate cell numbers for administration to cancer patients. Overall, these findings support the efficacy and feasibility of ex vivo IL-12-conditioning of TCR-modified human CD8+ T cells for adoptive transfer and cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adoptive cell therapy (ACT) has shown great potential for inducing curative responses in patients with advanced metastatic cancer. Recent findings provide additional optimism as it is apparent that tumor-reactive T cells can be rapidly and reliably generated by the transfer of tumor-reactive T cell receptor (TCR) and chimeric antigen receptor (CAR) genes [1–4]. Furthermore, it is becoming increasingly clear in murine studies that the conditioning of T cells prior to ACT is also a critical parameter affecting in vivo efficacy. For CD8+ T cells, polarization with IL-12, IL-4, or TGFβ/IL-6 leads to the generation of Tc1, Tc2, and Tc17 subsets, respectively [5–7]. These subsets each have unique functional properties and have demonstrated improved anti-tumor immunity when compared to unpolarized T cells (Tc0). Although there is a growing body of preclinical data demonstrating that polarized T cell subsets have improved durability and greater anti-tumor efficacy, current ACT clinical trials still use unpolarized T cells.
Perhaps the most therapeutically promising of the CD8+ T cell subsets are Tc1 cells. Early studies demonstrated that human CD8+ T cells activated and cultured with IL-12 were skewed toward a phenotype associated with enhanced function and cytotoxicity, as indicated by their ability to secrete IFNγ and degranulate upon antigen recognition [8–11]. These results were expanded upon using mouse T cells, leading to the formal identification of Tc1 cells [12–14]. Then, in a series of mouse studies, Dutton and colleagues showed that Tc1 cells can mediate effective anti-tumor immunity in mice in a variety of settings and in a manner unique to that of other polarized CD8+ T cell subsets [15–19]. Importantly, in most models, Tc1 mediate much more effective anti-tumor immunity than Tc2 or Tc17 cells [18–21].
While most studies have focused on a comparison of Tc1 cells with other effector cell subsets, Mescher and colleagues have more precisely defined the role of IL-12 in this process [22]. Using mouse T cells, they demonstrated that the presence of IL-12 during activation dramatically enhanced the functionality of CD8+ T cells as indicated by their ability to produce IFNγ, to be cytotoxic, to persist in vivo, and to mediate anti-tumor immunity. Chang et al. [23] demonstrated similar findings to Mescher and colleagues and also showed using a mixture of wild-type and IL-12Rβ1−/− T cells that IL-12 acts directly on CD8+ T cells. Interestingly, in all these studies, control CD8+ T cells cultured without IL-12 also produced IFNγ upon antigen stimulation, albeit less than with the inclusion of IL-12. These results demonstrate that IL-12 can not only promote a Tc1 phenotype, but also IL-12 can fundamentally improve the functional quality of an activated CD8+ T cells already producing IFNγ.
In our previous work [24], we used an approach similar to Mescher and colleagues to assess the impact of ex vivo IL-12-conditioning on tumor-reactive CD8+ T cells from pmel-1 TCR transgenic mice. Pmel-1 CD8+ T cells express a TCR that recognizes the H-2Db-restricted gp10025–33 epitope, an endogenous B16 tumor antigen [25]. Using peptide stimulation, we activated pmel-1 CD8+ T cells with (pmelIL-12) or without (pmelsham) IL-12-conditioning. We found that pmelIL-12 CD8+ T cells did not merely exhibit improved function in vitro, but also mediated robust regression of established melanoma when infused into mice lymphodepleted with cyclophosphamide (CTX) [24]. Thus, the combination of both ex vivo IL-12 conditioning of donor CD8+ T cells and host lymphodepletion led to synergistically enhanced anti-tumor immunity.
Here, we expand upon our previous findings by mechanistically defining how IL-12-conditioning augments the function and anti-tumor activity of CD8+ T cells. Further, we demonstrate the ability to generate an IL-12-conditioned cellular product in support of a clinical trial platform. First, using mouse pmel-1 CD8+ T cells, we find that IL-12-conditioning improves persistence and anti-tumor efficacy tenfold–100-fold. The enhanced effectiveness of IL-12-conditioning was associated with maintenance in functional avidity. In studies with human CD8+ T cells, we genetically modified T cells with a tyrosinase-reactive TCR, TIL 1383I, which recognizes the HLA-A2-restricted tyrosinase368–376 epitope, an antigen expressed on a high frequency of melanoma tumors [26, 27]. (This TIL 1383I TCR is being used in an ongoing ACT clinical trial (NCT01586403) at Loyola Medical Center in Chicago(coauthor G.S.).) Using TIL 1383I-modified CD8+ T cells, we found that IL-12-conditioning led to enhanced functional activity, including elevated expression of granzyme B and ability to degranulate, as indicated by surface CD107a expression in response to relevant antigen. Importantly, this enhanced functional ability was maintained during the 3-week period of expansion required for the CD8+ T cells to reach numbers adequate for patient administration.
Materials and methods
Mice
C57BL/6 (B6), B6.PL (Thy1.1), pmel-1 TCR transgenic [25], HLA-A2 transgenic, IFNγ−/−, and NSG mice were obtained from Jackson Laboratory (Bar Harbor, ME). We have described the generation of h3T TCR transgenic mice previously [28]. Pmel-1 mice were maintained by crossing a pmel-1 (male) to a Thy1.1 (female) generating hemizygous offspring. We generated pmel-1/IFNγ−/− mice in our colony. All animals were housed under specific pathogen-free conditions in accordance with institutional and federal guidelines at the Medical University of South Carolina.
Cell cultures
B16-F1 tumor cells were obtained from ATCC (Manassas, VA, USA) and cultured as previously described [24]. T2-A2 cells are a TAP-deficient hybridoma expressing HLA-A2. For generation of mouse gp100-reactive T cells, pmel-1 TCR transgenic splenocytes (1.5 × 106 cells/well in 1.5 ml) were stimulated with 1 µg/ml H-2Db-restricted human gp10025–33 peptide (KVPRNQDWL, American Peptide Company) for 3 days with or without mIL-12 (10 ng/ml, Shenandoah Biotechnology, Warwick, PA, USA) to generate pmelIL-12 or pmelsham T cells, respectively. In some experiments, we generated pmelIL-2 cells by substituting hIL-2 (200 ng/ml) for IL-12 during the 3-day culture. For generation of mouse tyrosinase-reactive T cells, h3T TCR transgenic splenocytes (1.5 × 106 cells/well in 1.5 ml) were cultured with irradiated HLA-A2 transgenic splenocytes (3.8 × 106 cells/well) and stimulated with 1 µg/ml HLA-A2-restricted human tyrosinase368–376 (hTyr) peptide (YMDGTMSQV, American Peptide Company) for 3 days with or without mIL-12 (10 ng/ml) to generate h3TIL-12 or h3Tsham T cells, respectively. For analysis of functional avidity, pmelIL-12, pmelsham, h3TIL-12, or h3Tsham were restimulated with the indicated concentration of relevant peptide for 6 h and assessed for IFNγ expression. For pmel-1 experiments, 105 T cells were co-cultured with 105 irradiated B6 splenocytes. For h3T experiments, 105 T cells were co-cultured with 105 irradiated T2-A2 cells.
Human T cells were obtained from Research Blood Components (Boston, MA, USA) and cultured using one of the following two protocols. For generation of TCR-modified human T cells, we used a modification of a previously described protocol [29–31]. On day 1, human PBMC were stimulated with soluble anti-CD3 mAb (OKT3, NCI preclinical repository) for 48 h. Except during the spinoculation step, cells were cultured with hIL-2 (30 IU/ml) and hIL-15 (100 ng/ml) and maintained between 1 and 2 × 106 cells/ml. On day 3, activated T cells were transduced by co-culture with 50 % retroviral supernatant from PG13 packaging cells transfected with the TIL 1383I TCR/CD34t construct. Transduction was done with retronectin-coated plates and spinoculation (2,000 g for 2 h at 32 °C). On day 8, cells underwent a rapid expansion protocol (REP) by incubation in an upright T175 flask of 1 × 106 transduced T cells with 2 × 108 irradiated (5,000 rad) allogeneic feeder cells from human donors. Soluble anti-CD3 mAb (OKT3, 30 ng/ml) was also added to the cultures. On day ~20, cultures were harvested and analyzed by flow cytometry or in functional assays. For assessment of CD107a degranulation, human T cells (105) were mixed with T2-A2 cells (105) with or without 1 µg/ml hTyr peptide. As control, we added PMA/ionomycin. Antibody against CD107a was added to the culture during the last 5 h of the 6-h culture. For expansion of non-genetically modified T cells for transfer into NSG mice, on day 1 PBMCs were stimulated with anti-CD3 mAb (OKT3) for 48 h. Beginning on day 3, cells were cultured in hIL-2 and hIL-15 and maintained between 1 and 2 × 106 cells/ml. Throughout the culture, cells were grown with or without hIL-12 (10 ng/ml). Cells were phenotyped and injected on day 7 of culture.
Flow cytometry
For flow cytometry, cells were analyzed as previously described [32]. For intracellular flow cytometry, cells were stained using the BD Cytofix/Cytoperm with Golgistop kit (BD Bioscience, San Jose, CA, USA). Flow cytometry was performed on a BD LSRII or a BD Accuri, and data were analyzed using Flowjo software (TreeStar, Ashland, OR, USA) and CFlow software (BD Bioscience).
RNA isolation and real-time PCR
Total cellular RNA was isolated from pmelIL-12 and pmelsham CD8+ T cells using Trizol (Invitrogen). cDNA was generated from total RNA (1 µg/sample) using the High-Capacity cDNA Reverse Synthesis Kit (Bio-Rad, Hercules, CA, USA). The resulting cDNA was amplified by PCR using primer pairs for the BCL3 (forward 5′-CCGGAGGCCCTTTACTACC-3′; reverse 5′-GAGTAGGCAGGTTCAGCAGC-3′), 18S (forward 5′-CCAGAGCGAAAGCATTTGCCAAGA-3′; reverse 5′-TCGGCATCGTTTATGGCTGGAACT-3′), and the beta-actin (forward 5′-ACGTAGCCATCCAGGCTGGTG-3′; reverse 5′-TGGCGTGAGGGAGAGCAT-3′) using 2× Sso advance SYBR green on CFX96 Touch real-time PCR detection system (Bio-Rad). The levels of Bcl-3 cDNA in each sample were normalized to beta-actin and 18S cDNA. The final relative expression of mRNA species was calculated using the comparative Ct method.
Tumor challenge, cyclophosphamide (CTX) preconditioning, and adoptive T cell transfer
For tumor experiments, B6 mice were injected (s.c.) with 2.5 × 105 B16-F1 tumor cells. Tumor growth was measured blindly by caliper twice per week and tumor surface area (mm2) was calculated by length x width. For CTX injections, mice were given 4 mg by i.p. injection. For adoptive transfer experiments, T cells were injected i.v. at the doses indicated. For antibody mediated depletion of IFNγ, mice received 150 µg anti-IFNγ mAb (XMG1.2 clone, Bioxcell) on days 0, 2, 4, and 6, after adoptive transfer.
Statistical methods
Data from all experiments were graphically displayed to identify the need for transformation. When analyzing number or % donor cells, a squareroot transformation was used to adhere to assumptions of ANOVA and other regression models (i.e., assumption of constant variance of residuals). Although conditions were compared using transformed endpoints, data are displayed on the original scale. Data shown in Fig. 1a, b were analyzed using ANOVA. Data in Fig. 1c, d were analyzed using random effects linear regression where random intercepts were included per mouse and main effects of time, group, and their interactions were included. To adequately represent the nonlinear pattern over time, both time and time2 were included in the models. Likelihood ratio tests were used to evaluate significance of conditions. Time-to-kill data are graphically displayed using Kaplan–Meier curves (Suppl. Figure 1), and groups were compared using a Gehan–Wilcoxon test (the modified Peto-Peto version). Conditions in Suppl. Figure 3A were compared using a random effects model where random intercepts were included for experiment and a fixed effect of IL-12 (vs. sham). p < 0.05 was considered significant for all experiments.
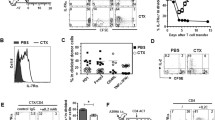
IL-12 conditioning improves CD8+ T cell persistence in lymphodepleted hosts at least 100-fold. a B6 mice were treated with or without cyclophosphamide (CTX), and 1 day later adoptively transferred with 3 × 106 pmelIL-12 or pmelsham T cells. On day 10 after adoptive transfer, spleens were harvested and the number of donor T cells (CD8+ Thy1.1+) was determined. Each triangle represents an individual mouse, and the bar indicates the mean. b As in a, except liver (top) and lymph node (bottom) cells were harvested and the frequency of donor T cells was determined. In a, b, pmelsham + CTX and pmelIL-12 + CTX conditions (denoted with an asterisk) were significantly different (p < 0.0001). c As in a, except titrated numbers of pmelIL-12 or pmelsham were injected. Blood was harvested at the indicated time points. Each point represents the mean of 3–6 mice. The % donor T cells differed significantly across time for all three conditions with CTX (p < 0.0001). d Similar to c with the transfer of pmelIL-2 cells. Each point represents the mean of 3–5 mice. There was a significant difference (p < 0.0001) between pmelIL-12 and pmelIL-2. There was no difference between pmelsham and pmelIL-2 (p = 0.40). Data are representative of at least two independent experiments
Results
Ex vivo IL-12-conditioning improves T cell persistence
To better characterize how ex vivo IL-12-conditioning of T cells improves the anti-tumor responses upon transfer into mice after host lymphodepletion, we initially assessed T cell persistence in non-tumor-bearing mice. Mice were pre-treated with or without cyclophosphamide (CTX, 4 mg, i.p.) and then adoptively transferred with 3 × 106 pmelIL-12 or pmelsham CD8+ T cells. Only the combination of IL-12 conditioning and lymphodepletion led to a significantly enhanced donor CD8+ T cell persistence (Fig. 1a). Importantly, this improved persistence was systemic in nature as it was also observed in the liver and lymph nodes (Fig. 1b). Titration of adoptively transferred cells demonstrated that the IL-12-conditioned CD8+ T cells (pmelIL-12) in the peripheral blood persisted at least 100-fold better than control CD8+ T cells (pmelsham) (Fig. 1c). Finally, the enhanced persistence induced by IL-12-conditioning was not seen when the T cells were cultured with a high dose of IL-2 (pmelIL-2) for 72 h (Fig. 1d), indicating that IL-12 imparts a unique signal.
Ex vivo IL-12-conditioning improves anti-tumor efficacy
To determine whether improved T cell persistence correlated with enhanced anti-tumor efficacy, we treated mice bearing subcutaneous B16 tumor cells with CTX and titrated numbers of adoptively transferred pmelIL-12 or pmelsham cells. PmelIL-12 cells exhibited at least a tenfold improvement in efficacy compared with pmelsham cells as indicated by reduced tumor growth (Fig. 2) and enhanced survival (Sup. Fig. 1). As we have observed previously, anti-tumor immunity was dependent on both ex vivo IL-12 conditioning and lymphodepletion [24].
IL-12 conditioning improves CD8+ T cell anti-tumor immunity by least tenfold. Mice with s.c. B16 tumors were treated on day 7 with cyclophosphamide (CTX). On day 8, mice were adoptively transferred with pmelIL-12 or pmelsham cells. In mice receiving T cells, 4 × 106 T cells were injected except 4 × 105 T cells in the condition designated (1:10). Tumors were measured blindly twice per week, and each line represents one mouse (n = 8–10/group). Similar results were obtained in an independent experiment shown as a survival curve in supplemental figure 1. Using a Gehan–Wilcoxon test, time-to-kill (mice were killed when tumors reached 400 mm2) was compared across groups using data from this figure and supplemental figure 1. There were significant differences between certain groups, and notably between pmelsham + CTX and pmelIL-12 (1:10) + CTX (p = 0.004)
To understand the mechanism by which pmelIL-12 cells mediated anti-tumor immunity after adoptive transfer into a lymphodepleted host environment, we assessed the role of donor cell IFNγ. In some tumor models, Tc1 cells require IFNγ [16, 33], while in other models, IFNγ is dispensable [15, 34]. As our experiments are unique in that donor pmelIL-12 were transferred into a lymphopenic environment, we assessed the role of IFNγ in our model and generated pmelIL-12 T cells from wild-type or IFNγ−/− mice. As shown in Fig. 3, effective anti-tumor immunity was dependent on the ability of donor T cells to produce IFNγ, as pmelIL-12-lacking IFNγ exhibited markedly inferior anti-tumor immunity.
IL-12-conditioned CD8+ T cells depend on IFNγ for optimal anti-tumor immunity. Mice with s.c. B16 tumors were treated on day 7 with cyclophosphamide (CTX). On day 8, mice were adoptively transferred with 3 × 106 pmelIL-12, pmelIL-2, or pmelsham cells from wild-type or IFNγ−/− pmel-1 mice. Tumors were measured blindly twice per week, and each line represents one mouse (n = 8–10/group). A similar requirement for IFNγ was also observed in B16-tumor-bearing mice given 6 Gy (in lieu of CTX), adoptively transferred with pmelIL-12, and injected with or without 150 µg of anti-IFNγ mAb (clone XMG1.2) on days 0, 2, 4, and 6
We also assessed whether IL-2 could substitute for IL-12 during the priming of pmel-1 CD8+ T cells. Similar to our findings in Fig. 1, pmelIL-2 cells failed to mediate effective anti-tumor immunity compared with pmelIL-12 cells (Fig. 3). PmelIL-2 and pmelsham cells were not distinguishable. Thus, IL-12 induces a qualitatively unique signal in these CD8 T cells that cannot be achieved by increasing the level of IL-2 signaling.
IL-12-mediated STAT4 phosphorylation requires T cell activation
The ability of IL-12 to improve the function of tumor-reactive T cells is thought to be most efficient on activated T cells, as activation (or TCR engagement) leads to upregulation of the IL-12 receptor (IL-12R) subunits [35–37]. To validate that activated T cells are more responsive to IL-12 compared with resting T cells on a per cell basis, we used flow cytometry to assay for STAT4 phosphorylation, which is immediately downstream of the IL-12R [38, 39]. Resting mouse or human CD8+ T cells cultured with IL-12 failed to induce the phosphorylation of STAT4 (Sup. Fig. 2, left), which is consistent with resting T cells not expressing a functional IL-12R. In contrast, IL-12 culture of activated mouse or human CD8+ T cells induced significant phosphorylation of STAT4 (Sup. Fig. 2, right). These results are consistent with T cell responsiveness to IL-12 that is dependent on T cell activation.
IL-12-conditioning improves T cell survival and avidity
To better understand the qualitative changes induced by IL-12 conditioning, we compared the survival of pmelIL-12 and pmelsham CD8+ T cells in the absence of exogenous growth factors such as IL-2 or IL-15. PmelIL-12 exhibited better short-term viability as compared with pmelsham after washing and overnight culture without addition of cytokines (Sup. Fig. 3A). In comparison, culture with a low dose of IL-15 (1 ng/ml) improved the viability of both pmelsham and pmelIL-12 cells (Sup. Fig. 3B). These results suggest that the improved functional efficacy of pmelIL-12 cells may be due to their ability to survive or function at least initially in the absence of critical T cell growth factors in the host. These results are consistent with previous findings, and the enhanced survival may be mediated by IL-12-induced upregulation of Bcl-3 [23, 40]. In fact, we observed elevated Bcl-3 in pmelIL-12 versus pmelsham cells (Sup. Fig. 3C).
A property critical to optimal anti-tumor immunity is the ability to recognize even nominal amounts of antigen on a tumor cell. As IL-12-conditioning was reported to reduce the ability of T cells to bind relevant tetramer [23], it was important to determine whether IL-12-conditioning impacted the ability to functionally recognize limiting amounts of antigen as indicated by the production of IFNγ. PmelIL-12 showed a slightly enhanced ability to produce IFNγ in response to low amounts of antigen as compared to pmelsham cells (Fig. 4a). We also found that pmelIL-12 cells bound relevant tetramer comparably to pmelsham cells (Fig. 4b) and that this was not temperature dependent (Fig. 4c). Using a second TCR, from h3T TCR transgenic mice (which expresses a murine version of TIL 1383I [28]), we observed a similar trend toward enhancement of IFNγ production in response to low amounts of antigen (Fig. 4d, e). Cumulatively, our results demonstrate that conditioning of T cells with IL-12 does not impair their ability to recognize the limiting amounts of antigen that may be present on tumor cells.
IL-12 conditioning maintains the functional avidity of activated CD8+ T cells. a PmelIL-12 or pmelsham cells were co-cultured for 6 h at a 1:1 ratio with naïve B6 splenocytes and titrated amounts of peptide and then intracellular IFNγ was assessed by flow cytometry. Shown is the % maximal IFNγ as a percentage based on maximal MFI. b Representative staining for a at the maximal peptide concentration. The shaded region shows staining of cells not stimulated with peptide. c PmelIL-12 or pmelsham cells were incubated with titrated tetramer at either 4 °C (top) or 37 °C (bottom). d Representative tetramer staining at the maximum concentration (2.5 µg/ml). The shaded region shows control staining without the addition of tetramer. e As in a, except we compared h3TIL-12 versus h3Tsham T cells
IL-12 improves the functional qualities of human T cells genetically modified with a tyrosinase-reactive TCR and expanded using a clinically relevant protocol
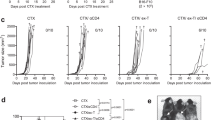
As a next step in translating our approach, we defined the functional parameters of human T cells cultured with or without IL-12 using conditions that would facilitate translation to a clinical trial for cancer patients. As part of this protocol, we genetically modified human T cells with the TIL 1383I TCR, generating tumor-reactive T cells reactive against the melanoma tumor antigen, tyrosinase. We used a modified version of a T cell expansion protocol that has been successfully used to generate sufficient numbers of receptor-modified cells for patients in a variety of trials [29–31] including an ongoing trial at Loyola Medical Center in Chicago using the TIL 1383I TCR platform. However, in contrast with the ongoing Loyola trial using lentiviral vectors, we used retroviral vectors encoding the same three gene products, which include the TIL 1383I TCR α and β, and a truncated CD34 gene [41]. Expression of the truncated CD34 gene enables the enrichment and in vivo detection of genetically modified cells. Using this established clinical protocol as a starting point, we cultured human T cells with or without IL-12 for ~3 weeks and monitored the cellular expansion. At the end of the culture, we assayed cells phenotypically and by functional readouts.
As shown in Fig. 5a, addition of IL-12 to the expansion protocol did not compromise our ability to generate sufficient numbers of cells for patient infusion, although there was a trend toward lower cellular expansion with IL-12 in the culture. There were high frequencies of genetically modified T cells with or without IL-12 culture (Fig. 5b). Similar to mouse cells, we observed higher levels of granzyme B in the presence of IL-12 (Fig. 5b). In response to relevant peptide, IL-12-conditioned CD34hi cells degranulated to a greater extent than CD34hi unconditioned counterparts (Fig. 5c). Cumulatively, our results indicate that the addition of IL-12 did not impede the generation of sufficient numbers of genetically modified T cells needed for patient infusion, and furthermore, IL-12-conditioned human T cells have the characteristics consistent with improved in vivo functional activity.
IL-12 conditioning enhances the functional ability of human T cells genetically modified with the TIL 1383I, tyrosinase-reactive T cell receptor. a Human PBMCs were stimulated with anti-CD3 mAb and retrovirally modified with the TIL 1383I TCR/CD34t construct encoding TIL 1383I α and β, and CD34t. T cells then underwent a rapid expansion protocol (REP) prior to phenotyping. As indicated, cells were maintained in either IL-2 or IL-2/IL-15, and also with or without hIL-12 throughout the culture. b Cells from a maintained in IL-2/IL-15 were analyzed by flow cytometry after conclusion of the REP. Shown are cells gated on CD8+ expression and depicting CD34 and granzyme B expression. c Cells in b were stimulated for 6 h with peptide, PMA/ionomycin(I), or control. Shown are cells gated on CD8+ expression and depicting CD34 expression and CD107a binding during culture. Results are representative of at least two independent experiments
IL-12-conditioned human T cells persist in immunodeficient NSG mice
In an attempt to understand how IL-12-conditioned cells would engraft in cancer patients, we next sought to determine how IL-12-conditioning impacts the ability of human T cells to persist in immunodeficient mice. In addition to functional capability, T cell persistence correlates with the therapeutic effectiveness of ACT therapy [5, 42]. Thus, we used a simplified version of our clinical protocol and activated human T cells with anti-CD3 mAb for 2 days followed by culture for 1 week with hIL-2 and hIL-15. Human T cells were cultured with or without hIL-12 during the entire length of the culture and then harvested for phenotypic analysis and subsequently injected in vivo into immunodeficient NSG mice [43]. As observed in our clinical T cell transduction culture protocol, culture with IL-12 enhanced granzyme B expression (Sup. Fig. 4A). Human donor cells exhibited efficient engraftment (Sup. Fig. 4B), and furthermore, IL-12 conditioned cells appeared to preferentially persist. However, in addition to the initial engraftment, there appeared to be a second rapid expansion phase beginning about 2–3 weeks post-injection, possibly suggesting graft versus host disease. Despite the potential of graft versus host disease, we noted that the ex vivo IL-12-conditioned donor T cells exhibited a trend toward elevated frequencies of donor cells as compared to non-conditioned cells when transferred into NSG hosts.
Discussion
The field of adoptive T cell therapy is developing the ability to reliably generate TCR and CAR gene transfer platforms with an increasing capability of inducing curative responses in patients with advanced metastatic cancer. It is becoming clear, however, that the conditioning of T cells with cytokines capable of affecting the functional and phenotypic fingerprint of transferred T cells prior to ACT is also a critical means of improving in vivo efficacy and durability. Despite this growing body of preclinical data, current ACT therapies have yet to leverage this dimension by incorporating these potentially more sophisticated T cells into phase I studies. While it would seem that translation of these concepts into human trials would be relatively straightforward, there are significant conceptual and logistical barriers that must be addressed. These hurdles include identifying a cytokine-conditioning methodology that can have a durable impact in vivo. Furthermore, it will be critical to develop the methodology for reliably and efficiently integrating the application of cytokine preconditioning into the complex methodology of T cell transduction that needs to be considered in order to reliably deploy a TCR transduction clinical protocol that meets FDA standards.
Among several therapeutically promising phenotypes, including Tc2 and Tc17 cells, we have focused on Tc1 cells because early studies by several investigators have clearly demonstrated that CD8+ T cells cultured with IL-12, and skewed to a Tc1 phenotype, had enhanced functional ability and anti-tumor efficacy [8–11, 15–24]. The ex vivo dimension of these findings are especially cogent for IL-12 where significant in vivo clinical toxicity [44, 45] has limited the utilization of this promising cytokine and led investigators to look for non-systemic approaches. With the goal of translating these observations into a safe and effective therapy for cancer patients, we have characterized the impact of ex vivo IL-12-conditioning on both mouse and human T cells. We found that IL-12-conditioned mouse T cells exhibit tenfold–100-fold improvement in persistence and anti-tumor immunity when transferred into lymphopenic hosts. Furthermore, IL-12-conditioned cells demonstrate improved survival in the absence of T cell growth factors and also retention of T cell avidity. Importantly, when applied to a clinical expansion protocol, we found that IL-12-cultured and TCR-modified human T cells are capable of being expanding to the numbers necessary for patient administration. Furthermore, IL-12-conditioning of these TCR-modified human T cells improved their functionality as indicated by granzyme B and antigen-specific CD107a degranulation.
One important aspect of our study was the direct titration of cell numbers transferred and the benefits of IL-12-conditioning on the persistence and function of mouse T cells. IL-12-conditioned mouse T cells exhibited at least a tenfold increase in anti-tumor activity versus control cells. Similarly, we observed that the ability of these IL-12-conditioned CD8+ T cells to persist in vivo was improved approximately 100-fold by IL-12 conditioning. These beneficial effects were dependent on cyclophosphamide preconditioning, suggesting that the in vivo environment induced by lymphodepletion is critical for the optimal function of IL-12-conditioned T cells. This result suggests the existence of host factors that may selectively support the functional ability of IL-12-conditoned cells during lymphodepletion. Although not directly addressed in this body of work, the discernment of the mechanisms involved with this synergistic partnership will be important to understand as we work to thoughtfully build on our current ACT protocols.
In direct support of the potential of ex vivo IL-12-conditioning in patient care, we utilized a modification of a currently used clinical protocol for the expansion and genetic modification of human TIL 1383I-modified T cells (NCT01586403). Variations of this protocol have been used by several groups to induce efficient engraftment of TCR- and CAR-modified T cells, and in some cases anti-tumor responses [29–31]. However, in these prior studies, IL-12 was not incorporated into the culture methodology. Our findings show that addition of IL-12 to the 3 weeks of culture did not impair our ability to generate sufficient numbers of T cells for patient administration. Furthermore, the T cells generated exhibited functional qualities which would predict enhanced anti-tumor efficacy in vivo including higher levels of granzyme B and the improved ability to mobilize CD107a in response to relevant antigen.
To assess the impact of IL-12-conditioning on persistence of human T cells in vivo, we used the NSG immunodeficient mouse model [43]. These mice, which are on a NOD, SCID, and IL-2Rγ−/− background, are thought to represent one of the best models for assaying the function of human immune cells in mice. We cultured human T cells using a simplified ~9-day protocol involving soluble anti-CD3 mAb stimulation with or without continual IL-12. Unfortunately, engraftment of the human cells into NSG mice was only successful in 1 of 3 experiments independent of IL-12-conditioning. In the one experiment where engraftment could be assessed (Sup. Fig. 4), we observed a trend of enhanced persistence in cells cultured with IL-12. Thus, at a minimum, IL-12 conditioning was not detrimental to T cell persistence. Despite the limitations of our model, these results provide a basis for future experiments involving the transfer of human T cells into NSG mice and identification of the factors important for achieving stable engraftment. Moving forward in the NSG mouse model, our goal is to determine whether IL-12-conditioned human T cells genetically modified with the TIL 1383I TCR and adoptively transferred will not only persist longer but mediate enhanced anti-tumor immunity.
It is relevant to note that one other group has reported the transfer of IL-12-conditioned human T cells into immunodeficient mice [10]. Using a 3-week culture protocol but no REP as in our experiments, the authors demonstrated that IL-12-conditioned human T cells genetically modified with a CAR recognizing CEA led to enhanced anti-tumor immunity compared with human T cells cultured without IL-12. The authors did not assess the persistence of the human T cells in the immunodeficient mice, but demonstrated in vitro a functional improvement in human T cells following culture with IL-12. Our findings build on these earlier observations and demonstrate the ability to generate adequate numbers of cells for patient administration.
One alternative IL-12-based approach for augmenting T cell-mediated immunity warrants review. Several groups have recently reported that genetically modifying tumor-reactive CD8+ T cells to express IL-12 significantly improves anti-tumor immunity [46–49]. While these results are exciting and the approach is being evaluated clinically, we note that the mechanism is likely distinct from that described in our study. Thus, Kerkar et al. [46] found that CD8+ T cells deficient in functional IL-12 receptor still exhibited improved anti-tumor ability when genetically modified to express IL-12. These authors concluded that IL-12 was not acting on the donor T cells, and instead, IL-12 was modifying the myeloid cell compartment to facilitate the function of the donor T cells. In contrast, in our studies the impact of IL-12 is only on the donor T cells and IL-12 is removed prior to T cell infusion. Given the clinical toxicities that have been reported with IL-12, this methodological distinction is important.
In summary, we find that IL-12-conditioning of murine and human tumor-reactive CD8+ T cells can lead to a significant enhancement in function and anti-tumor immunity. We also demonstrate for the first time that TCR-modified human T cells can be effectively expanded with IL-12-conditioning to numbers sufficient for patient administration using a clinically relevant protocol. Our findings demonstrate a pathway for the safe clinical application of IL-12 which could have application in tumor immunology and other domains of clinical immunology.
Abbreviations
- ACT:
-
Adoptive cell therapy
- CAR:
-
Chimeric antigen receptor
- CTX:
-
Cyclophosphamide
- IL-12:
-
Interleukin-12
- REP:
-
Rapid expansion protocol
- Tc0:
-
CD8+ T cells not polarized
- Tc1:
-
CD8+ T cells polarized with IL-12
- TCR:
-
T cell receptor
References
Kershaw MH, Westwood JA, Darcy PK (2013) Gene-engineered T cells for cancer therapy. Nat Rev Cancer 13(8):525–541
Restifo NP, Dudley ME, Rosenberg SA (2012) Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12(4):269–281
Barrett DM, Singh N, Porter DL, Grupp SA, June CH (2014) Chimeric antigen receptor therapy for cancer. Annu Rev Med 65:333–347
Jensen MC, Riddell SR (2014) Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol Rev 257(1):127–144
Muranski P, Restifo NP (2013) Essentials of Th17 cell commitment and plasticity. Blood 121(13):2402–2414
Shrikant PA, Rao R, Li Q, Kesterson J, Eppolito C, Mischo A, Singhal P (2010) Regulating functional cell fates in CD8 T cells. Immunol Res 46(1–3):12–22
Woodland DL, Dutton RW (2003) Heterogeneity of CD4(+) and CD8(+) T cells. Curr Opin Immunol 15(3):336–342
Mehrotra PT, Wu D, Crim JA, Mostowski HS, Siegel JP (1993) Effects of IL-12 on the generation of cytotoxic activity in human CD8+ T lymphocytes. J Immunol 151(5):2444–2452
Gately MK, Desai BB, Wolitzky AG, Quinn PM, Dwyer CM, Podlaski FJ, Familletti PC, Sinigaglia F, Chizonnite R, Gubler U et al (1991) Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol 147(3):874–882
Emtage PC, Clarke D, Gonzalo-Daganzo R, Junghans RP (2003) Generating potent Th1/Tc1 T cell adoptive immunotherapy doses using human IL-12: harnessing the immunomodulatory potential of IL-12 without the in vivo-associated toxicity. J Immunother 26(2):97–106
Halverson DC, Schwartz GN, Carter C, Gress RE, Fowler DH (1997) In vitro generation of allospecific human CD8+ T cells of Tc1 and Tc2 phenotype. Blood 90(5):2089–2096
Sad S, Marcotte R, Mosmann TR (1995) Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity 2(3):271–279
Croft M, Carter L, Swain SL, Dutton RW (1994) Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med 180(5):1715–1728
Fowler DH, Breglio J, Nagel G, Hirose C, Gress RE (1996) Allospecific CD4+, Th1/Th2 and CD8+, Tc1/Tc2 populations in murine GVL: type I cells generate GVL and type II cells abrogate GVL. Biol Blood Marrow Transplant 2(3):118–125
Dobrzanski MJ, Reome JB, Dutton RW (2000) Type 1 and type 2 CD8+ effector T cell subpopulations promote long-term tumor immunity and protection to progressively growing tumor. J Immunol 164(2):916–925
Dobrzanski MJ, Reome JB, Hollenbaugh JA, Dutton RW (2004) Tc1 and Tc2 effector cell therapy elicit long-term tumor immunity by contrasting mechanisms that result in complementary endogenous type 1 antitumor responses. J Immunol 172(3):1380–1390
Hollenbaugh JA, Dutton RW (2006) IFN-gamma regulates donor CD8 T cell expansion, migration, and leads to apoptosis of cells of a solid tumor. J Immunol 177(5):3004–3011
Helmich BK, Dutton RW (2001) The role of adoptively transferred CD8 T cells and host cells in the control of the growth of the EG7 thymoma: factors that determine the relative effectiveness and homing properties of Tc1 and Tc2 effectors. J Immunol 166(11):6500–6508
Garcia-Hernandez Mde L, Hamada H, Reome JB, Misra SK, Tighe MP, Dutton RW (2010) Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol 184(8):4215–4227
Yu Y, Cho HI, Wang D, Kaosaard K, Anasetti C, Celis E, Yu XZ (2013) Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol 190(4):1873–1881
Ye Z, Tang C, Xu S, Zhang B, Zhang X, Moyana T, Yang J, Xiang J (2007) Type 1 CD8+ T cells are superior to type 2 CD8+ T cells in tumor immunotherapy due to their efficient cytotoxicity, prolonged survival and type 1 immune modulation. Cell Mol Immunol 4(4):277–285
Curtsinger JM, Mescher MF (2010) Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol 22(3):333–340
Chang J, Cho JH, Lee SW, Choi SY, Ha SJ, Sung YC (2004) IL-12 priming during in vitro antigenic stimulation changes properties of CD8 T cells and increases generation of effector and memory cells. J Immunol 172(5):2818–2826
Rubinstein MP, Cloud CA, Garrett TE, Moore CJ, Schwartz KM, Johnson CB, Craig DH, Salem ML, Paulos CM, Cole DJ (2012) Ex vivo interleukin-12-priming during CD8(+) T cell activation dramatically improves adoptive T cell transfer antitumor efficacy in a lymphodepleted host. J Am Coll Surg 214(4):700–707; discussion 707–708
Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP (2003) Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med 198(4):569–580
Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J (2006) Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res 12(3 Pt 1):764–771
Cormier JN, Abati A, Fetsch P, Hijazi YM, Rosenberg SA, Marincola FM, Topalian SL (1998) Comparative analysis of the in vivo expression of tyrosinase, MART-1/Melan-A, and gp100 in metastatic melanoma lesions: implications for immunotherapy. J Immunother 21(1):27–31
Mehrotra S, Al-Khami AA, Klarquist J, Husain S, Naga O, Eby JM, Murali AK, Lyons GE, Li M, Spivey ND, Norell H, Martins da Palma T, Onicescu G, Diaz-Montero CM, Garrett-Mayer E, Cole DJ, Le Poole IC, Nishimura MI (2012) A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self immunity in mice. J Immunol 189(4):1627–1638
Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, Nath A, Wang T, Bielekova B, Wuest SC, Akula N, McMahon FJ, Wilde S, Mosetter B, Schendel DJ, Laurencot CM, Rosenberg SA (2013) Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36(2):133–151
Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA (2006) Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314(5796):126–129
Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA (2012) B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119(12):2709–2720
Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW (2008) IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood 112(9):3704–3712
Hollenbaugh JA, Reome J, Dobrzanski M, Dutton RW (2004) The rate of the CD8-dependent initial reduction in tumor volume is not limited by contact-dependent perforin, Fas ligand, or TNF-mediated cytolysis. J Immunol 173(3):1738–1743
Macgregor JN, Li Q, Chang AE, Braun TM, Hughes DP, McDonagh KT (2006) Ex vivo culture with interleukin (IL)-12 improves CD8(+) T-cell adoptive immunotherapy for murine leukemia independent of IL-18 or IFN-gamma but requires perforin. Cancer Res 66(9):4913–4921
Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, Gately MK (1992) IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol 148(10):3125–3132
Szabo SJ, Dighe AS, Gubler U, Murphy KM (1997) Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med 185(5):817–824
Li Q, Eppolito C, Odunsi K, Shrikant PA (2010) Antigen-induced Erk1/2 activation regulates Ets-1-mediated sensitization of CD8+ T cells for IL-12 responses. J Leukoc Biol 87(2):257–263
Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN (1996) Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382(6587):171–174
Kaplan MH, Sun YL, Hoey T, Grusby MJ (1996) Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 382(6587):174–177
Valenzuela JO, Hammerbeck CD, Mescher MF (2005) Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J Immunol 174(2):600–604
Norell H, Zhang Y, McCracken J, Martins da Palma T, Lesher A, Liu Y, Roszkowski JJ, Temple A, Callender GG, Clay T, Orentas R, Guevara-Patino J, Nishimura MI (2010) CD34-based enrichment of genetically engineered human T cells for clinical use results in dramatically enhanced tumor targeting. Cancer Immunol Immunother 59(6):851–862
Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ Jr, Rosenberg SA (2004) Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol 173(12):7125–7130
Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R (2005) Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 174(10):6477–6489
Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL (1997) Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 90(7):2541–2548
Colombo MP, Trinchieri G (2002) Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 13(2):155–168
Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, Trinchieri G, Rosenberg SA, Restifo NP (2011) IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 121(12):4746–4757
Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, Rosenberg SA, Morgan RA (2011) Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther 19(4):751–759
Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ (2012) Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119(18):4133–4141
Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, Rosenberg SA (2012) Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res 18(6):1672–1683
Acknowledgments
We thank Daniel Neitzke for critical review of this manuscript. Grant funding for this project was provided by the following grants from the National Institutes of Health and the National Cancer Institute: P01CA54778-01, R01CA138930, R01CA133503, and R01CA175061. This work was also supported in part by pilot research funding and also the Cell Evaluation and Therapy Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30CA138313).
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rubinstein, M.P., Su, E.W., Suriano, S. et al. Interleukin-12 enhances the function and anti-tumor activity in murine and human CD8+ T cells. Cancer Immunol Immunother 64, 539–549 (2015). https://doi.org/10.1007/s00262-015-1655-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1655-y