Abstract
FoxP3+ CD4+ regulatory T cells (Tregs) are important mediators of peripheral immune tolerance, acting via multiple mechanisms to suppress cellular immunity including antitumor responses. Although therapeutic strategies have been proposed to deplete Tregs in patients with breast cancer and other malignancies, dynamic changes in the Treg compartment as a function of stage and treatment of breast cancer remain poorly understood. Here, we evaluated peripheral blood CD4+ T cells and FoxP3+ CD4+ T cells from 45 patients with early or late stage breast cancer and compared percentages, absolute counts, and Treg function to those from healthy volunteers (HV) of comparable age. Patients having completed adjuvant chemotherapy and patients with metastatic cancer exhibited significantly lower absolute CD4 counts and significantly higher percentages of FoxP3+ CD4+ T cells. In contrast, the absolute counts of circulating FoxP3+ CD4+ T cells did not differ significantly among early stage patients, late stage patients, or HV. Functionally, FoxP3+ CD4+ T cells from all donor groups similarly expressed CTLA-4 and failed to secrete IFN-γ in response to stimulation. Thus, although Tregs comprise an increased percentage of circulating CD4+ T cells in patients with metastatic breast cancer and patients in remission after completing the adjuvant chemotherapy, the systemic Treg pool, as measured by absolute counts, appears relatively constant regardless of disease stage or treatment status. Total CD4+ T cell counts are not constant, however, suggesting that homeostatic mechanisms, or susceptibility to cytotoxic or malignant insults, fundamentally differ for regulatory and non-regulatory CD4+ T cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regulatory T cells (Tregs) represent an important CD4+ T cell subset that contributes to the maintenance of immunological self-tolerance and comprises about 5% of CD4+ T cells in the peripheral blood of humans [1–4]. Tregs inhibit autoimmune reactions, impede antitumor immunity, and prevent the expansion of other T cells in vivo [1–4]. In particular, human Tregs markedly inhibit CD8 T cell activation [5, 6], and contribute to immune dysfunction in cancer patients [7–10]. The forkhead/winged-helix transcription factor FoxP3 has been shown to control the development and function of Tregs in mice [4], and mutation of FoxP3 leads to fatal lymphoproliferative disease in mice [11] and profound and life-threatening autoimmunity in humans [12]. FoxP3 expression in humans is not entirely specific for Tregs (e.g., certain activated T cells transiently express FoxP3 [13–15]), but is generally considered superior to CD25 as a marker for Tregs, because some FoxP3+ CD4+ T cells in humans are CD25neg.
Tregs play a critical role in the inhibition of tumor immune surveillance. In mice, the depletion of Tregs in vivo enhances tumor immunity in mouse models of cancer [16, 17]. Low-dose cyclophosphamide, for example, potentiates the antitumor effects of therapeutic cancer vaccines in mice with breast carcinoma by selectively deleting or inhibiting the cycling population of Tregs [18]. In another strategy, injection of mice with CD25 antibody depletes animals of CD25+ T cells and markedly enhances response to vaccination [19–21]. In humans, it has been widely reported that there is an increased pool of Tregs in the peripheral blood of cancer patients, prompting attempts to deplete Tregs in cancer patients in vivo to augment immune surveillance or immunotherapy [22–28]. Nevertheless, many of the preclinical studies have been limited by the exclusive use of percentages (rather than absolute counts), the use of CD25 rather than FoxP3 to identify Tregs, or comparisons to healthy volunteers of younger age. Overall, in humans, dynamic changes in the Treg compartment as a function of stage and treatment of disease remain poorly understood, potentially hindering the optimal design of cancer immunotherapy.
In this study, we evaluated peripheral blood Tregs from patients with early or late stage breast cancer and compared the results to healthy volunteers of comparable age. We focused on breast cancer because Tregs exhibit potent immunosuppressive functions and are known to infiltrate primary tumors and draining lymph nodes [8, 9]. Moreover, it has been demonstrated that within primary breast tumors, higher numbers of Tregs are associated with a poor prognosis [29, 30]. Indeed, the ineffectiveness of numerous immunotherapy strategies in breast cancer may be related in part to the negative effects of Tregs. We, therefore, assessed the percentages, absolute counts, and function of FoxP3+ CD4+ T cells in the peripheral blood of 45 patients and identified the dynamic changes in the Treg and non-Treg compartments as a function of stage and treatment.
Materials and methods
Subjects and samples
Peripheral blood was obtained after signed, informed consent using protocols approved by Institutional Review Board of the Hospital of the University of Pennsylvania or the Philadelphia Veterans Affairs Medical Center. Blood was obtained from four groups of donors: (1) healthy volunteers (HV) (n = 14); (2) patients with stages I–III breast cancer in remission who had completed initial surgery and were about to begin adjuvant chemotherapy at the time of phlebotomy (n = 11); (3) stages I–III patients in remission who had completed adjuvant chemotherapy within the past 3 months (n = 11); and (4) stage IV patients with active disease and who had not received chemotherapy for at least 30 days prior to phlebotomy (n = 23). Subject characteristics are described in Supplemental Table 1. The mean (±SD) age of all subjects was 52 ± 12 years, and there was no significant difference in age among groups (P = 0.18).
All adjuvant patients (groups 2, 3) received combination chemotherapy as determined by their primary oncologist. All but one patient in groups 2 and 3 had stages II or III breast cancer. The remaining patient had a 1.7 cm primary tumor that was negative for hormone receptor and HER2/neu expression without lymph node involvement. Adjuvant chemotherapy was not homogenous, but all patients received regimens that are standard of care. In the post-chemotherapy group (group 3), a majority of these patients (7/11, 64%) received doxorubicin plus cyclophosphamide followed by a taxane [31] while the others (4/11, 36%) received taxane-based regimens without anthracyclines [32]. In group 3, phlebotomy was performed at a median of 29 days after the completion of chemotherapy (range 0–90 days); two of these patients had initiated radiation therapy prior to the time of phlebotomy. Absolute lymphocyte count (ALC) was obtained from a complete blood count and differential. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll centrifugation (Amersham Pharmacia Biotech, Uppsala, Sweden).
Monoclonal antibodies
Fluorochrome-conjugated mAb used were PE-Cy7-CD3 clone SK7, FITC- or APC-CD4 clone RPA-T4, PerCP-CD4 clone SK3, PE-CD25 clone M-A251 or 4E3, PerCP- or APC- or APC-Cy7-CD14 clone MφP9, PerCP-CD19 clone 4G7, PE-CD27 clone M-T271, PE-CD45RA clone HI100, PE-CD45RO clone UCHL1, and PE-CD152 clone BNI3 (BD Biosciences, San Jose, CA); Alexa fluor 488-anti-FoxP3 clone 259D and Alexa fluor 488-conjugated isotype control clone MOPC-21 (Biolegend, San Diego, CA); APC-anti-FoxP3 clone PCH101, APC-anti-IFN-γ clone 4S.B3, and APC-conjugated isotype control clone MOPC-21 (eBioscience, San Diego, CA); and PE-CCR7 clone 150503 (R&D Systems, Minneapolis, NM).
Flow-cytometric analysis
Peripheral blood mononuclear cells in PBS with 5% heat-inactivated fetal calf serum were then stained for the surface markers CD3 (PE-Cy7), CD4 (APC), CD14 (PerCP), and CD25 (PE), followed by intracellular staining for FoxP3 (clones PCH101 or 259D) using a fixation/permeabilization kit (eBioscience), according to the manufacturer’s instructions. Cells were washed twice in staining buffer and analyzed immediately using a FACSCanto cytometer and FACSDiva software (BD Biosciences). Quadrants and box gates were set in reference to isotype controls, and percentages of the parent CD4+ population were calculated.
Intracellular cytokine assay
Peripheral blood mononuclear cells in complete media comprising RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated human AB serum, 20 mM HEPES (Sigma-Aldrich), and 2 mM l-glutamine (Invitrogen) were stimulated with or without phorbol 12-myristate 13-acetate (PMA) (5 ng/ml) and ionomycin (1 μM) (Sigma, St. Louis, MO) for 4 h in the presence of 1 μM monensin (BD Biosciences). PBMC were then washed twice, re-suspended in staining buffer and stained for the surface markers CD3, CD4, CD14, and CD25 followed by intracellular staining for FoxP3 and IFN-γ using a fixation/permeabilization kit (eBioscience), according to the manufacturer’s instructions. PBMC were washed twice in staining buffer and analyzed immediately by flow cytometry.
Treg suppressive assay
CD25high CD4+ T cells were isolated using a Treg isolation kit (Miltenyi Biotec, Bergisch-Gladbach, Germany) from peripheral blood of patients or healthy volunteers. The number of Tregs quantified for the immunosuppressive assay was based on the total number of isolated cells, 70–80% of which expressed FOXP3 as determined by post-sort flow cytometry. The responder cells were total PBMC isolated from a healthy volunteer. Tregs (1.0 × 106 per ml) were co-cultured with responder PBMC (1.0 × 106 per ml) labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Carlsbad, CA) in the presence of 1.3 CD3-conjugated M-450 tosylactivated Dynabeads (Dynal Biotech, Hamburg, Germany) per PBMC for 4 days, as previously described [33]. CD8+ T cell proliferation was determined by dilution of CFSE among labeled cells compared with control PBMCs cultured without Tregs.
Statistical analysis
Analysis of variance (ANOVA) was employed to compare the mean values of a variable among the four blood donor groups (e.g., healthy volunteers, stages I–III breast cancer patients prior to adjuvant chemotherapy, stages I–III breast cancer patients after adjuvant chemotherapy, and breast cancer patients with metastatic disease). Once a significant difference among the donor groups was established, pairwise post hoc comparisons were performed using Scheffé’s test at a P < 0.05 significance level, which controls for multiple paired testing. The magnitude of linear correlation between two variables was assessed by Pearson’s correlation coefficient. Prior to testing, the normality assumption was assessed for each variable using a histogram and normal probability plot. If a variable was not considered to be normally distributed, then a natural log transformation was applied and the normality assessment was repeated. To control the overall type-I error rate, the Benjamini–Hochberg procedure [34] for multiple testing was employed. Taking into consideration the total number of ANOVA tests performed, P < 0.03 was considered to be statistically significant. All statistical tests were two-sided and were performed using STATA v10.0 software (StataCorp).
Results
FoxP3+ CD4+ T cells in healthy volunteers and patients with breast cancer
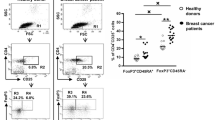
To explore the hypothesis that Treg homeostasis is altered in patients with breast cancer, we used multi-parameter flow cytometry to evaluate T cells in unmanipulated PBMC from donors in each of four donor groups: (1) healthy volunteers (HV) (n = 14); (2) patients with stages I–III breast cancer immediately prior to the initiation of adjuvant chemotherapy (n = 11); (3) stages I–III patients in remission who had completed adjuvant chemotherapy (n = 11); and (4) stage IV patients with active disease. Subject characteristics are described in Supplemental Table 1 and further detailed in “Materials and methods”. Peripheral blood FoxP3+ CD4+ T cells were identified for all donors in each group (Fig. 1). Two monoclonal antibodies (mAb) were used to evaluate FoxP3 expression in light of recent controversy in this area [35, 36], but the pattern of FoxP3 expression and the percent positivity was nearly identical when comparing the anti-FoxP3 mAb clone PCH101 versus 259D (data not shown).
FoxP3 expression on peripheral blood CD4+ T cells. PBMC were analyzed by flow cytometry and gated on CD3+, CD4+, and CD14neg events. Boxes indicate percentages of CD4+ population and drawn according to isotype control. a Healthy volunteer, b early stage patient having completed initial surgery and about to begin adjuvant chemotherapy, c early stage patient having completed adjuvant chemotherapy, d stage IV patient having not received chemotherapy for 30 days. Data shown are representative of 14 healthy volunteers, 11 pre-chemotherapy patients, 11 post-chemotherapy patients, and 23 stage IV patients
To further characterize and compare FoxP3+ CD4+ T cells in healthy volunteers and patients, we then analyzed FoxP3+ CD4+ T cells for expression of CTLA4, CD45RO, CD27, CD45RA, and CCR7. We found no significant differences in the phenotype of CD4+ FoxP3+ T cells among the four donor types (Fig. 2). In particular, the expression of CTLA4, which has been implicated in mediating function of Tregs in humans [37], was similarly positive in each case.
Expression of surface and intracellular markers on FoxP3+ CD4+ T cells from healthy volunteers and patients. Peripheral blood from healthy volunteers (HV; n = 3), stages I–III patients having completed initial surgery and about to begin adjuvant chemotherapy (PreC; n = 2), stages I–III patients having completed adjuvant chemotherapy during the past 3 months (postC; n = 2), and stage IV patients having not received chemotherapy for at least 30 days (Met; n = 2) was evaluated via flow cytometry for the expression of CD25, CTLA-4, CD45RO, CD27, CD45RA and CCR7. Cells were gated on FoxP3+ CD4+ T cells. Light gray histograms represent isotype controls; dark gray histograms represent experimental results. Mean fluorescence intensity after background subtraction is indicated in the upper right corner
To evaluate the functional status of FoxP3+ CD4+ T cells, we first measured IFN-γ secretion from FoxP3+ and FoxP3neg CD4+ T cells following in vitro stimulation with PMA/ionomycin. The failure to produce effector cytokines after mitogenic stimulation is an intrinsic property of Treg populations, and as has been shown recently by other investigators [38, 39], can be measured in an assay that evaluates total FoxP3+ CD4+ T cells using minimal numbers of cells rather than just the CD25-expressing subset of CD4+ T cells isolated from a large number of cells. We found that for all donor groups tested, FoxP3neg CD4+ T cells included a large subset of cells able to secrete IFN-γ (no significant difference among donor groups), but FoxP3+ CD4+ T cells did not secrete IFN-γ (Fig. 3). These observations are consistent with the expected anergic properties recognized as a general phenomenon of Tregs [9, 38, 39].
Functional analysis of FoxP3+ CD4+ T cells. Cells were stimulated with PMA/ionomycin in the presence of monensin and stained for surface markers followed by intracellular staining for FoxP3 and IFN-γ. PBMC gated were CD3+, CD4+, and CD14neg events. Quadrants show percentages of the CD4+ gate and are drawn according to isotype controls. a Healthy volunteer, b early stage patient having completed initial surgery and about to begin adjuvant chemotherapy, c early stage patient having completed adjuvant chemotherapy, d stage IV patient having not received chemotherapy for 30 days. Representative plots of three subjects from each donor group
We then directly measured functional suppressive activity of Tregs by isolating viable CD25-expressing CD4+ T cells from donors and coculturing these cells with CD3-stimulated responder T cells. Although this assay is limited because not all FoxP3+ CD4+ T cells express CD25 and a measurable fraction of non-regulatory (FoxP3neg) T cells do express CD25, FoxP3 expression is mostly confined to CD25high cells in humans [39]. (It was not possible to isolate FoxP3-expressing CD4+ T cells for this assay because cellular permeabilization and fixation required to detect intracellular FoxP3 kills the cells.) We found that Tregs isolated from either normal donors or patients with metastatic disease suppressed CD3-mediated proliferation of normal CD8+ T cells (Supplemental Fig. 1), consistent with the known function of Tregs. We did not have sufficient cells from enough patients to explore if patient Tregs suppressed more potently than healthy volunteer Tregs.
Differences in the percentage, but not absolute counts of FoxP3 cells
Five parameters were then compared among the four donor groups: (1) ALC, (2) the percentage of CD4+ T cells among all lymphocytes, (3) the absolute count of CD4+ T cells (cells/μl), (4) the percentage of FoxP3+ CD4+ T cells among all CD4+ T cells and (5) the absolute counts of FoxP3+ CD4+ T cells (Table 1; Fig. 4). For ALC, there was a highly significant difference among donor groups (P = 0.0003, Fig. 4a). There was no significant difference between healthy volunteers and patients pre-chemotherapy, but ALC was significantly lower both for patients post-chemotherapy and those with metastatic disease (P < 0.05). For the percentage of CD4+ T cells, there was no significant difference among groups (P = 0.55, Fig. 4b), but because of changes in the ALC, the average absolute count of CD4+ T cells was significantly different among donor groups (P = 0.0019, Fig. 4c). In patients with metastatic disease, the average count was significantly lower than in healthy or patients pre-chemotherapy (P < 0.05). In addition, there was a trend toward lower CD4+ T cell counts in patients post-chemotherapy. Indeed, CD4+ counts were <200 cells/μl, which defines AIDS in HIV-infected persons, in 27% of patients post-chemotherapy and 22% of patients with metastatic disease compared with 0% of healthy individuals or patients pre-chemotherapy. Overall, these results suggest that after chemotherapy and/or in the context of metastatic tumor burden, breast cancer patients can be relatively lymphopenic and moderately to severely compromised with regard to CD4+ T cell numbers.
FoxP3+ CD4+ T cells in healthy volunteers and breast cancer patients. Peripheral blood was evaluated from healthy volunteers (HV; 14), stages I–III patients having completed initial surgery and about to begin adjuvant chemotherapy (PreC; n = 11), stages I–III patients having completed adjuvant chemotherapy during the past 3 months (postC; n = 11), and stage IV patients having not received chemotherapy for at least 30 days (Met; n = 23). a lymphocyte count per μl of blood, b percent CD4+ T cells of total lymphocytes, c number of CD4+ T cells per μl of blood; d percentage of FoxP3+ CD4+ T cells among total CD4+ T cells, e number of FoxP3+ CD4+ T cells per μl of blood. Each symbol represents one donor, and bars represent means. *P < 0.05 for the comparison shown; ***P < 0.001
In contrast to the percentage of total CD4+ T cells, which did not differ among groups, the percentage of FoxP3+ CD4+ T cells among all CD4+ T cells was highly significantly different among donor groups (P < 0.0001, Fig. 4d). It was higher in patients post-chemotherapy (P < 0.05) and in patients with metastatic disease (P < 0.001) compared with either healthy volunteers or patients pre-chemotherapy. The absolute number of FoxP3+ CD4+ T cells, however, was not significantly different among donor groups (P = 0.84, Fig. 4e). This steady state of FoxP3+ CD4+ T cell numbers appeared to be accomplished by an inverse correlation of percent FoxP3+ CD4+ and ALC; that is, an elevated percentage of FoxP3+ CD4+ T cells significantly correlated with a lower ALC (r = −0.405, P = 0.002). Thus, in contrast to CD4+ T cell counts which are relatively lower in patients after chemotherapy or patients with metastatic disease, counts of FoxP3+ CD4+ are similar among donor groups despite the important differences in treatment history and tumor burden.
CD25+ FoxP3+ CD4+ T cell subset shows similar pattern as total FoxP3+ CD4+ T cells
We also evaluated whether the patterns we observed for FoxP3+ CD4+ T cell homeostasis among the four donor groups also applied to the CD25+ subset of Treg. In humans, not all FoxP3+ CD4+ T cells express CD25, and it remains unclear whether these cells differ functionally from CD25low CD4+ FoxP3+ T cells in vivo in humans. We found, using multi-parameter flow cytometry, that CD25bright FoxP3+ CD4+ T cells were readily detected in donors from each group (Fig. 5a) and that this subpopulation was equally anergic as total FoxP3+ CD4+ T cells with regard to mitogen-induced IFN-γ secretion (data not shown). The percentage of CD25bright FoxP3+ CD4+ T cells was highly significantly different among donor groups (P < 0.0001, Table 1; Fig. 5b). The percentage was higher in patients with metastatic breast cancer when compared with healthy volunteers (P < 0.001) or patients either pre-chemotherapy (P < 0.001) or post-chemotherapy (P = 0.003); however, similar to that for total FoxP3+ CD4+ T cells, there was no significant difference in the absolute count of CD25bright FoxP3+ CD4+ T cells among donor groups (P = 0.21, Fig. 5c).
FoxP3 and CD25 expression on peripheral blood CD4+ T cells. a PBMC from a patient with stage IV disease were analyzed by flow cytometry and gated on CD3+, CD4+, and CD14neg events. Quadrants show percentages of the CD4+ gate, b percentage of CD25+ FoxP3+ CD4+ T cells among total CD4+ T cells for the four donor groups, c number of CD25+ FoxP3+ CD4+ T cells per μl of blood. For (b) and (c), each symbol represents one donor, and bars represent means. *P < 0.05 for the comparison shown; ***P < 0.001
Discussion
It is now generally accepted that Tregs frustrate productive tumor immune surveillance and represent a major obstacle for the successful development of cancer immunotherapy. In patients with breast cancer, increased Treg infiltration of primary tumors independently predicts a worse clinical course [29, 30]. Ex vivo, Tregs isolated from patients with breast cancer inhibit T cell proliferation [8, 9, 40]. In mice genetically engineered to develop invasive breast cancer, therapeutic elimination of Tregs markedly improves the efficacy and curative potential of tumor vaccination [18], further underscoring the importance of Treg biology in the natural history and therapy of breast and other cancers. In this study, we have determined the relative and absolute numbers of FoxP3+ CD4+ T cells in the peripheral blood of patients with early or late stage breast cancer compared with healthy volunteers to understand the dynamic changes in the Treg compartment as a function of stage and treatment. Although the percentage of FoxP3+ CD4+ T cells among all CD4+ T cells was significantly higher in patients with metastatic breast cancer and those with stages I–III breast cancer who had completed chemotherapy, the absolute counts of FoxP3+ CD4+ T cells in peripheral blood were stable in all donor groups evaluated, despite the variations in total absolute CD4 counts. Similar results were obtained for the CD25+ subset of FoxP3+ CD4+ T cells. Overall, these findings demonstrate that the circulating Treg pool as measured by absolute counts is no higher in patients than in healthy individuals, and that a homeostatic mechanism appears to govern a stable absolute count of FoxP3+ CD4+ T cells regardless of disease stage or treatment status.
Several prior studies have examined circulating Tregs in cancer patients by exclusively employing percentages and not absolute counts of Tregs in blood, using CD25 rather than FoxP3 to identify Tregs, or performing comparisons to normal donors of unstated ages. The latter is particularly important because Treg prevalence appears to increase with age in both mice and humans [41–43], which might confound analyses if adult cancer patients who are generally older are compared with samples from younger blood donors. In this study, we calculated both percentages and absolute counts of Tregs, using FoxP3 as the primary Treg marker, and compared the results to a group of healthy volunteers that was not statistically different in age to three patient groups. Moreover, functional anergy for FoxP3+ CD4+ T cells, but not FoxP3neg CD4+ T cells, was demonstrated for cells from each type of donor in this study. Measuring Tregs in peripheral blood, as we have done here, may not optimally reflect the physiology of Tregs at the tissue level, especially within the tumor microenvironment. Immunohistology analysis comparing tumor tissue to peripheral blood may help reveal whether changes in the percentage of Tregs in PBMC has an influence on the composition of tumor infiltrate. However, in this study, we did not evaluate intratumoral Tregs either because tumor tissue was not available for this purpose or there was no tumor to evaluate (e.g., healthy volunteers or patients in remission after surgery and/or adjuvant chemotherapy).
Although a higher percentage of FoxP3+ CD4+ T cells among total CD4+ T cells in peripheral blood of patient with metastatic breast cancer has been previously observed [8, 9], our observation that breast cancer patients after adjuvant chemotherapy (but not before) also exhibit a relatively higher percentage of FoxP3+ CD4+ T cells has not been previously reported. This relative increase in FoxP3+ CD4+ T cells was not related to tumor burden because all patients were in complete remission. Some of the agents administered to our patients in the adjuvant setting, cyclophosphamide in particular, are considered Treg toxic, even selectively so [18]. However, it does not appear likely from our data that Tregs are depleted systemically by standard adjuvant chemotherapy either in relative or absolute numbers. This notion carries important clinical implications: for example, vaccines and other immunotherapies delivered to patients on completion of adjuvant therapy may need to include strategies aimed expressly at overcoming the immunosuppressive effects of Tregs even though patients achieve minimal residual disease. Overall, we observed reductions in total lymphocytes and in conventional (non-Treg) CD4+ T cells in patients compared with healthy volunteers, and this might be due to the effects of chemotherapy or tumor burden. Reductions in such cells may also explain the apparent relative increase in Tregs, as measured as a percentage of total CD4+ T cells. However, when the absolute count of Tregs was measured, we found a relatively constant number within the four donor populations.
Finally, our data suggest that homeostatic mechanisms governing the peripheral blood count of FoxP3+ CD4+ T cells fundamentally differ from those governing the total CD4+ T cell count; such differential regulation would explain, for example, why the average absolute CD4 count in patients with metastatic disease or those post-chemotherapy was lower than that for healthy volunteers, yet the absolute count of FoxP3+ CD4+ T cells was not. Regulatory and non-regulatory T cells might be regulated differentially by cytokines such as IL-2 or exhibit differential susceptibility to effects of chemotherapy or macroscopic tumor burden. Although our data do not allow us to distinguish among these possibilities, the manifestation of this physiology is a circulating concentration of Tregs that is no higher in early or late stage breast cancer patients than in healthy individuals. In future studies, it would be interesting to perform a comprehensive serum cytokine analysis to explore this hypothesis; however, such an analysis may be revealing only at the microenvironmental level. An alternative approach to explore the role of cytokines in Treg modulation would be to deliver in vivo monoclonal antibody that blocks cytokines or their receptors. Along these lines, we have initiated a clinical trial to deliver anti-CD25 monoclonal antibody to patients (in order to block IL-2) and subsequently measure effects on Tregs in vivo. In preliminary data recently reported [44], we have found that treatment with anti-CD25 monoclonal antibody results in a protracted loss of Tregs from peripheral blood of patients with stage IV breast cancer. Studies to investigate the mechanism of this effect (IL-2 deprivation vs. antibody-mediated cytotoxicity vs. other) are ongoing.
References
Baecher-Allan C, Viglietta V, Hafler DA (2004) Human CD4+ CD25+ regulatory T cells. Semin Immunol 16:89–98
Shevach EM (2006) From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 25:195–201
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133:775–787
Camara NO, Sebille F, Lechler RI (2003) Human CD4+ CD25+ regulatory cells have marked and sustained effects on CD8+ T cell activation. Eur J Immunol 33:3473–3483
Thornton AM, Shevach EM (2000) Suppressor effector function of CD4+ CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol 164:183–190
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H et al (2001) Regulatory CD4(+)CD25(+) T cells in tumors from patients with early- stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 61:4766–4772
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E et al (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9:606–612
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
Khattri R, Cox T, Yasayko SA, Ramsdell F (2003) An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol 4:337–342
Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL et al (2001) X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27:18–20
Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY et al (2006) Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA 103:6659–6664
Ahmadzadeh M, Antony PA, Rosenberg SA (2007) IL-2 and IL-15 each mediate de novo induction of FOXP3 expression in human tumor antigen-specific CD8 T cells. J Immunother 30:294–302
Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE (2007) Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol 37:129–138
DiGiacomo A, North RJ (1986) T cell suppressors of antitumor immunity: the production of Ly-1-, 2+ suppressors of delayed sensitivity precedes the production of suppressors of protective immunity. J Exp Med 164:1179–1192
Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S et al (2004) Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med 200:771–782
Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD et al (2005) Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med 201:1591–1602
Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T et al (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 59:3128–3133
Shimizu J, Yamazaki S, Sakaguchi S (1999) Induction of tumor immunity by removing CD25+ CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 163:5211–5218
Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME et al (2001) Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med 194:823–832
Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D et al (2005) Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 115:3623–3633
Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA (2005) Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother 28:582–592
Ruter J, Barnett BG, Kryczek I, Brumlike MJ, Daniel BJ et al (2006) Manipulating T regulatory cells in cancer immunotherapy. Exp Rev Derm 1:589–597
Mahnke K, Schonfeld K, Fondel S, Ring S, Karakhanova S et al (2007) Depletion of CD4+ CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer 120:2723–2733
Powell DJ Jr, Attia P, Ghetie V, Schindler J, Vitetta ES et al (2008) Partial reduction of human FoxP3+ CD4 T cells in vivo after CD25-directed recombinant immunotoxin administration. J Immunother 31:189–198
Rasku MA, Clem AL, Telang S, Taft B, Gettings K et al (2008) Transient T cell depletion causes regression of melanoma metastases. J Transl Med 6:12
Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D et al (2008) Depletion of human regulatory T cells specifically enhances antigen specific immune responses to cancer vaccines. Blood 112:610–618
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF et al (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380
Wolf AM, Rumpold H, Wolf D, Gastl G, Reimer D et al (2007) Role of forkhead box protein 3 expression in invasive breast cancer. J Clin Oncol 25:4499–4500
Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP et al (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21:1431–1439
Jones SE, Savin MA, Holmes FA, O’Shaughnessy JA, Blum JL et al (2006) Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24:5381–5387
Golovina TN, Mikheeva T, Suhoski MM, Aqui NA, Tai VC et al (2008) CD28 costimulation is essential for human T regulatory expansion and function. J Immunol 181:2855–2868
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57:289–300
Pillai V, Karandikar NJ (2008) Attack on the clones? Human FOXP3 detection by PCH101, 236A/E7, 206D, and 259D reveals 259D as the outlier with lower sensitivity. Blood 111:463–464
Tran DQ, Ramsey H, Shevach EM (2007) Induction of FOXP3 expression in naive human CD4+ FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110:2983–2990
Zheng Y, Manzotti CN, Burke F, Dussably L, Qureshi O et al (2008) Acquisition of suppressive function by activated human CD4+ CD25- T cells is associated with the expression of CTLA-4 not FoxP3. J Immunol 181:1683–1691
Ahmadzadeh M, Felipe-Silva A, Heemskerk B, Powell DJ Jr, Wunderlich JR et al (2008) FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood 112:4953–4960
Kryczek I, Liu R, Wang G, Wu K, Shu X et al (2009) FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res 69:3995–4000
Delong P, Carroll RG, Henry AC, Tanaka T, Ahmad S et al (2005) Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol Ther 4:342–346
Dejaco C, Duftner C, Schirmer M (2006) Are regulatory T-cells linked with aging? Exp Gerontol 41:339–345
Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N et al (2005) The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol 140:540–546
Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G et al (2008) Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol 181:1835–1848
Rech AJ, Vonderheide RH (2009) Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci 1174:99–106
Acknowledgments
We thank Theresa Colligon and Adrianna Recio, RN, and Drs. Daniel J. Powell, Jr., Tatiana Golovina, and James Riley for helpful discussions. This study was supported by grants from the Breast Cancer Research Foundation and the NCI (R01 CA111377) and a gift from Mark and Paula Solomon.
Conflict of interest statement
The authors declare that no conflict of interests exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rech, A.J., Mick, R., Kaplan, D.E. et al. Homeostasis of peripheral FoxP3+ CD4+ regulatory T cells in patients with early and late stage breast cancer. Cancer Immunol Immunother 59, 599–607 (2010). https://doi.org/10.1007/s00262-009-0780-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0780-x