Abstract
Background
The compartments of memory T cells play a fundamental role in the immune system by substantiating specific and acquired immunity. A new subset of memory cells, T stem cell memory (TSCM) cells, with stem cell-like properties, a high capacity to proliferate, a long survival, and an ability to differentiate into all effector and memory cells has recently been introduced. In the present study, we aimed to determine the frequency of CD4+ TSCM and other T memory cell subsets in tumor draining lymph nodes of breast cancer patients.
Materials and methods
Mononuclear cells were obtained from axillary lymph nodes of 52 untreated patients with breast cancer (BC) and stained with fluorochrome conjugated anti-CD4, −CCR7, −CD45RO and -CD95 antibodies to detect different subtypes of memory cells in CD4+ lymphocyte populations. Data were acquired using a four-color FACSCalibur flow cytometer and analyzed using CellQuest Pro software.
Results
We found that >70% of CD4+ lymphocytes in draining lymph nodes of BC patients exhibited a memory phenotype of which 7.04 ± 1.04% had a TSCM phenotype (CD4+CCR7+CD45RO−CD95+). The frequency of TSCM cells was significantly higher in tumor positive lymph nodes compared to tumor negative lymph nodes (p = 0.026) as well as among those patients who had at least one affected lymph node (p = 0.012). Moreover, we found that the total frequency of central memory T cells (TCM) with a low expression of CD45RO was significantly higher among these patients. The percentage of CD45ROLow TCM cells was also found to increase with tumor progression from stage I to stage III (p = 0.020). On the other hand, we found that the percentage of CD95Hi effector memory T cells (TEM) was significantly decreased in involved lymph nodes (p = 0.009).
Conclusion
Our data suggest that following long-term exposure to putative tumor antigens, TSCM cells proliferate to generate a pool of committed memory and effector T cells. As the tumor progresses, the immunosuppressive milieu induced by tumor cells may slow down the differentiation of CD45ROLow TCM cells to more functional sub-populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The immunological memory pool is regarded as an important component of adoptive immune responses. Having faced the antigen, naïve T cells become active to proliferate and differentiate, gradually gaining – throughout the maturation process – the effector functionality, thereby losing the capability to self-renew and survive. During this process, a small portion of the cells evolves into subsets of T memory cells exhibiting a high longevity. These cells subsequently settle in secondary lymphatic organs and tissues where they turn into effector T cells upon encountering the antigen, and provide effective secondary immunologic responses against pathogens and tumors [1,2,3,4,5].
The subsets of memory T cells are heterogeneous and can be divided into two main categories of central (TCM) and effector (TEM) memory cells based on the expression of surface markers, i.e., molecules responsible for lymphocyte homing and functionality [6]. TCM cells highly express CCR7 and CD62L and circulate inside the lymphatic organs. They exhibit a relative high longevity, but can be converted into more short-lived TEM cells [6, 7]. TEM cells, in contrast, do not express CD62L, CCR7 and CD45RA, but are CD45RO positive and circulate mainly in non-lymphatic organs. These cells have a low capacity to proliferate and are actually in the end-stage of differentiation [4, 8].
A number of complex issues have arisen as a result of recent studies that introduced a new subset of memory cells, T stem cell memory (TSCM) cells [9]. In healthy individuals, these cells make up only 2–3% of the T cells population. However, their similarity to stem cells – i.e., self-renewability and multipotency to differentiate into various cell lineages – has made them of significant importance [10,11,12]. Currently, the presence of TSCM cells in mice, humans and primates has been proven. These cells have markers in common to naïve T cells (CCR7+CD45RO−CD95+) and express CD62L at high levels. They can, however, be distinguished from naïve T cells through the expression of CD95 and CD122, markers that are also expressed by memory cells [9, 13, 14]. Their memory functionality, including a rapid production of cytokines after T cell receptor (TCR) stimulation and longtime survival, suggests that they may be responsible for immunity throughout human lifetime [15]. Their high capacity to proliferate, their long survival and their ability to change into all effector and memory cell subgroups, grant TSCM cells the possibility to be highly effective in tumor destruction with a small number of cells compared to other T cell subsets. The role of this T cell memory subset in various pathologic conditions, such as AIDS, has amply been studied [10], whereas investigations aimed at the role of these cells in cancer are still in a preliminary stage [16, 17]. In the present study, we determined the frequency of CD4+ TSCM cells in tumor draining lymph nodes (TDLNs) of breast cancer patients. In addition, we investigated putative associations of these cells with various clinical and pathological features of these patients.
2 Materials and methods
2.1 Patient samples
Fifty-two patients with breast cancer (BC) were recruited for the study before receiving any treatment. Following routine pathological examination, parts of axillary lymph nodes were referred to our laboratory. All samples were obtained after giving informed consent. Tumor infiltration to the nodes was histologically determined by pathologists. Lymph nodes that were infiltrated by tumor cells were classified as node positive. The patients were considered lymph node positive when at least one resected regional lymph node was found to be infiltrated by tumor cells (LN+ patients). All positive lymph nodes had macro-metastases (0.5–4.5 mm). Clinical and pathological information was obtained from patient files. Patient stages were determined using the TNM system according to the 7th edition of the AJCC cancer staging manual.
2.2 Preparation of lymph node mononuclear cells
To obtain single cell suspensions, fresh lymph nodes were mechanically minced into small pieces in complete culture medium containing 10% fetal bovine serum (FBS; Gibco, USA), 100 units/ml penicillin and 100 μg/ml streptomycin (Biosera, UK) and filtered through a 40 μm cell strainer (BD Biosciences, USA). Next, mononuclear cells were separated using Ficoll-Hypaque (Biosera, UK) gradient centrifugation in 600×g at 20 °C for 20 min. The mononuclear ring was harvested, washed twice and dissolved in 1× phosphate buffered saline (PBS) for further analysis. A Trypan Blue dye (Biosera, UK) exclusion test was used to determine the number of viable cells. Finally, the cells were transferred at a 250 × 103 concentration to polystyrene round bottom flow cytometric tubes (BD Biosciences, USA) in 50 μl PBS.
2.3 Cell staining and flow cytometry
To determine the frequency of CD4+ memory T cell subsets, the following antibodies were used: PerCP–conjugated anti-CD4, clone: Sk3, FITC–conjugated anti-CCR7, clone: 3D12, PE–conjugated anti-CD95, clone: Dx2 and APC–conjugated anti-CD45RO, clone: UCHL1. The isotype controls used were: PerCP–conjugated mouse IgG1, FITC–conjugated mouse IgG2a, PE–conjugated mouse IgG1 and APC–conjugated mouse IgG2a. All antibodies were purchased from BD Biosciences, USA. The mononuclear cells were surface-labeled with appropriate antibodies for 20 min at room temperature. Next, the cells were washed twice with 1× PBS to remove unbound antibodies after which ~100 × 103 events were acquired on a four-color FACSCalibur flow cytometer (BD Biosciences, USA). The data were analyzed using the CellQuest Pro software tool (BD Biosciences, USA). The mean fluorescent intensity (MFI) of CD45RO as well as CD95 normalized to MFI of negative cells, was reported as mean expression of these molecules on memory cells.
2.4 Statistical analysis
Nonparametric Mann–Whitney U test and Kruskal-Wallis H were used to determine statistical differences between subgroups. Correlations between the prevalence of each memory T cell subset and tumor size were assessed by Spearman’s rank correlation. SPSS15 software (SPSS GmbH Software, Germany) was used for all statistical analyses and p < 0.05 (two-tailed) was considered significant. GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA) was used for drawing the graphs.
3 Results
3.1 Patient inclusion characteristics
After pathological confirmation, 52 untreated patients with BC (mean age = 48.38 ± 11.40 years) were recruited for this study. In total 53 lymph nodes were acquired from these patients (two lymph nodes were obtained from one patient, one was involved and one was free). According to the pathological reports, in 24 of 53 (45.28%) lymph nodes tumor cells were seen (involved LNs). Infiltrative ductal carcinoma was the most frequent tumor type (80.39%). Most patients were in stage II (52.83%) and none of them showed distant metastases at the time of diagnosis. The main clinicopathological characteristics of the patients are listed in Table 1.
3.2 Frequency of CD4+ memory subsets in tumor draining lymph nodes of breast cancer patients
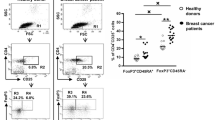
To determine the frequencies of the various T cell memory subsets after selecting CD4+ cells in the lymphocyte gate (Fig. 1a), the phenotypes of the different subsets were defined based on the expression of CCR7, CD45RO and CD95 (Fig. 1b). CD95+CD4+ lymphocytes simultaneously expressing CCR7 and CD45RO were considered as TCM cells (Fig. 1c and d). A small population with a CCR7−CD45RO+CD95+CD4+ phenotype was considered as TEM cells (Fig. 1e), whereas CCR7+CD45RO− cells that did not express CD95 were considered as naïve T cells. A subgroup of cells with a naïve phenotype (CCR7+CD45RO−) but positive for CD95 was coined as TSCM cells (Fig. 1f). CD95 and CD45RO were found to exhibit a gradient of expression ranging from low to high. Accordingly, two main subgroups were defined among the TCM subpopulation for each marker: CD95+ and CD95Hi cells and CD45ROHi and CD45ROLow cells (Fig. 1c and d). The average frequencies of the different memory T cell subtypes along with the mean CD95 fluorescent intensities (MFI) on the surface of these cells were recorded in the CD4+ lymphocyte population (listed in Table 2).
Phenotypic determination of CD4+ T cell memory subsets in TDLNs of breast cancer patients. After selecting CD4+ positive cells in the lymphocyte gate (a), the phenotypes of the different subsets were defined based on the expression of CCR7, CD45RO and CD95 (b). CD95+CD4+ lymphocytes with a CCR7+CD45RO+ phenotype were considered as TCM cells in both CD95+ and CD95Hi populations (c and d), whereas lymphocytes with a CCR7−CD45RO+CD95+CD4+ phenotype were considered as TEM cells (e) and CCR7+CD45RO− cells that did not express CD95 were considered as naïve T cells (f). A subgroup of lymphocytes with naïve phenotype (CCR7+CD45RO−) but positive for CD95 was coined as TSCM cells (f). TSCM: Stem Cell Memory T cell; TCM: Central Memory T cell; TEM: Effector Memory T cell; TN: Naïve T cell
3.3 Memory CD4+ T cell subsets in breast cancer patients with different clinical and pathological characteristics
3.3.1 CD4+ TSCM cells
The percentage of cells with a CD4+CCR7+CD45RO−CD95+ phenotype, TSCM, was found to be 7.04 ± 1.04 in the draining lymph nodes of the BC patients. We also found that the frequencies of these cells significantly increased in involved LNs (p = 0.026) (Fig. 2a). In addition, regarding CD95 expression, both CD95+ (p = 0.012) and CD95Hi (p = 0.041) TSCM cells were found to be elevated in patients with a lymphovascular invasion (Fig. 2c, p = 0.015 for CD95+ and p = 0.006 for CD95Hi cells). The frequency of CD95Hi TSCM cells was also found to be elevated in patients with perineural invasions (Fig. 2d, p = 0.048). The frequencies of CD4+ TSCM cells did not show any significant changes in patients at different stages of the disease (Fig. 2b) nor in patients with other clinical and pathological characteristics. Similar results were obtained in patients with an invasive ductal carcinoma (IDC) tumor type.
Frequency of TSCM in TDLNs of breast cancer patients with different pathological conditions. Percentages of the various TSCM memory cell subsets in TDLNs of BC patients with tumor cell-involved lymph nodes (a), at different stages (I-III) (b) with lymphovascular invasion (LVI) (c) and with perineural invasion (PI) (d). The data are presented as mean ± SEM. *Difference significant at 0.05 level (2-tailed), **Difference significant at 0.01 level (2-tailed)
3.3.2 CD4+ TCM cells
Central memory T cells with a CD4+CCR7+CD45RO+CD95+ phenotype were found to represent the most frequent CD4+ memory subset in the draining lymph nodes of BC patients (56.54 ± 2.09). We also found that the percentage of CD45ROLow expressing TCM sub-populations with different CD95 expression levels were significantly increased in tumor cell-involved lymph nodes compared to the tumor cell-free ones (p = 0.004 and p = 0.019 for CD95+ and CD95HiCD45ROLow TCM cells, respectively; Fig. 3a). Considering the number of involved lymph nodes, the frequency of the various TCM cell subgroups and CD95 MFI on these cells were found to be generally elevated, with increases in the number of tumor cell-involved lymph nodes (Fig. 3b).
Frequency of TCM cells in TDLNs of breast cancer patients with different pathological condition. Percentages of the various CD4+ TCM memory cell subsets and mean CD95 intensities on these cells in TDLNs of patients with involved lymph nodes (a), number of involved lymph nodes (b), stages (I-III) (c) and lymphovascular invasion (LVI) (d). The data are presented as mean ± SEM. *Difference significant at 0.05 level (2-tailed), **Difference significant at 0.01 level (2-tailed), *** Difference significant at 0.001 level (2-tailed). N0: free (0), N1: 1–3, N2: 4–9 and N3: >9
Similarly, we found after comparison of CD4+ TCM cells in the patients at different stages of the disease that, while the tumor progresses from stage I to stage III, the percentage of CD95HiCD45ROLow TCM cells significantly increases. Further analysis indicated that these cells were more frequent in patients at stage II (p = 0.012) and stage III (p = 0.010) than in patients at stage I. We also found that the CD95 MFI in CD95HiCD45ROLow TCM cells were increased in patients at stage II and III (p = 0.004 and p = 0.0005, respectively) compared to those at stage I (Fig. 3c). Again, we found that the results were similar in patients with an IDC tumor type.
Regarding other clinical and pathological parameters, we found that the percentages of the various sub-populations of TCM cells generally increased in patients positive for lymphovascular invasion, although the frequency of CD95MedCD45ROHi TCM cells was found to be decreased in these patients (Fig. 3d). The percentages of the different TCM sub-populations in patients with other clinical and pathological parameters, including histological tumor grade, tumor site as well as estrogen, progesterone and human epidermal growth factor receptor expression, did not show any significant differences.
3.3.3 CD4+ TEM cells
The frequency of TEM cells with a CD4+CCR7−CD45RO+CD95+ phenotype was found to be 2.82 ± 0.45, the lowest frequency among the memory subsets in TDLNs of the breast cancer patients tested. Based on CD95 expression, we found that the percentage of the various sub-populations of TEM cells was significantly decreased in involved lymph nodes (p = 0.014 and p = 0.040, respectively for the CD95Hi and CD95Low/Med sub-populations; Fig. 4a). In addition, we found that the frequency of this subset showed a significant reduction in patients with lymphovascular invasion (Fig. 4c). The mean expression of CD95 on CD95Hi TEM cells in patients with progesterone receptor expressing tumors (PR+) was found to be significantly increased in comparison to the PR negative ones (78.84 ± 6.28 versus 67.41 ± 8.72, p = 0.041, Supplementary Fig. 1). No significant differences were observed in the frequencies of the various TEM sub-populations in patients at different stages of the disease (Fig. 4b) nor in patients with other clinical and pathological characteristics.
Frequency of TEM in TDLNs of breast cancer patients with different pathological condition. Percentages of the various CD4+ TEM subsets in TDLNs of patients with a different node status (a), stages (I-III) (b) lymphovascular invasion (LVI) (c) and perineural invasions (PI) (d).The data are presented as mean ± SEM. *Difference significant at 0.05 level (2-tailed), **Difference significant at 0.01 level (2-tailed)
3.3.4 Other T cell subsets in draining lymph nodes of breast cancer patients
Along with the different memory subsets, the frequencies of naïve T cells with a CD4+CCR7−CD45RO−CD95− phenotype and expression of CD45RO, CCR7 and CD95 in the total lymphocyte population, as well as CD4+ cells, were determined in draining lymph nodes of BC patients. By doing so, we found that CD45RO+ lymphocytes were increased in patients with lymphovascular invasion (p = 0.025, Fig. 3d). The other subsets did not show any significant differences in patients with various clinical and pathological characteristics. The results were found to be similar in patients with an IDC tumor type.
4 Discussion
We investigated the presence of CD4+ T memory subsets in tumor draining lymph nodes of patients with breast cancer (BC) and found that >35% of the lymphocytes in the draining lymph nodes exhibited a memory phenotype, of which the frequency rises to >70% in the CD4+ population. The frequency of CD4+ lymphocytes with a memory phenotype also showed a significant increase in patients with tumor free lymph nodes. It is well documented that the generation of an appropriate memory response against tumor antigens may prevent tumor relapse in patients with different types of cancers, including BC [18,19,20]. Concordantly, high frequencies of CD45RO+ cells among BC tumor infiltrating lymphocytes have been found to correlate with a smaller size of the tumor, a lower stage, a smaller number of involved lymph nodes, less invasion into the lymphatic system and a longer survival following treatment [21]. These observations confirm the notion that memory cells may efficiently prevent tumor relapse. Accordingly, the observed increase in the frequency of CD4+ memory cells in TDLNs of BC patients in our study may suggest a protective role for these cells in preventing metastasis to lymph nodes and, hence, tumor progression. In the only study available so far on lymph nodes, Oberg et al. [19] showed that in patients with colon cancer higher percentages of CD45RO+ cells may be indicative of a better prognosis with a 5-year survival rate of ~66%. One of the major limitations of previous studies on memory cells is the exclusive use of CD45RO as a marker for the memory T cell phenotype, mostly by immunohistochemistry which, besides its low sensitivity, in its own cannot properly distinguish memory cells from other effector subsets. Memory cells can be divided into different subgroups according to their functionality and their cytokine expression. This is the first study investigating various CD4+ memory T cell sub-populations within lymph nodes of BC patients with a specific focus on TSCM cells.
We found that CD4+ TSCM cells with a CD4+CCR7+CD45RO−CD95+ phenotype represent ~7% of the lymphocyte population within the TDLNs of BC patients. Our analyses indicated that the percentage of these cells within the lymph nodes was increased, regardless of whether the investigated lymph nodes were positive or negative for tumor cells. This increase was also found to be accompanied by invasion into the lymphovascular and neural systems. TSCM cells are, in fact, a new subgroup of memory T cells that have only been recognized in recent years. Phenotypically speaking, they are more similar to naïve T cells, but they can be distinguished through expression of the CD95 and CD122 memory markers [9, 13]. These cells exhibit a high proliferative capacity, a high self-renewal capacity, and a multipotency to differentiate into other memory T cell subsets. These properties have turned these cells into invaluable tools in the design of adoptive T cell therapies in cancer [10,11,12]. The majority of studies on memory stem cells have been conducted in the context of AIDS. In the only study on breast cancer so far, published as an abstract by Pincha et al. [22], TSCM cells with a CCR7+CD45RO−CD127+CD45RA+CD95+CXCR3+IL-2Rβ+ phenotype were found to be present in the peripheral blood and bone marrow of BC patients among both CD4+ and CD8+ cell populations. Compared to healthy individuals, the frequency of TSCM cells was found to be elevated up to threefold in BC patients, particularly among CD8+ cells. These authors also reported that > 95% of the TSCM cells did not express exhaustion markers, i.e., PD-1 and LAG-3, indicating less exhaustion of these cells compared to other cells. We observed an increase in the frequency of TSCM cells in TDLNs of BC patients along with tumor progression as well as tumor cell metastasis to draining lymph nodes. Such an increase may possibly be attributed to long-term contact with tumor antigens, resulting in both central and effector memory cell pools during anti-tumor immune responses. As a result of tumor-induced suppression or factors released by tumor cells, however, the effector cells may be tightly regulated in such a way that they cannot competently control tumor growth and/or spread. This assumption is in line with Feuerer et al. [23, 24] who found that in BC patients the number of bone marrow memory cells was higher after tumor metastasis to the bone marrow. To substantiate this notion, however, further studies are required to assess the differentiation capacities and functions of TSCM cells in BC patients. In another study, Nagai et al. [17] showed that TSCM cells were increased in patients with acute adult T cell leukemia. In these patients the TSCM cells, along with other memory cells, may express specific HTLV-1 receptors, which indicates that these cells are also prone to viral contamination and, therefore, deemed as a reservoir for HTLV-1 viruses. On the other hand, Gattinoni et al. [15] found that in a transgenic mouse model of melanoma the adoptive transfer of a very small number of TSCM cells could cause tumor regression. They also showed that the TSCM cells exhibited a high capacity to both proliferate and survive after transfer to immunodeficient nod scid gamma (NSG) mice. Stronger anti-tumor responses and longer survival rates were found to be induced by TSCM cells compared to both TCM and TEM cells [9].
We also investigated the frequency of CD4+ TCM cells with a CCR7+CD45RO+CD95+ phenotype. We found that these cells were most abundant among the memory T cell subsets (as much as 56%) within BC draining lymph nodes. Besides variation in the CD95 expression level, these cells could clearly be divided into two groups based on CD45RO expression: one subgroup with a high CD45RO expression (CD45ROHi) and another subgroup with a low to moderate CD45RO expression (CD45ROLow/Med). Assessment of the total frequency of TCM cells and their subsets revealed that the percentage of CD45ROLow/MedCD95+ TCM cells in the involved lymph nodes was significantly increased. The frequency of CD45ROLow/MedCD95+ TCM cells was also found to be positively correlated with the number of involved lymph nodes, and progression of the disease from stage I to stage III. Meanwhile, the frequency of the CD45ROHi TCM sub-population in patients without lymph node involvement and/or any invasion to lymphatic vessels was found to be higher. Together, these data suggest that a high frequency of CD4+ TCM cells with a low-to-moderate expression of CD45RO is associated with a more advanced stage of the disease and with tumor progression. In contrast, we found that the occurrence of more differentiated CD4+ TCM cells, discriminated by a higher CD95 expression, is associated with a better clinical status and a decreased tumor growth. Despite the fact that the role of memory T cells in the generation of an appropriate immune response in pathologic conditions such as cancer is well documented and that they have been associated with better prognosis in many cases, the role of TCM cells in cancer has not been appropriately studied yet. Results from a phase II colon cancer clinical trial revealed that in patients receiving granulocyte macrophage colony-stimulating factor (GM-CSF) and Aldesleukin (GOLFIG-1) a continuous increase in the frequency of CD4+ and CD8+ TCM cells was accompanied by a decline in the percentage of suppressive regulatory T cells (Treg), as well as activation of cytotoxic T cells. This finding was found to be correlated with anti-tumor activity, although in our study more differentiated forms of CD4+ TCM cells with a higher expression of CD45RO and CD95 have been noted [25].
We observed that CD4+ TEM cells (CD4+CCR7−CD45RO−CD95+) exhibited the lowest frequency among the subgroups of memory T cells in TDLNs of BC patients. This observation is consistent with the role reported for these cells in the migration to inflammatory sites. We found a higher percentage of TEM cells in tumor free lymph nodes and also in LN− patients. It seems that by generating an appropriate immune response against tumor antigens, TEM cells may play an important role in inhibiting tumor progression and spread to lymph nodes. Concordant with our results in breast cancer, in colon cancer the percentage of memory cells with a similar phenotype to TEM (CD45RO+CCR7−CD27−CD28−) has been found to be increased during tumor progression, whereas a high infiltration was found to be correlated with absence of metastasis and less advanced stages of the disease [26]. The total frequency of TEM cells with more CD8+ T cells than CD4+ T cells was also found to be increased in BC patients vaccinated with an E57 peptide [27]. In patients with hepatocellular carcinoma it has been found that CD4+ TEM cells constitute the most abundant memory T cell sub-population [28]. All in all it seems that, similar to CD8+ TEM cells, an increase in CD4+ TEM cells in tumor free lymph nodes and LN− patients, along with an increase in patients without vascular and perineural invasion, may be indicative of an important role of these cells in preventing tumor cell metastasis to lymph nodes and, hence, tumor progression.
5 Conclusions
Our results indicate that in breast cancer patients the percentages of TSCM and CD45ROLow TCM cells are increased in involved lymph nodes, whereas the percentage of CD4+ TEM cells is increased in tumor free lymph nodes, implying a protective role in preventing tumor metastasis to lymph nodes and in preventing tumor progression. A similar result as for TEM cells was obtained with more differentiated CD4+ TCM forms, which can be distinguished based on a higher level of CD95 expression. Collectively, our observations may indicate that, due to suppressive conditions resulting from the presence of tumor cells within lymph nodes [29], TCM cell differentiation may be blocked in primary stages at which CD45RO is expressed at a low level. As a result, we hypothesize that the formation of an appropriate anti-tumor immune response may fail, but this hypothesis should be tested using additional functional studies and/or larger patient sample sizes. In addition, the investigation of tumor infiltrating lymphocytes in respect to significance of CD4+ TSCM merits further investigation.
References
K. Tokoyoda, A.E. Hauser, T. Nakayama, A. Radbruch, Organization of immunological memory by bone marrow stroma. Nat Rev Immunol 10, 193–200 (2010). https://doi.org/10.1038/nri2727
N.S. Butler, J.T. Harty, The role of inflammation in the generation and maintenance of memory T cells. Adv Exp Med Biol 684, 42–56 (2010)
M.K. MacLeod, J.W. Kappler, P. Marrack, Memory CD4 T cells: Generation, reactivation and re-assignment. Immunology 130, 10–15 (2010). https://doi.org/10.1111/j.1365-2567.2010.03260.x
N.P. Weng, Y. Araki, K. Subedi, The molecular basis of the memory T cell response: Differential gene expression and its epigenetic regulation. Nat Rev Immunol 12, 306–315 (2012). https://doi.org/10.1038/nri3173
T. Sathaliyawala, M. Kubota, N. Yudanin, D. Turner, P. Camp, J.J. Thome, K.L. Bickham, H. Lerner, M. Goldstein, M. Sykes, T. Kato, D.L. Farber, Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38, 187–197 (2013). https://doi.org/10.1016/j.immuni.2012.09.020
M. Takeshita, K. Suzuki, Y. Kassai, M. Takiguchi, Y. Nakayama, Y. Otomo, R. Morita, T. Miyazaki, A. Yoshimura, T. Takeuchi, Polarization diversity of human CD4+ stem cell memory T cells. Clin Immunol 159, 107–117 (2015). https://doi.org/10.1016/j.clim.2015.04.010
F. Sallusto, A. Langenkamp, J. Geginat, A. Lanzavecchia, Functional subsets of memory T cells identified by CCR7 expression. Curr Top Microbiol Immunol 251, 167–171 (2000). https://doi.org/10.1007/978-3-642-57276-0_21
S.C. Jameson, D. Masopust, Diversity in T cell memory: An embarrassment of riches. Immunity 31, 859–871 (2009). https://doi.org/10.1016/j.immuni.2009.11.007
L. Gattinoni, E. Lugli, Y. Ji, Z. Pos, C.M. Paulos, M.F. Quigley, J.R. Almeida, E. Gostick, Z. Yu, C. Carpenito, E. Wang, D.C. Douek, D.A. Price, C.H. June, F.M. Marincola, M. Roederer, N.P. Restifo, A human memory T cell subset with stem cell-like properties. Nat Med 17, 1290–1297 (2011). https://doi.org/10.1038/nm.2446
J.K. Flynn, P.R. Gorry, Stem memory T cells (TSCM)-their role in cancer and HIV immunotherapies. Clin Transl Immunol 3 (2014). https://doi.org/10.1038/cti.2014.16
L. Gattinoni, Memory T cells officially join the stem cell club. Immunity 41, 7–9 (2014). https://doi.org/10.1016/j.immuni.2014.07.003
C.J. Luckey, C.T. Weaver, Stem-cell-like qualities of immune memory; CD4+ T cells join the party. Cell Stem Cell 10, 107–108 (2012). https://doi.org/10.1016/j.stem.2012.01.011
Y.D. Mahnke, T.M. Brodie, F. Sallusto, M. Roederer, E. Lugli, The who's who of T-cell differentiation: Human memory T-cell subsets. Eur J Immunol 43, 2797–2809 (2013). https://doi.org/10.1002/eji.201343751
S.M. Kaech, Celebrating diversity in memory T cells. J Immunol 192, 837–839 (2014). https://doi.org/10.4049/jimmunol.1303268
L. Gattinoni, X.S. Zhong, D.C. Palmer, Y. Ji, C.S. Hinrichs, Z. Yu, C. Wrzesinski, A. Boni, L. Cassard, L.M. Garvin, C.M. Paulos, P. Muranski, N.P. Restifo, Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med 15, 808–813 (2009). https://doi.org/10.1038/nm.1982
A. Scherpereel, B.D. Grigoriu, M. Noppen, T. Gey, B. Chahine, S. Baldacci, J. Trauet, M.C. Copin, J.P. Dessaint, H. Porte, M. Labalette, Defect in recruiting effector memory CD8+ T-cells in malignant pleural effusions compared to normal pleural fluid. BMC Cancer 13, 1471–2407 (2013). https://doi.org/10.1186/1471-2407-13-324
Y. Nagai, M. Kawahara, M. Hishizawa, Y. Shimazu, N. Sugino, S. Fujii, N. Kadowaki, A. Takaori-Kondo, T memory stem cells are the hierarchical apex of adult T-cell leukemia. Blood 125, 3527–3535 (2015). https://doi.org/10.1182/blood-2014-10-607465
K. Hotta, M. Sho, K. Fujimoto, K. Shimada, I. Yamato, S. Anai, N. Konishi, Y. Hirao, K. Nonomura, Y. Nakajima, Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer 105, 1191–1196 (2011). https://doi.org/10.1038/bjc.2011.368
A. Oberg, S. Samii, R. Stenling, G. Lindmark, Different occurrence of CD8+, CD45R0+, and CD68+ immune cells in regional lymph node metastases from colorectal cancer as potential prognostic predictors. Int J Color Dis 17, 25–29 (2002). https://doi.org/10.1007/s003840100337
K. Enomoto, M. Sho, K. Wakatsuki, T. Takayama, S. Matsumoto, S. Nakamura, T. Akahori, T. Tanaka, K. Migita, M. Ito, Y. Nakajima, Prognostic importance of tumour-infiltrating memory T cells in oesophageal squamous cell carcinoma. Clin Exp Immunol 168, 186–191 (2012). https://doi.org/10.1111/j.1365-2249.2012.04565.x
R. Yajima, T. Yajima, T. Fujii, Y. Yanagita, T. Fujisawa, T. Miyamoto, S. Tsutsumi, M. Iijima, H. Kuwano, Tumor-infiltrating CD45RO(+) memory cells are associated with a favorable prognosis breast cancer. Breast Cancer 23, 668–674 (2016). https://doi.org/10.1007/s12282-015-0622-y
M. Pincha, P. Boonsawat, C. Domschke, P. Beckhove, Characterizing tumor-specific memory stem like T cells in blood and bone marrow of breast cancer patients. Cancer Res 74, 4076 (2014). https://doi.org/10.1158/1538-7445.AM2014-4076
V. Schirrmacher, M. Feuerer, P. Beckhove, T. Ahlert, V. Umansky, T cell memory, anergy and immunotherapy in breast cancer. J Mammary Gland Biol Neoplasia 7, 201–208 (2002). https://doi.org/10.1023/A:1020308104613
M. Feuerer, M. Rocha, L. Bai, V. Umansky, E.F. Solomayer, G. Bastert, I.J. Diel, V. Schirrmacher, Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer 92, 96–105 (2001). https://doi.org/10.1002/1097-0215(200102)9999:9999<::AID-IJC1152>3.0.CO;2-Q
P. Correale, P. Tagliaferri, A. Fioravanti, M.T. Del Vecchio, C. Remondo, F. Montagnani, M.S. Rotundo, C. Ginanneschi, I. Martellucci, E. Francini, M.G. Cusi, P. Tassone, G. Francini, Immunity feedback and clinical outcome in colon cancer patients undergoing chemoimmunotherapy with gemcitabine + FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukin (GOLFIG-1 trial). Clin Cancer Res 14, 4192–4199 (2008). https://doi.org/10.1158/1078-0432.ccr-07-5278
F. Pages, A. Berger, M. Camus, F. Sanchez-Cabo, A. Costes, R. Molidor, B. Mlecnik, A. Kirilovsky, M. Nilsson, D. Damotte, T. Meatchi, P. Bruneval, P.H. Cugnenc, Z. Trajanoski, W.H. Fridman, J. Galon, Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353, 2654–2666 (2005). https://doi.org/10.1056/NEJMoa051424
M.T. Hueman, A. Stojadinovic, C.E. Storrer, Z.A. Dehqanzada, J.M. Gurney, C.D. Shriver, S. Ponniah, G.E. Peoples, Analysis of naive and memory CD4 and CD8 T cell populations in breast cancer patients receiving a HER2/neu peptide (E75) and GM-CSF vaccine. Cancer Immunol Immunother 56, 135–146 (2007). https://doi.org/10.1007/s00262-006-0188-9
Y. Xie, B.-W. Luo, X.-D. Yuan, P.-K. Tian, X. Ou, Z.-W. Lin, X.-P. Liu, J.-K. Liu, Expression characteristics of surface markers of memory T cells, CD45RO, CCR7 and CD62L, in tumor-infiltrating lymphocytes in liver cancer tissues of patients with hepatocellular carcinomas. J Clin Cell Immunol 4, 181 (2013). https://doi.org/10.4172/2155-9899.1000181
Z. Faghih, N. Erfani, M.R. Haghshenas, A. Safaei, A.R. Talei, A. Ghaderi, Immune profiles of CD4+ lymphocyte subsets in breast cancer tumor draining lymph nodes. Immunol Lett 158, 57–65 (2014). https://doi.org/10.1016/j.imlet.2013.11.021
Funding
This study was financially supported by grants from the Shiraz University of Medical Sciences, Shiraz, Iran [Grant No. 94–7484] and Shiraz Institute for Cancer Research [ICR-100-500], and was part of an MSc thesis written by Yasmin Vahidi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures used involving human participants were in accordance with the ethical standards of the Ethical Committee of Shiraz University of Medical Sciences and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Electronic supplementary material
Supplementary Fig. 1.
Frequency of TEM in TDLNs of breast cancer patients with positive and negative progesterone receptor (PR) tumors. The mean expression of CD95 on CD95HiTEM cells in patients with progesterone receptor expressing tumors (PR+) in comparison to PR negative ones. The data are presented as mean ± SEM. *Difference significant at 0.05 level (2-tailed). (DOCX 64.6 kb)
Rights and permissions
About this article
Cite this article
Vahidi, Y., Faghih, Z., Talei, AR. et al. Memory CD4+ T cell subsets in tumor draining lymph nodes of breast cancer patients: A focus on T stem cell memory cells. Cell Oncol. 41, 1–11 (2018). https://doi.org/10.1007/s13402-017-0352-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-017-0352-6