Abstract
Objectives
Indoleamine-2,3-Dioxygenase (IDO) is an immunosuppressive molecule inducible in various cells. In addition to classic IDO (IDO1), a new variant, IDO2, has recently been described. When expressed in dendritic cells (DCs) or cancer cells, IDO was thought to suppress the immune response to tumors. A novel therapeutic approach in cancer envisages inhibition of IDO with 1-methyl-tryptophan (1MT). The levo-isoform (l-1MT) blocks IDO1, whereas dextro-1MT (d-1MT), which is used in clinical trials, inhibits IDO2. Here we analyze IDO2 expression in human cancer cells and the impact of both 1-MT isoforms on IDO activity.
Methods
Surgically extirpated human primary tumors as well as human cancer cell lines were tested for IDO1 and IDO2 expression by RT-PCR. IDO1 activity of Hela cells was blocked by transfection with IDO1-specific siRNA and analysed for tryptophan degradation by RP-HPLC. The impact of d-1MT and l-1MT on IDO activity of Hela cells and protein isolates of human colon cancer were studied.
Results
Human primary gastric, colon and renal cell carcinomas constitutively expressed both, IDO1 and IDO2 mRNA, whereas cancer cells lines had to be induced to by Interferon-gamma (IFN-γ). Treatment of Hela cells with IDO1-specific siRNA resulted in complete abrogation of tryptophan degradation. Only l-1MT, and not d-1MT, was able to block IDO activity in IFN-γ-treated Hela cells as well as in protein isolates of primary human colon cancer.
Conclusions
Although IDO2 is expressed in human tumors, tryptophan degradation is entirely provided by IDO1. Importantly, d-1MT does not inhibit the IDO activity of malignant cells. If ongoing clinical studies show a therapeutic effect of d-1MT, this cannot be attributed to inhibition of IDO in tumor cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
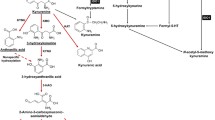
Tumors have developed various strategies to escape immune attack [15]. Recently, indoleamine 2,3-dioxygenase (IDO)—a molecule capable of preventing T cell-driven rejection of allogeneic fetuses during pregnancy [9]—has attracted the attention of scientists. If IDO plays a role in tolerance induction to tumors, it might constitute a novel candidate for targeted anti-cancer therapy.
Two observations led to this assumption: First, dendritic cells (DCs), which act as major antigen presenting cells in the induction of tumor-specific immune responses, are able to express IDO [12]. Second, Fuchs et al. as well as others showed that various malignant cells express this immunoregulatory protein [2, 13, 14]. Consequently, two mutually non-exclusive models emerged, in an effort to explain the involvement of IDO in tumor-directed immunosuppression [7]. One hypothesizes that IDO-expressing DCs, which are located in tumor-draining lymph nodes, suppress or anergize tumor-reactive T cells responding to antigens presented by these DCs. The other model claims that tumor cells expressing IDO inhibit the immune response. Pioneering work in this field has been performed by van den Eynde et al. [13]. In a series of elegant experiments, they demonstrated that mouse tumor cells transfected with IDO became resistant to immunologic rejection, even in recipients that had been preimmunized against the tumor. The participation of IDO in tumor development was given further support by the finding that the expression of this potentially suppressive molecule by cancer cells correlated with poor clinical prognosis in ovarian carcinoma [10], endometrial carcinoma [4] and colon carcinoma [2]. On the basis of all these findings, it was only a small step to the proposal of a therapeutic approach aimed at inducing tumor rejection by abrogation of the IDO activity. Van den Eynde′s group [13] showed that preimmunized mice bearing IDO-expressing tumors presented a significant reduction of tumor size and an increased number of tumor-reactive T cells when treated with levo-1-methyl tryptophan (l-1MT). An even stronger effect was obtained in mouse tumor models with the dextro isomer of 1-methyl tryptophan (d-1MT), especially if combined with chemotherapy [3]. On the basis of these findings, d-1MT is now being used in a clinical phase I trial. However, in contrast to l-1MT, d-1MT exhibited only little biochemical activity as an IDO inhibitor in certain in vitro test systems [3]. This raised the question of how it affects tumor development if it does not inhibit IDO activity (or only poorly). The dilemma was seemingly resolved by the discovery of a novel gene called IDO2 [1] as selective target for d-1MT [6]. It was suggested that a therapeutic effect of d-1MT in humans could be expected from its ability to block IDO2 at the two potential sites of tolerance induction, namely, the tumor itself or the DCs residing in tumor-draining lymph nodes [8]. We have recently shown [5] that IDO2 is expressed by human DCs, but it is inactive and consequently not affected by d-1MT. The current series of experiments addresses the questions whether tumor cells express IDO2 and if so, whether the IDO2 activity is abrogated by d-1MT.
Methods
Human primary tumors and tumor cell lines
Preparation and analysis of human primary cancerous material was approved by the ethical board of the University of Tübingen. Surgically removed tumors were frozen, dissected and verified by microscopy. Tumor cell lines were cultured in IMDM (Gibco, Germany) containing 2 mM l-glutamine (Gibco), 10% FCS (Lonza, Germany) and 50 μg/ml gentamicin (Gibco).
siRNA transfection of tumor cell lines
Hela cells were transfected with reagent alone (Mock), 100 nM control (CTR siRNA) or IDO1-specific siRNA (IDO1 siRNA) using Dharmafect1 reagent (Dharmacon, Germany) according to the manufacturer’s instructions. After 4 h, cells were stimulated with 200 U/ml IFN-γ for 48 h.
Qualitative and quantitative IDO expression, IDO activity assay
Results and discussion
First, we analyzed whether IDO2 is expressed in human malignant cells. To this end, tumor samples were collected during surgery, micro-dissected and microscopically verified. Figure 1a shows that gastric, colon and renal carcinomas all express various amounts of IDO2 and IDO1 mRNA. In a parallel experiment (Fig. 1b), pancreas- (Capan-1), colon- (HCT116), cervix- (Hela), hepatocellular- (HepG2), and renal carcinoma (RCC68) cell lines were studied. Although none of them constitutively expressed IDO2 or IDO1, expression of both genes could be induced in carcinoma cells by treatment with IFN-γ, with the exception of HepG2 cells (Fig. 1b).
Expression of IDO1 and IDO2 in human primary tumors and tumor cell lines. a Micro-dissected human gastric-, colon-, and renal cell carcinomas were analyzed for IDO1 and IDO2 expression by RT-PCR. Water instead of cDNA was used as negative (NC), IFN-γ-treated DCs [5] as positive control (PC). PCR products (β-Actin: 407 bp, IDO1: 321 bp, IDO2: 371 bp) were separated on agarose gel. b Human pancreas- (Capan-1), colon- (HCT116), cervix- (Hela), hepatocellular- (HepG2), and renal carcinoma (RCC68) cell lines either treated with IFN-γ or untreated were analyzed as described earlier. c Hela cells were treated either with transfection reagent instead of siRNA (Mock), 100 nM control siRNA (CTR siRNA), or siRNA specific for IDO1 (IDO1 siRNA) and stimulated with IFN-γ. IDO1 and IDO2 transcription was detected as described earlier. d The relative expression level of IDO1 after siRNA treatment was analyzed in Hela cells by qRT-PCR. IDO1 expression levels of transfected cells were correlated with those of untransfected cells (mean value ± SD)
Although primary tumor samples were carefully inspected for non-malignant cell infiltrations, one can never exclude the possibility that undetected, contaminating inflammatory cell infiltrates might contribute to the IDO activity. Therefore, established tumor cell lines were used for subsequent functional studies. It is well known that IDO expression does not automatically imply the functional activity [12]. Consequently, in spite of its expression, IDO2 might not unfold the enzymatic activity. To elucidate the extent to which IDO1 and IDO2 contribute to tryptophan degradation, IDO1 expression was blocked by treating the tumor cells with IDO1-specific siRNA. As expected, IDO1 transcription became undetectable, as measured by qualitative (Fig. 1c, lane 3) and quantitative RT-PCR (Fig. 1d, column 4) whereas IDO2 transcription remained unaffected (Fig. 1c, lane 3). If the IDO activity is contributed exclusively by IDO1, one would expect complete abrogation of kynurenine production following IDO1-specific siRNA transfection. Figure 2a shows that this was indeed the case.
IDO activity of Hela cells treated with IDO1-siRNA and of Hela cells or human primary tumors treated with 1-MT stereoisomers. a Tryptophan degradation and kynurenine accumulation of siRNA-transfected cells was measured in cell culture supernatants (n = 5). b Hela cells were stimulated with IFN-γ to induce IDO. d-1MT and l-1MT (Sigma) were tested at 200 µM and 1 mM for their potential to block the enzymatic activity (n = 5, mean value ± SD). c Human primary colon carcinoma (CC) incubated with either 1 mM d-1MT or l-1MT was tested for IDO activity as described earlier [11]. IFN-γ treated peripheral blood mononuclear cells (PBMCs) served as positive control
It was previously reported that d-1MT blocks the IDO2 activity and l-1MT blocks the IDO1 activity [6]. Because IDO2 was not expressed in a functionally active form in tumor cells, we expected that d-1MT would not block their IDO function. Figure 2b shows that d-1MT is completely inactive, whereas l-1MT effectively inhibits the IDO activity of tumor cells. In situ, tumor tissues may harbor, in addition to malignant cells, leukocyte infiltrates as well as normal organ-specific cells. All these may contribute to the IDO activity. To simulate the in vivo constellation as closely as possible, we prepared whole protein extracts of surgically extirpated colon carcinoma tumor. Figure 2c shows that the extracts generated the IDO activity, but the activity was inhibited only by l-1MT whereas d-1MT was completely inactive. The findings obtained with colon carcinoma samples are of special interest since previous clinical studies revealed a correlation between the IDO activity of tumor specimens, T cell infiltration, and liver metastases, suggesting that IDO plays a role in this type of tumor [2]. On the basis of our observations, one would not expect a therapeutic effect of d-1MT by IDO inhibition.
Interestingly, our study shows that primary tumor samples directly expressed IDO, whereas IDO expression in cell lines had to be induced. Our data indicate that the IDO activity in the analyzed tumor specimens either reflects constitutive IDO protein expression of malignant cells or, more probably, induction as a consequence of the inflammatory tumoral microenvironment. Whatever the reason for the IDO activity might be, the IDO2-blocker d-1MT has no influence on it because its proposed target IDO2 is expressed in a functionally inactive variant. Of course, we are aware of the fact that the IDO2 primers used are located at the 3′ end of the gene and thus might also detect incomplete transcripts not coding for the entire IDO2 protein. But first, this region was found to be common to all human IDO2 cDNAs [6] and second, additional activity studies on IDO2 after transfection of Hela cells with siRNA specific for IDO1, did not reveal tryptophan degradation by IDO2. This demonstrates that IDO2, for whatever reason, is not expressed as a functional protein in human cancer cell lines. The high frequency of polymorphisms of the IDO2 gene in Caucasians [6] leading to drastically reduced enzymatic activity, additionally increases doubts about the clinical use of d-1MT. If d-1MT indeed reduces tumor growth in clinical studies, since it does not inhibit the IDO activity of human tumor cells or DCs (Fig. 3a, b), one has to consider alternative immunological or non-immunological mechanisms for its action.
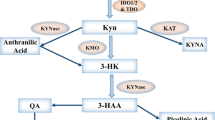
Synoptic view of the IDO activity in tumor cells and DCs: impact of l-1MT and d-1MT. a Tumor cells either constitutively express IDO1 or IDO2 mRNA or are induced by inflammatory cytokines. Only IDO1 is expressed as a functional protein, IDO2 being inactive. This process can be reversed by l-1MT but not by d-1MT. b DCs residing in the tumor-draining lymph nodes can express IDO1 and IDO2 mRNA, but only IDO1 contributes as an active enzyme to generation of a tryptophan-depleted and kynurenine-enriched environment. IDO activity of DCs is blocked only by l-1MT
The present results led us to conclude that although tumor cells express IDO2, it is produced in a functionally inactive form. Most importantly, d-1MT does not block the IDO activity of tumor cells (Fig. 3a). Whatever the therapeutic results of ongoing clinical studies with d-1MT in cancer patients will be, they can be attributed to inhibition of the IDO activity in neither DCs [5] nor in tumor cells. Consequently, we propose the use of the active l-isoform of 1-MT as IDO-blocker for further clinical studies.
References
Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH (2007) Characterization of an indoleamine 2, 3-dioxygenase-like protein found in humans and mice. Gene 396(1):203–213
Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Gobel G, Margreiter R, Konigsrainer A, Fuchs D, Amberger A (2006) Prognostic value of indoleamine 2, 3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res 12(4):1144–1151
Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH (2007) Inhibition of indoleamine 2, 3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res 67(2):792–801
Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S, Nagasaka T, Takikawa O, Kikkawa F (2006) Indoleamine 2, 3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer 95(11):1555–1561
Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P (2008) Levo- but not dextro–1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 111(4):2152–2154
Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC (2007) Prendergast, Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2, 3-dioxygenase inhibitory compound d-1-methyl-tryptophan. Cancer Res 67(15):7082–7087
Munn DH, Mellor AL (2007) Indoleamine 2, 3-dioxygenase and tumor-induced tolerance. J Clin Invest 117(5):1147–1154
Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL (2004) Expression of indoleamine 2, 3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest 114(2):280–290
Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL (1998) Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281(5380):1191–1193
Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, Ishii N, Yanaihara N, Yamada K, Takikawa O, Kawaguchi R, Isonishi S, Tanaka T, Urashima M (2005) Indoleamine 2, 3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res 11(16):6030–6039
Takikawa O, Kuroiwa T, Yamazaki F, Kido R (1988) Mechanism of interferon-gamma action. Characterization of indoleamine 2, 3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem 263(4):2041–2048
Terness P, Chuang JJ, Opelz G (2006) The immunoregulatory role of IDO-producing human dendritic cells revisited. Trends Immunol 27(2):68–73
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nat Med 9(10):1269–1274
Widner B, Fuchs D (2000) Immune activation and degradation of tryptophan. Mod Asp Immunobiol 1:105–108
Zou W (2005) Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 5(4):263–274
Acknowledgments
S.L. was supported by a Fortüne grant of the University of Tübingen (1636-0-0). The authors thank Lynne Yakes for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Löb, S., Königsrainer, A., Zieker, D. et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother 58, 153–157 (2009). https://doi.org/10.1007/s00262-008-0513-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0513-6