Abstract
Indoleamine 2, 3-dioxygenase 1 (IDO1) is a rate-limiting metabolic enzyme that converts the essential amino acid tryptophan (Trp) into downstream catabolites known as kynurenines. Coincidently, numerous studies have demonstrated that IDO1 is highly expressed in multiple types of human cancer. Preclinical studies have further introduced an interesting paradox: while single-agent treatment with IDO1 enzyme inhibitor has a negligible effect on decreasing the established cancer burden, approaches combining select therapies with IDO1 blockade tend to yield a synergistic benefit against tumor growth and/or animal subject survival. Given the high expression of IDO1 among multiple cancer types along with the lack of monotherapeutic efficacy, these data suggest that there is a more complex mechanism of action than previously appreciated. Similar to the dual faces of the astrological Gemini, we highlight the multiple roles of IDO1 and review its canonical association with IDO1-dependent tryptophan metabolism, as well as documented evidence confirming the dispensability of enzyme activity for its immunosuppressive effects. The gene transcript levels for IDO1 highlight its strong association with T-cell infiltration, but the lack of a universal prognostic significance among all cancer subtypes. Finally, ongoing clinical trials are discussed with consideration of IDO1-targeting strategies that enhance the efficacy of immunotherapy for cancer patients.
Similar content being viewed by others
Introduction
Within the last decade, there has been incredible success regarding application of immune checkpoint inhibitors, with an emphasis on targeting CTLA-4 and/or PD-(L)1, to improve patient survival for otherwise untreatable melanoma,1 non-small-cell lung2 and renal3 cancers. This progress has motivated medical oncologists and/or tumor immunologists to better understand the regulation, role and functions of co-inhibitory pathways that are expressed by immune and cancer cells.4 Preclinical and clinical studies have demonstrated a trend of immunotherapeutic combination strategies to confer a greater survival benefit over single-agent approaches.5,6 There are, however, notable exceptions to the general belief that more is better, and several recent studies have highlighted the fact that multi-therapy approaches do not universally provide an advantage to the host and/or immune system.7,8 These considerations reflect the complicated regulatory network that governs the human immune system’s response to cancer, its failure to have a ‘one-size-fits-all’ approach, the toxicities induced by certain immunotherapeutic combinations and the critical need to further understand why checkpoint therapies (i) provide benefits to some patients, but not others; (ii) enhance tumoricidal effects against some cancers, but not others; and (iii) beneficially stimulate immune responses with certain combinations, but not others.
In this review, we focus on the novel immune checkpoint target, indoleamine 2, 3-dioxygenase 1 (IDO1), which is characterized as a rate-limiting metabolic enzyme that converts tryptophan (Trp), into downstream kynurenines (Kyn) (Figure 1). IDO1 is interferon-inducible and has been associated with mediating potently immunosuppressive effects in cancer.9,10 While a growing body of data suggest that there is a lack of therapeutic efficacy when targeting IDO1 alone, there is strong support for combination approaches to provide a synergistic benefit.11,12,13,14 Due to the limited effect(s) of single agents, a growing number of active clinical trials utilize IDO1 as an adjuvant alongside other cancer treatment modalities (Vacchelli et al. 15 and Table 1), which raises the question of whether immunological therapies somehow ‘activate’ IDO1 so that it becomes therapeutically targetable. These developments highlight additional questions that have yet to be answered, including the following: (i) Is enzyme metabolism the sole characteristic that endows IDO1 with immunosuppressive activity? (ii) Why is IDO1 enzyme inhibition not effective as a monotherapy16 given that IDO1 is highly expressed in a variety of human cancers?10,17 (iii) Why does coupling radiotherapy and/or chemotherapy tend to synergize with IDO1 inhibition?18 (iv) How do IDO1 pathway inhibitors that have no effect on converting Trp into Kyn,19 such as indoximod (dextrorotatory 1-methyl-Trp; D-1-MT), affect IDO1-mediated immune suppression?20 (v) Do select IDO1 inhibitors confer gain-of-function toxicity under certain therapeutic contexts? (vi) Are there immunosuppressive effects of IDO1 in cancer cells that are independent of enzyme activity and similar to effects that have been reported in innate immune cells?21
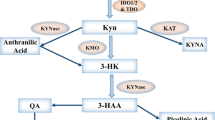
Tryptophan (Trp) catabolic pathways. In addition to being used as a building block for protein synthesis, the majority of dietary Trp (95%) is catabolized via the Trp→Kyn pathway (red arrows). Other minor pathways include conversion to tryptamine or melatonin. Within the Kyn pathway, the underlined metabolites can cross the blood brain barrier (BBB). IDO1 (and TDO) are highlighted in black boxes. ACMSD: 2-amino-3-carboxymuconate semialdehyde carboxylase; 3-HAO, 3-hydroxyanthranilate 3, 4-dioxygenase; IDO1, indoleamine 2, 3-dioxygenase 1; KAT, kynurenine aminotransferase (I, II, III); KMO, kynurenine 3-monooxygenase; KYNU, kynureninase; MAO, monoamine oxidase; QPRT, quinolinic-acid phosphoribosyl transferase; TDO, tryptophan 2,3-dioxygenase.
Trp dioxygenases
L-Trp, which is the least abundant essential amino acid, can be metabolized via four distinct mechanisms: decarboxylation to tryptamine; protein synthesis; the serotonergic pathway; and the Kyn pathway (Figure 1).22 Kyn pathway metabolism accounts for ~95% of all mammalian dietary Trp.23 The first rate-limiting step required for conversion of Trp into Kyn is oxidative cleavage of a 2,3-indole ring double bond that forms N-formylkynurenine, which is almost immediately converted to L-Kyn. Three different Trp dioxygenases have been identified in mammals, including IDO1, tryptophan 2,3-dioxygenase (TDO) and IDO2. Human IDO1 is a monomeric heme-containing protein encoded by chromosome 8p12 and has high enzyme activity for Trp (Km~20 μM).24 TDO is located on chromosome 4 and forms a tetrameric heme-containing complex with lower enzyme activity for Trp (Km~190 μM)25 compared to IDO1. IDO2 is a genetic paralog of IDO1 and is directly adjacent to IDO1 on the same chromosome.26 Although it can allegedly convert Trp into Kyn, it has an almost 1000-fold lower enzyme activity (Km~6.8 mM).27
In addition to the differences in structure and rates of enzyme conversion, the three Trp catabolic enzymes have varying substrate specificities, tissue distribution and regulation of expression. While TDO is only capable of metabolizing the L-Trp isomer,25 IDO1 mediates oxidative cleavage of several indole substrates, including D- and L-Trp, tryptamine, 5-hydroxy-L-tryptophan, serotonin and melatonin (Figure 1).28 Compared to IDO1 and TDO, the exceptionally low enzyme activity of IDO2 raises the question of whether L-Trp is a relevant physiological substrate.24 Under normal conditions, TDO is primarily expressed in the liver, placenta and brain, which likely reflects its professional role of satisfying the energetic needs required by the body. In contrast, IDO1 is detected throughout mammalian tissues at various levels, including the central nervous system, epididymis, intestine, thymus, respiratory tract, spleen, pancreas, placenta, lens, kidney, myeloid cells and endothelial cells,17 and has been shown to be increased in select tissues with age.29 Interestingly, IDO1 is noticeably absent from the liver. Constitutive expression of murine IDO2 mRNA and protein is detected in the liver, epididymis and brain, but little information has been reported for human IDO2 due to the lack of antibody specificity and complexity of human IDO2 transcription.30 In addition to their expression in normal tissues, IDO1, TDO and IDO2 are selectively expressed in different types of human and mouse cancers.31 Notably, although IDO1 and TDO are expressed at a high level in many cancer subtypes, a recent gene expression profiling study evaluating two large RNA-sequencing data sets among 31 cancer subtypes revealed negligible expression of IDO2 in the majority of human cancers (>99%), which possibly indicates that IDO2 has a less important role in supporting tumorigenesis due to is reduced expression and/or activity.30
Previous mechanistic studies demonstrated that expression of TDO is regulated by glucocorticoid hormones and dietary Trp levels,32,33 whereas IDO1 is regulated and expressed in response to a variety of inflammatory stimuli, including interferon (IFN)-α,34 IFN-γ,35 lipopolysaccharide (LPS),36,37 interleukin-1 (IL-1), tumor necrosis factor,38 CpG oligodeoxynucleotides (CpG-ODN)39 and prostaglandin-E2 (PGE2).40 IDO2 is also increased by treating cells with IFN-γ, IL-10, LPS and PGE2, although its expression is less robust than that of IDO1.41 Coincidently, activation of the aryl hydrocarbon receptor (AhR), which is a transcription factor that Kyn has been proposed to serve as a physiologically relevant ligand for,42 has been shown to be upregulated through an IDO2-dependent pathway in dendritic cells (DCs).43,44
The involvement of multiple Trp-catabolizing enzymes contributes to Kyn metabolite generation and/or accumulation, which raises a potential challenge for therapeutic strategies that target this metabolic pathway. A clear question for the field is determining whether inhibiting a single player is sufficient for enhancing immune-mediated antitumor effects or whether simultaneous inhibition of all three enzymes is required. A complete answer to this question will likely depend on the following: (i) the type of cancer under investigation; (ii) expression levels and metabolic activity of IDO1, TDO and IDO2; (iii) intratumoral and serological levels of Trp and Kyn; and (iv) cellular origin of the expressed functional gene products. To date, an IDO1–TDO dual inhibitor has been discovered, although the in vivo relevance of this agent in antitumor therapy has yet to be revealed.45 Furthermore, while addressing whether inhibition of all three Trp dioxygenases is interesting, it is important to note that the more limited anatomical expression of TDO as well as the full role of IDO2 may detract from the undeniably immunosuppressive effects of IDO1, which is a clear therapeutic priority for achieving greater immune-mediated antitumor efficacy.
Kyn pathway and tumor immune escape
The relationship between cancer and elevated Trp catabolism was recognized as early as the 1950s.46 Since Trp is the least abundant amino acid and must be ingested through the diet, IDO1 was originally thought to be part of an ancient, innate mechanism that was designed to slow the growth of neoplastic tissues and/or infectious agents that require Trp stores for continued metabolic activity.27 In support of this hypothesis, Munn et al. demonstrated that female mice pregnant with allogeneic pups and treated with 1-methyl Trp (1-MT) resulted in maternal immune-mediated rejection.47 Later studies of experimental autoimmune encephalomyelitis suggested that Trp catabolites and their derivatives contribute to a shift in primarily Th1-mediated disease to a Th2-associated non-pathological condition.48 Collectively, these studies indicate an important role for IDO1 in general mechanisms that support immune tolerance. The role of IDO1 in immune-mediated evasion of cancer was first introduced in 2002 when Friberg et al. 49 showed that Lewis lung carcinoma (LLC) cells stimulated a more robust allogeneic T-cell response when cultured in the presence of an IDO1 inhibitor, which commensurately delayed LLC tumor growth after systemic treatment in vivo.
Currently, three major hypothetical mechanisms are proposed to explain the role of IDO1 in tumor-associated immunosuppression. First, enzyme activity results in local depletion of Trp, which results in an increase of uncharged transfer RNA in neighboring T cells and activation of the amino-acid-sensitive GCN2 and mTOR stress-kinase pathways. In turn, GCN2 signaling causes cell cycle arrest and induction of anergy in responding T cells. An additional hypothesis is that downstream Kyns, including L-Kyn, 3-hydroxy-L-Kyn, 3-hydroxyanthranilate (3HAA) and quinolinic acid, have an immune modulatory effect that acts by inducing effector T-cell arrest or apoptosis, both in vitro and in vivo. 50 Downstream Kyn accumulation may also contribute to the conversion of naive CD4+ T cells into immunosuppressive FOXP3-expressing regulatory T cells (Treg, CD3+CD4+CD25+FOXP3+) by virtue of the interaction between L-Kyn and Ahr.51 It is important to note, however, that this mechanism is unlikely to represent the majority of Treg in solid tumors, since the infiltrating component is primarily thymus-derived natural Treg (nTreg)52,53 combined with the absence of Ahr expression in nTreg.54 Collectively, these mechanisms may play a role in contributing to the suppression of tumor immunity via IDO1 expression in cancer.
The above hypotheses are supported by several lines of experimental evidence, with the majority of observations derived from an in vitro cell culture-based investigation. The limitation of cell culture analyses for studying the effects of Trp depletion and/or Kyn accumulation is that there is an obvious lack of physiological relevance unless the observations can be mirrored in vivo. This limitation has introduced a potential form of ‘in vitro bias’ that leaves researchers with a series of challenges and questions. For example, previous work has demonstrated that to inhibit T-cell proliferation in vitro, Trp concentrations are required to be below 0.5–1 μM.55 While this observation is interesting and scientifically well-supported, its physiological significance should be considered. Notably, plasma Trp levels range from 50 to 100 μM in humans, and local Trp reservoirs can be rapidly replenished by diffusion and/or active transport across the large amino-acid transporter from surrounding blood vessels. The Trp depletion theory incorporates the premise that non-T cells are more resistant to Trp starvation and is partially explained by the identification of a high-affinity Trp transporter that is selectively expressed on myeloid-derived macrophages, but not in T cells.56 It is not clear whether other types of IDO1-expressing cells, including tumor cells, use the same mechanism to survive Trp depletion. Further questions arise that need to be answered to determine how downstream Kyns suppresses T cells, with hypotheses suggesting that phosphoinositide-dependent protein kinase 1 (PDK1) acts as a direct target for 3HAA and 3HAA-mediated PDK1 inhibition and is responsible for the induction of type 2 T helper cell (TH2 cell) dysfunction and apoptosis.57
Novel aspects of ido1 in cancer immunity
The majority of experimental data supporting a non-enzymatic immunosuppressive role for IDO1 is derived from studies in mouse plasmacytoid DCs, which is a type of professional antigen-presenting cell. In these studies, 1-MT was used as an IDO1 enzyme inhibitor to demonstrate that IDO1-mediated immune suppression was independent of Trp catabolism.58,59,60 The mechanism of action involved two IDO1-intrinsic immunoreceptor tyrosine-based inhibitory motifs (ITIMs). Transforming growth factor-β (TGF-β) signaling caused phosphorylation of the ITIMs, which triggered noncanonical nuclear factor-κB (NF-κB) activation and phosphorylation of inhibitor for NF-κB subunit alpha and led to further autocrine reinforcement of IDO1 and TGF-β expression; ultimately, this signaling led to long-term tolerance of DCs. Although this unique IDO1 signaling pathway was not inhibited by 1-MT, it was abolished in cells lacking IDO1 expression.61
The relevance of non-enzyme IDO1 activity has yet to be addressed in a cancer setting. It is therefore unclear whether IDO1 possesses the same signaling circuitry in tumor cells as has been demonstrated in DCs. Coincidently, our recent work found that the intracranial engraftment of murine glioblastoma cells into syngeneic immunocompetent mice resulted in decreased tumor-infiltrating Treg (P<0.01) and increased animal subject survival (P<0.001) when the brain tumor cells were silenced for IDO1 expression with stably expressing small hairpin RNA.62 Notably, IDO1-silenced tumor cells kill animal subjects similar to IDO1-wild-type control cells when the cells are engrafted intracranially into mice deficient for either CD4+ and/or CD8+ T cells, which suggests that the mechanism regulating survival from tumor cells requires the suppression of IDO1 expression and is immune system-dependent. Interestingly, these outcomes were independent of the IDO1 effects on the Trp and Kyn levels mediated by tumor cells.63 Instead, the majority of IDO1 metabolism was mediated by non-tumor cells of the engrafted intracranial glioblastoma. Also, IDO1 metabolism did not decrease the endogenous Trp levels within the brain tumor compared to a naive mouse brain without tumor cells. Collectively, these data suggest that IDO1 in tumor cells and non-tumor cells possess different functions that are non-overlapping, which infers a potential difference in targetability with the current generation of IDO1 inhibitors primarily focused on enzyme activity.
One recent study revealed a novel role for IDO1 in which it affects tumor repopulating cell survival via induction of the tumor dormancy program.64 Unexpectedly, IFNγ treatment of differentiated tumor cells led to higher rates of apoptosis via STAT1-dependent signaling. By contrast, when IDO1 and Ahr were co-expressed in tumor cells with IFNγ treatment, STAT1 signaling was inhibited, which led to suppression of cell death and activation of the tumor cell dormancy program. Mechanistically, IFNγ induced high IDO1 and AhR expression as well as increased the Trp transporter levels in tumor-repopulating cells, which did not occur in differentiated cells. Mechanistically, IDO1/AhR pathway activation upregulated the cell cycle inhibitor p27, which diverted tumor-repopulating cells from the pro-apoptotic STAT1-dependent pathway toward the survival dormancy program. Therapeutically, L-1-MT-driven IDO1 enzyme inhibition diminished IFNγ-induced dormancy and suppressed tumor growth in vitro and in vivo. This latest finding further highlights the multi-faceted role of IDO1 in tumorigenesis and its complex mechanism of action with respect to cancer immunotherapy.
IDO1 and innate immune modulation in the tumor microenvironment
Over the last decade, interactions between IDO1 and a large range of immune modulators have been discovered. CpG-ODN induces expression of IDO1 through toll-like receptor 9 activation,39 with similar increases of expression by DNA nanoparticle-mediated activation of the stimulator of IFN genes (STING) adaptor pathway coupled with type I IFN (IFN-α/β) signaling.65 STING-mediated IDO1 induction in the setting of tumor immune evasion and tumor progression was demonstrated using a STING knockout mouse with engrafted LLC cells.66 Given the promising results of STING agonists in preclinical tumor immunotherapy67 and the potential commensurate induction of IDO1 via STING activation, it has become critical to evaluate STING-targeted cancer therapies for their potential synergistic potential with IDO1 inhibitors.
Another mechanism resulting in increased IDO1 expression is mediated through cell apoptosis and/or necrosis, which is a pathological hallmark in many cancers. The balance between immunogenic and tolerogenic cell death determines the outcome of the immune response within the tumor microenvironment.68 IDO1 appears to play a significant role in maintaining tolerance of apoptotic cells by the following: (i) altering the phenotype of macrophages and neighboring cells through upregulation of IL-10 and TGF-β as well as inhibition of IL-1; (ii) inducing phenotypic changes of local cross-presenting DCs; and (iii) recruiting Tregs.69,70,71 Furthermore, a subcutaneous injection of apoptotic tumor cells causes activation of IDO1, which induces suppressive phosphatase and tensin homolog (PTEN)-expressing Tregs72 and indicates that there is a potential role for IDO1 in apoptotic tumor cell-induced immune suppression.
Myeloid-derived suppressor cells (MDSCs) have been recognized as an important group of heterogeneous mediators in cancer that convey potent immunosuppressive effects on T cells.73 Recent studies have demonstrated that IDO1 is highly induced in tumor-infiltrating MDSCs and is responsible for MDSC-associated activation and/or recruitment of Tregs in human breast cancer, sarcoma and chronic lymphocytic leukemia.74,75,76 In addition to its role in immunotolerance, studies utilizing a mouse melanoma model also revealed that tumor-expressed IDO1 recruits and activates MDSCs through a Treg-dependent mechanism,77 which demonstrates the functional versatility of IDO1 in MDSC-associated immunoevasion.
IDO1 is also implicated in the inhibition of T-cell-dependent complement system activation, which was initially reported in an early study of allogeneic mouse fetal rejection.78 However, the linkage between IDO1 and the complement system in cancer was not discovered until recently. In a study of intracranial mouse glioblastoma, combination radiation and chemotherapy mediated extensive complement deposition when non-brain tumor cell IDO1 was targeted and/or inhibited through pharmacological and/or genetic methods, respectively. Importantly, complement deposition was mechanistically required for the pro-survival effect of an IDO1 pathway inhibitor.18 Given that we previously demonstrated that non-glioblastoma cells are the predominant mediators of IDO1-dependent Trp catabolism,8 the data collectively suggest that metabolically active IDO1 becomes targetable when other forms of cytotoxic therapy are used synergistically and highlights the IDO1-dependent non-tumor cell mechanisms that contribute to immunosuppression in solid tumors.
IDO1 and other key immune checkpoints
An increasing number of recognized immune checkpoints act to coordinately influence the local tumor-immune environment. To obtain the maximal therapeutic benefit with combination approaches that incorporate multiple forms of immunotherapy, a critical question is how IDO1 inhibition will interact with other key modulators of tumor-induced immune suppression (that is, CTLA-4 and/or PD-1 blockade)? Despite some preclinical cancer studies showing synergy when combining pharmacological IDO1 and CTLA-4/PD-1 blockade,13,14,79 the molecular mechanism of this relationship remains largely unknown. It has been reported that Treg cell-expressed CTLA-4 upregulates IDO1 expression by DCs,80 and there is reciprocal activation of Treg cells. In addition, IDO1 upregulates PD-1 expression on Tregs, which contributes to the maintenance of PTEN activity.72 One of our recent studies also demonstrated that treatment with a dual blockade of CTLA-4 and PD-L1 in glioblastoma-bearing mice resulted in increased tumor IDO1 mRNA expression commensurate with elevated transcripts of CD3ɛ, CD8α and IFNγ,63 possibly suggesting that tumor-infiltrating effector T cells (CD3+CD8+) activated by immune checkpoint inhibition increase IDO1 expression via IFNγ stimulation. Our recent analysis of the cancer genome atlas supports this hypothesis and found a correlation between increasing IDO1 levels with other immune checkpoints, including PD-1, PD-L1, PD-L2, CTLA-4, signal transducer and activator of transcription 3, CD39, B- and T-lymphocyte attenuator, lymphocyte-activation gene 3 and FoxP3 in surgically resected human glioblastoma.81 Taken together, the data suggest that as IDO1 expression increases in tumors, so do other immune checkpoints. It is therefore possible that combination strategies targeting multiple immune checkpoints may lead to greater synergistic effects in cancer immunotherapy, although the enhancement of host toxicity may also increase as well.
IDO1 in different cancer types: functional diversity?
Since the discovery of increased IDO1 levels in human cancer, there have been several reports correlating IDO1 expression with poor patient prognosis, including those diagnosed with acute myeloid leukemia,82 colorectal cancer,83 non-small-cell lung cancer,84,85 prostate cancer,86 ovarian carcinoma,87,88 endometrial cancer89 and esophageal cancer.90 Unexpectedly, high IDO expression levels in renal cell carcinoma and hepatocellular carcinoma patients are correlated with better survival outcomes.91,92,93 The complexity of outcomes associated with utilizing IDO1 expression as a stratifying factor for cancer patient prognosis likely reflects the complexity of IDO1 expression, regulation and functional effects within different types of human cancer. In support of this concept, our analysis of The Cancer Genome Atlas reveals that distinct IDO1 gene expression correlates with overall survival when comparing patients diagnosed with glioblastoma and melanoma.81 As shown in Figure 2, higher IDO1 transcript levels correlate with decreased glioblastoma patient survival, which is diametrically opposed to the correlation between increased IDO1 mRNA levels and its association with increased survival in melanoma patients. These results are somewhat surprising given the preclinical work suggesting that IDO1 inhibition synergizes with CTLA-4 blockade to mediate rejection of mouse melanoma.14 Further analysis demonstrates that IDO1 expression positively correlates with gene expression markers for CD8+ cytolytic T and Tregs, which are both found in glioblastoma and melanoma. Although there is a difference between these cancers regarding their correlation with IDO1 expression and survival, both diagnoses appear to have a strong correlation between the presence of intratumoral T cells and increased IDO1 expression. This led us to ask whether there is also a difference between T cell infiltration and survival outcomes. Whereas higher gene expression for cytolytic T cell markers was associated with decreased glioblastoma patient survival (Figure 2), the opposite trend was true for melanoma patients, which reflects the results of published studies.94,95
The Cancer Genome Atlas analysis reveals distinct correlations between patient survival, IDO1 transcript levels and markers for tumor-infiltrating lymphocytes between glioblastoma (GBM) and melanoma. Top panel: Kaplan–Meier analysis is based on the mRNA expression level of IDO1 in GBM (left column) and melanoma (right column). Expression of IDO1 is divided into low (blue) and high (red) groups as determined by the indicated cutoff value (calculated by Cutoff Finder, Supplementary Table and Methods). The sample size of each group is listed in the parenthesis. Middle panel: canonical correlation analysis (Supplementary Table and Methods) between IDO1 and tumor-infiltrating CD8+ T lymphocytes within GBM (left column) and melanoma (right column). Bottom panel: Kaplan–Meier analysis based on the mRNA expression level for the CD8+ T cell marker genes CD3E and CD8A in GBM (left column) and melanoma (right column). Expression of CD3E and CD8A are divided into low (blue) and high (red) groups, which were determined by the indicated cutoff value (calculated by Cutoff Finder, Supplementary Table and Methods). The patient sample size for each group is listed in parentheses. *P<0.05; **P<0.01; ****P<0.0001.
While these data are straightforward for patients diagnosed with glioblastoma, in regard to providing a rationale for including IDO1 adjuvant therapy in treatments that enhance T cell-mediated IDO1 expression increases, there are many questions about melanoma, including the following: (i) Does IDO1 play a negative role in the tumors of human patients with malignant skin cancer? (ii) Why does increased IDO1 expression correlate with increased patient survival? (iii) Will the threshold for immunotherapeutic intervention be different between patients diagnosed with glioblastoma and melanoma? (iv) What is the composition of IDO1 expression by tumor and stromal cells among different malignant subtypes? (v) If IDO1 possesses different functions among distinct cell types, do these differences contribute to the differences in outcomes between IDO1 expression and patient survival when glioblastoma and melanoma are compared?
IDO1 inhibitors in cancer immunotherapy
To date, IDO1 inhibitors have been designed, screened, and tested in preclinical models of disease (reviewed in Vacchelli et al. 15; Röhrig et al. 96). Currently, no IDO1-targeting agent is approved by the Food and Drug Administration as a standalone cancer therapeutic. However, the results of recent phase I–II studies suggest that the IDO1 pathway modulator indoximod (D-1-MT), the best-in-class IDO1 enzyme inhibitor INCB024360 (Epacadostat), and the IDO1 vaccine are well tolerated by cancer patients.97,98,99,100,101 With confirmation that targeting IDO1 is safe and well tolerated, the number of trials evaluating IDO1 inhibition in cancer therapy continue to grow (Table 1). Consistent with preclinical evaluation, the objective response rates for the non-enzyme-targeting IDO1 pathway inhibitor indoximod has yielded objective response rates of 10–18%.101,102 Combined with other immunotherapeutics, such as PD-1/PD-L1 or CTLA-4 inhibitors, this value ranges from 10 to 57% among different cancer types.103,104 One potential explanation for this wide range of variable response rates is based on the complexity of IDO1 functions among different cancer types, which suggests that the elucidation of IDO1 in different cancer subtypes is imperative for its efficacy as a therapeutic target.
Precision medicine initiatives that tailor targeted therapy against IDO1 may enhance the effectiveness of treatment, but this ideological concept still requires verification in clinical trials and across cancer diagnoses. This effect may also be a moving target since immunotherapies that enhance T-cell infiltration may also increase immunosuppressive molecule expression and activity. Furthermore, to effectively evaluate IDO1 inhibitors, target validation, pharmacodynamic properties on Trp and Kyn levels, as well as their impact on conformational activity and protein stability, are critical future requirements. While it is easy to measure the Trp and Kyn levels in vitro, quantification of IDO1 metabolism is more challenging in vivo. Recent developments for noninvasive in vivo metabolite imaging may provide a solution for this technical hurdle.105,106 It should be noted, however, that systemic Trp levels can be affected by TDO, which is expressed in the liver constitutively and induced in some types of cancer,42 suggesting that evaluation of this amino acid is not necessarily a sole reflection of IDO1 enzyme activity. Finally, understanding how IDO1 works in cancer cells, versus non-cancer cells, in terms of the enzyme and signal transduction properties, is essential for targeting the full effects of this pleiotropic mediator of immune suppression.
Concluding remarks
Substantial knowledge of IDO1 and its role in cancer has been generated over the past two decades. However, new questions continue to be raised regarding its full spectrum of function(s). Similar to the astrological description of Gemini, the Trp catabolic function of IDO1 appears to be one feature of a multifunctional player. Cell lines that express IDO1, but do not catabolize Trp, have become important tools for recognizing this phenomenon. Similarly, the IDO1 pathway inhibitor indoximod (D-1-MT), which does not convert Trp to Kyn,107,108 but is a potentially important and clinically meaningful treatment for cancer patients, has further highlighted the possibility that the non-enzyme activity of IDO1 is a relevant target in cancer immunotherapy. To address some of the questions raised earlier in this review, our group is currently constructing three novel transgenic mouse models that (i) possess a point mutation that nullifies IDO1 enzyme activity; (ii) have a 2-TA linker connecting the C terminus of endogenous IDO1, with an eGFP reporter; and (iii) contain a floxed STOP codon upstream of FLAG-tagged IDO, for future knock-in experiments under tissue-specific promoters. It is our hope that ongoing work by our team and others will answer some of the unanswered questions surrounding IDO1 in malignant cancers and, perhaps, other diseases as well.
References
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–133.
Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015; 16: 257–265.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1803–1813.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348: 56–61.
Smyth MJ, Ngiow SF, Ribas A, Teng MWL. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 2015; 13: 143–158 advance online publication.
Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 2016; 13: 273–290.
Shrimali RK, Shamim A, Verma V, Zeng P, Ananth S, Gaur P et al. Concurrent PD-1 blockade negates the effects of OX40 agonist antibody in combination immunotherapy through inducing T-cell apoptosis. Cancer Immunol Res 2017; 5: 755–766.
Zhai L, Ladomersky E, Dostal CR, Lauing KL, Swoap K, Billingham LK et al. Non-tumor cell IDO1 predominantly contributes to enzyme activity and response to CTLA-4/PD-L1 inhibition in mouse glioblastoma. Brain Behav Immun 2017; 62: 24–29.
Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol 2016; 37: 193–207.
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003; 9: 1269–1274.
Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res 2007; 67: 792–801.
Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan CL, Haley PJ et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther 2010; 9: 489–498.
Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim C, Tobias AL et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clini Cancer Res 2014; 20: 5290–5301.
Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 2013; 210: 1389–1402.
Vacchelli E, Aranda F, Eggermont A, Sautes-Fridman C, Tartour E, Kennedy EP et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 2014; 3: e957994.
Beatty GL, O'Dwyer PJ, Clark J, Shi JG, Bowman KJ, Scherle PA et al. First-in-human phase I study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin Cancer Res 2017; 23: 3269–3276.
Theate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res 2015; 3: 161–172.
Li M, Bolduc AR, Hoda MN, Gamble DN, Dolisca SB, Bolduc AK et al. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J Immunother Cancer 2014; 2: 21.
Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010; 115: 3520–3530.
Opitz CA, Litzenburger UM, Opitz U, Sahm F, Ochs K, Lutz C et al. The indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan upregulates IDO1 in human cancer cells. PLoS ONE 2011; 6: e19823.
Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 2011; 12: 870–878.
Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res 2009; 2: 45–60.
Beadle GW, Mitchell HK, Nyc JF. Kynurenine as an intermediate in the formation of nicotinic acid from tryptophane by neurospora. Proc Natl Acad Sci USA 1947; 33: 155–158.
Pantouris G, Serys M, Yuasa HJ, Ball HJ, Mowat CG. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids 2014; 46: 2155–2163.
Batabyal D, Yeh SR. Human tryptophan dioxygenase: a comparison to indoleamine 2,3-dioxygenase. J Am Chem Soc 2007; 129: 15690–15701.
Murray MF. The human indoleamine 2,3-dioxygenase gene and related human genes. Curr Drug Metab 2007; 8: 197–200.
Yuasa HJ, Ball HJ, Ho YF, Austin CJ, Whittington CM, Belov K et al. Characterization and evolution of vertebrate indoleamine 2, 3-dioxygenases IDOs from monotremes and marsupials. Comp Biochem Physiol B Biochem Mol Biol 2009; 153: 137–144.
Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov 2002; 1: 609–620.
Ladomersky E, Zhai L, Gritsina G, Genet M, Lauing KL, Wu M et al. Advanced age negatively impacts survival in an experimental brain tumor model. Neurosci Lett 2016; 630: 203–208.
van Baren N, Van den Eynde BJ. Tryptophan-degrading enzymes in tumoral immune resistance. Front Immunol 2015; 6: 1–9.
Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ et al. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res 2015; 21: 5427–5433.
Ott M, Litzenburger UM, Rauschenbach KJ, Bunse L, Ochs K, Sahm F et al. Suppression of TDO-mediated tryptophan catabolism in glioblastoma cells by a steroid-responsive FKBP52-dependent pathway. Glia 2015; 63: 78–90.
Knox WE. Two mechanisms which increase in vivo the liver tryptophan peroxidase activity: specific enzyme adaptation and stimulation of the pituitary adrenal system. Br J Exp Pathol 1951; 32: 462–469.
Hassanain HH, Chon SY, Gupta SL. Differential regulation of human indoleamine 2,3-dioxygenase gene expression by interferons-gamma and -alpha. Analysis of the regulatory region of the gene and identification of an interferon-gamma-inducible DNA-binding factor. J Biol Chem 1993; 268: 5077–5084.
Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 1991; 5: 2516–2522.
Yoshida R, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc Natl Acad Sci USA 1978; 75: 3998–4000.
Fujigaki S, Saito K, Sekikawa K, Tone S, Takikawa O, Fujii H et al. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism. Eur J Immunol 2001; 31: 2313–2318.
Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine 2000; 12: 588–594.
Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol 2005; 175: 5601–5605.
Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood 2005; 106: 2375–2381.
Prendergast GC, Metz R, Muller AJ, Merlo LM, Mandik-Nayak L. IDO2 in immunomodulation and autoimmune disease. Front Immunol 2014; 5: 585.
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011; 478: 197–203.
Vogel CFA, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun 2008; 375: 331–335.
Bankoti J, Rase B, Simones T, Shepherd DM. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol Appl Pharmacol 2010; 246: 18–28.
Mautino MR, Metz RA, Jaipuri F, Waldo J, Kumar S, Marcinowicz-Flick A et al. Abstract 1633: novel specific- and dual- tryptophan-2,3-dioxygenase (TDO) and indoleamine-2,3-dioxygenase (IDO) inhibitors for tumor immunotherapy. Cancer Res 2014; 74: 1633.
Boyland E, Williams DC. The estimation of tryptophan metabolites in the urine of patients with cancer of the bladder. Biochem J 1955; 60: v.
Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281: 1191–1193.
Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science 2005; 310: 850–855.
Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer 2002; 101: 151–155.
Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol 2003; 24: 242–248.
Wainwright DA, Dey M, Chang A, Lesniak MS. Targeting Tregs in malignant brain cancer: overcoming IDO. Front Immunol 2013; 4: 116.
Wainwright DA, Sengupta S, Han Y, Lesniak MS. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol 2011; 13: 1308–1323.
Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 2013; 339: 1219–1224.
Ye J, Qiu J, Bostick JW, Ueda A, Schjerven H, Li S et al. Aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep 2017; 21: 2277–2290.
Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 1999; 189: 1363–1372.
Seymour RL, Ganapathy V, Mellor AL, Munn DH. A high-affinity, tryptophan-selective amino acid transport system in human macrophages. J Leukoc Biol 2006; 80: 1320–1327.
Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci USA 2007; 104: 18619–18624.
Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Front Biosci 2012; 4: 734–745.
Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 2007; 117: 1147–1154.
Mellor AL, Munn DH. Ido expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 2004; 4: 762–774.
Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 2011; 12: 870–878.
Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon K-S, Auffinger B et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 2012; 18: 6110–6121.
Zhai L, Ladomersky E, Dostal CR, Lauing KL, Swoap K, Billingham LK et al. Non-tumor cell IDO1 predominantly contributes to enzyme activity and response to CTLA-4/PD-L1 inhibition in mouse glioblastoma. Brain Behav Immun 2017; 62: 24–29.
Liu Y, Liang X, Yin X, Lv J, Tang K, Ma J et al. Blockade of IDO-kynurenine-AhR metabolic circuitry abrogates IFN-gamma-induced immunologic dormancy of tumor-repopulating cells. Nat Commun 2017; 8: 15207.
Huang L, Li L, Lemos H, Chandler PR, Pacholczyk G, Baban B et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J Immunol 2013; 191: 3509–3513.
Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res 2016; 76: 2076–2081.
Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 2015; 11: 1018–1030.
Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12: 860–875.
Ravishankar B, Liu H, Shinde R, Chaudhary K, Xiao W, Bradley J et al. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc Natl Acad Sci USA 2015; 112: 10774–10779.
Ravishankar B, Shinde R, Liu H, Chaudhary K, Bradley J, Lemos HP et al. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc Natl Acad Sci USA 2014; 111: 4215–4220.
Ravishankar B, Liu H, Shinde R, Chandler P, Baban B, Tanaka M et al. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci USA 2012; 109: 3909–3914.
Sharma MD, Shinde R, McGaha TL, Huang L, Holmgaard RB, Wolchok JD et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv 2015; 1: e1500845.
Solito S, Pinton L, Damuzzo V, Mandruzzato S. Highlights on molecular mechanisms of MDSC-mediated immune suppression: paving the way for new working hypotheses. Immunol Invest 2012; 41: 722–737.
Yu J, Du W, Yan F, Wang Y, Li H, Cao S et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 2013; 190: 3783–3797.
Zhang H, Maric I, DiPrima MJ, Khan J, Orentas RJ, Kaplan RN et al. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood 2013; 122: 1105–1113.
Jitschin R, Braun M, Buttner M, Dettmer-Wilde K, Bricks J, Berger J et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood 2014; 124: 750–760.
Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP et al. Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep 2015; 13: 412–424.
Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol 2001; 2: 64–68.
Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer 2014; 2: 3.
Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol 2003; 4: 1206–1212.
Zhai L, Ladomersky E, Lauing KL, Wu M, Genet M, Gritsina G et al. Infiltrating T cells increase IDO1 expression in glioblastoma and contribute to decreased patient survival. Clin Cancer Res 2017; 23: 6650–6660.
Chamuleau ME, van de Loosdrecht AA, Hess CJ, Janssen JJ, Zevenbergen A, Delwel R et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica 2008; 93: 1894–1898.
Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clinical Cancer Res 2006; 12: 1144–1151.
Astigiano S, Morandi B, Costa R, Mastracci L, D'Agostino A, Ratto GB et al. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia 2005; 7: 390–396.
Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 2010; 67: 361–365.
Feder-Mengus C, Wyler S, Hudolin T, Ruszat R, Bubendorf L, Chiarugi A et al. High expression of indoleamine 2,3-dioxygenase gene in prostate cancer. Eur J Cancer 2008; 44: 2266–2275.
Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res 2005; 11: 6030–6039.
Takao M, Okamoto A, Nikaido T, Urashima M, Takakura S, Saito M et al. Increased synthesis of indoleamine-2,3-dioxygenase protein is positively associated with impaired survival in patients with serous-type, but not with other types of, ovarian cancer. Oncol Rep 2007; 17: 1333–1339.
Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K et al. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer 2006; 95: 1555–1561.
Sakurai K, Enomoto K, Amano S, Kimura T, Sugito K, Kimizuka K et al. [Study of indoleamine 2,3-dioxygenase expression in patients of esophageal squamous cell carcinoma]. Gan To Kagaku Ryoho 2004; 31: 1780–1782.
Riesenberg R, Weiler C, Spring O, Eder M, Buchner A, Popp T et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res 2007; 13: 6993–7002.
Ishio T, Goto S, Tahara K, Tone S, Kawano K, Kitano S. Immunoactivative role of indoleamine 2,3-dioxygenase in human hepatocellular carcinoma. J Gastroenterol Hepatol 2004; 19: 319–326.
Pan K, Wang H, Chen MS, Zhang HK, Weng DS, Zhou J et al. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol 2008; 134: 1247–1253.
Piras F, Colombari R, Minerba L, Murtas D, Floris C, Maxia C et al. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer 2005; 104: 1246–1254.
Zhai L, Ladomersky E, Lauing KL, Wu M, Genet M, Gritsina G et al. Infiltrating T cells increase IDO1 expression in glioblastoma and contribute to decreased patient survival. Clin Cancer Res 2017; 23: 6650–6660.
Röhrig UF, Majjigapu SR, Vogel P, Zoete V, Michielin O. Challenges in the Discovery of Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitors. J Med Chem 2015; 58: 9421–9437.
Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res 2014; 20: 221–232.
Soliman HH, Jackson E, Neuger T, Dees CE, Harvey DR, Han H et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget 2014; 5: 8136–8146.
Perez RP, Riese MJ, Lewis KD, Saleh MN, Daud A, Berlin J et al. Epacadostat plus nivolumab in patients with advanced solid tumors: preliminary phase I/II results of ECHO-204. J Clin Oncol 2017; 35: 3003–3003.
Siu LL, Gelmon K, Chu Q, Pachynski R, Alese O, Basciano P et al. Abstract CT116: BMS-986205, an optimized indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab (nivo) in advanced cancers in a phase 1/2a trial. Cancer Res 2017; 77: CT116–CT116.
Soliman HH, Jackson E, Neuger T, Dees EC, Harvey RD, Han H et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget 2014; 5: 8136–8146.
Soliman HH, Minton SE, Han HS, Ismail-Khan R, Neuger A, Khambati F et al. A phase I study of indoximod in patients with advanced malignancies. Oncotarget 2016; 7: 22928–22938.
Spira AI, Hamid O, Bauer TM, Borges VF, Wasser JS, Smith DC et al. Efficacy/safety of epacadostat plus pembrolizumab in triple-negative breast cancer and ovarian cancer: Phase I/II ECHO-202 study. J Clin Oncol 2017; 35: 1103–1103.
Gangadhar TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Luke JJ et al. Preliminary results from a phase I/II study of epacadostat (incb024360) in combination with pembrolizumab in patients with selected advanced cancers. J Immunother Cancer 2015; 3: O7.
Tang T, Gill HS, Ogasawara A, Tinianow JN, Vanderbilt AN, Williams SP et al. Preparation and evaluation of L- and D-5-[18F]fluorotryptophan as PET imaging probes for indoleamine and tryptophan 2,3-dioxygenases. Nucl Med Biol 2017; 51: 10–17.
Xin Y, Cai H. Improved radiosynthesis and biological evaluations of L- and D-1-[18F]fluoroethyl-tryptophan for pet imaging of ido-mediated kynurenine pathway of tryptophan metabolism. Mol Imaging Biol 2017; 19: 589–598.
Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 2008; 111: 2152–2154.
Löb S, Königsrainer A, Zieker D, Brücher BDM, Rammensee H-G, Opelz G et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother 2009; 58: 153–157.
Acknowledgements
This work was supported by NIH grants R00 NS082381 (DAW) and R01 NS097851-01 (DAW), the Cancer Research Institute—Clinic and Laboratory Integration Program (DAW), the Robert H. Lurie Comprehensive Cancer Center—Zell Scholar Program of the Zell Family Foundation Gift (DAW) and the Northwestern Brain Tumor Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhai, L., Ladomersky, E., Lenzen, A. et al. IDO1 in cancer: a Gemini of immune checkpoints. Cell Mol Immunol 15, 447–457 (2018). https://doi.org/10.1038/cmi.2017.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2017.143
- Springer Nature Limited
This article is cited by
-
Comprehensive analysis of the prognostic value and immunological role of IDO1 gene in pan-cancer
European Journal of Medical Research (2024)
-
Association between metabolites in tryptophan-kynurenine pathway and inflammatory bowel disease: a two-sample Mendelian randomization
Scientific Reports (2024)
-
Increased coexpression of PD-L1 and IDO1 is associated with poor overall survival in patients with NK/T-cell lymphoma
Leukemia (2024)
-
Longitudinal molecular profiling elucidates immunometabolism dynamics in breast cancer
Nature Communications (2024)
-
Exploring a repurposed candidate with dual hIDO1/hTDO2 inhibitory potential for anticancer efficacy identified through pharmacophore-based virtual screening and in vitro evaluation
Scientific Reports (2024)