Abstract
Interleukin (IL)-21 is a recently discovered cytokine in early clinical development, which has shown anti-tumor activity in various animal models. In the present study, we examine the anti-tumor activity of IL-21 protein therapy in two syngeneic tumor models and its effect on the density of tumor infiltrating T cells. We treated mice bearing established subcutaneous B16 melanomas or RenCa renal cell carcinomas with intraperitoneal (i.p.) or subcutaneous (s.c.) IL-21 protein therapy and subsequently scored the densities of tumor infiltrating CD4+ and CD8+ T cells by immunohistochemistry. Whereas both routes of IL-21 administration significantly inhibited growth of small, established RenCa and B16 tumors, only s.c. therapy significantly inhibited the growth of large, established tumors. We found a greater bioavailability and significant drainage of IL-21 to regional lymph nodes following s.c. administration, which could account for the apparent increase in anti-tumor activity. Specific depletion of CD8+ T cells with monoclonal antibodies completely abrogated the anti-tumor activity, whereas NK1.1+ cell depletion did not affect tumor growth. In accordance, both routes of IL-21 administration significantly increased the density of tumor infiltrating CD8+ T cells in both B16 and RenCa tumors; and in the RenCa model s.c. administration of IL-21 led to a significantly higher density of tumor infiltrating CD8+ T cells compared to i.p. administration. The densities of CD4+ T cells were unchanged following IL-21 treatments. Taken together, these data demonstrate that IL-21 protein has anti-tumor activity in established syngeneic tumors, and we show that IL-21 therapy markedly increases the density of tumor infiltrating CD8+ T cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Successful immune-based cancer therapy needs to enhance the interaction and/or reactivity between the immune system and the cancer cells. In tumor immunity, tumor infiltrating lymphocytes (TILs) are regarded as the primary effector cells, and the number of TILs has previously been correlated with prolonged survival in cancer patients [6,18]. The number of tumor infiltrating CD8+ T cells and the CD8+/CD4+ T cell ratio have been shown to be independent prognostic factors for improved survival in several different human cancers [21,22,28,30]. Thus, novel treatments able to increase the number of tumor-specific, tumor-infiltrating immune effector cells could hold a promising clinical future.
IL-21 is the latest member of the common γ-chain-dependent cytokine family and is currently in early clinical development for the treatment of cancer. IL-21 was discovered as a product of activated CD4+ T helper cells, and its unique receptor IL-21R has been identified on a broad range of immune cells including B, T, NK, and dendritic cells [3,23]. IL-21 has pleiotropic immune modulatory activity, which has shown encouraging anti-tumor effects in several different animal models [16]. CD8+ cytotoxic T lymphocytes (CTLs), NK cells, or both have been identified as the main mediators of IL-21 anti-tumor activity [16], and in this respect IL-21 has been suggested to play a role in the transition from innate to adaptive immunity [14]. However, it remains to be shown whether its anti-tumor activity in vivo is mediated by modulation of the tumor infiltrating effector cell populations. In vitro, IL-21 stimulation induced the differentiation and maturation of human NK cells from progenitor cells [23,31] and also increased the cytolytic activity of mature activated human NK cells [23]. Murine NK cells have shown more biphasic responses to IL-21 stimulation in vitro, depending on IL-21 concentration, co-stimuli, and cell maturation stage [14,24,36]. In mice and humans, IL-21 has been shown to increase the expansion of antigen stimulated CD8+ cytotoxic T cells as well as enhance their cytolytic activity in vitro [14,17,39]. In vivo, IL-21 has been shown to enhance the expansion, activity, and long-term survival of ovalbumin-specific CD8+ T cells detected in lymph nodes (LNs) in mice bearing ovalbumin-expressing E.G7 lymphomas [20]. Based on these results we anticipate that IL-21 in vivo is able to increase the number and/or reactivity of TILs.
In previous studies of IL-21-mediated anti-tumor activity, the cytokine was primarily administered via plasmid gene delivery [38], tumor cell secretion [7,8,19,37], or used in systems where the immune response was enhanced by introduction of foreign antigens or adoptive transfer of tumor specific lymphocytes [13,20,26,39]. Clearly the effects of IL-21 protein therapy also need to be investigated in simple syngeneic models using more conventional routes of administration, which are more clinically relevant. The use of IL-21 protein therapy in a native syngeneic model could help to determine whether IL-21 is able to modulate TILs in vivo. The evaluation of s.c. administration of IL-21 is of major interest for the clinical application of IL-21 because of patient convenience and since increased tolerability and sustained efficacy has been shown in clinical trials with IL-2 by this route of administration [11].
In this study, we demonstrate the anti-tumor effects of s.c. and i.p. IL-21 protein therapy in two syngeneic tumor models. Our data indicate that IL-21 protein via both routes of administration can inhibit established tumor growth and that s.c. administration could be applicable in the clinic. Furthermore, we show that IL-21 therapy strongly increases the density of CD8+ TILs without changing the CD4+ TILs, and that the CD8+ T cells are essential for the IL-21-induced anti-tumor activity.
Materials and methods
Mice
Wild type (WT) female C57BL/6 and BALB/c mice were purchased from Taconic Europe A/S, Lille Skensved, Denmark, whereas female C57BL/6 nude mice (B6.Cg/Ntac-Foxn1 nu N9) were acquired from Taconic, Hudson, NY, USA. The animals were 6 weeks old on arrival and were allowed to acclimatize for at least one week before start of experiments. WT mice were housed in a standard animal facility whereas nude mice were isolated in an immunodeficient facility separated by a barrier. Light was controlled on a 12-h light–dark cycle, and the animals were given free access to food and drinking water. The animals were observed daily for clinical signs and their body weights were recorded regularly. All experiments were conducted in accordance with corporate and governmental policies.
Cell lines
C57BL/6 derived B16 (F0) melanoma cells (American Type Culture Collection (ATCC), CRL-6322) and BALB/c derived RenCa renal cell carcinoma cells (kindly provided by Dr. Robert H. Wiltrout, NCI at Frederick, MD, USA) were cultured in RPMI 1640 with GlutaMAXTM supplemented with 10% heat-inactivated FCS, sodium pyruvate (RenCa only), non-essential amino acids (RenCa only), and 5% penicillin-streptomycin (all from GIBCO Cell Culture, Invitrogen, Denmark).
Reagents and antibodies
Recombinant murine IL-21 (IL-21) protein was provided by Novo Nordisk A/S, Denmark and Zymogenetics, Inc., WA, USA and used in all experiments. The stock solutions contained IL-21 in a concentration of 5.5–10 mg/ml and working preparations were diluted in PBS. Radioactive 125I conjugated IL-21 was produced at Novo Nordisk A/S, Denmark. The depleting anti-mouse CD8 (clone 2.43, TIB-210 from ATCC) and anti-mouse NK1.1 (clone PK136, HB191 from ATCC) monoclonal antibodies were obtained from supernatants of hybridomas cultured at Novo Nordisk A/S, Denmark. The antibodies were purified in-house by affinity chromatography. For the histological examination we used rat anti-mouse CD4 (L3T4, clone RM4–5) (BD Pharmingen, CA, USA), rat anti-mouse CD8 (Ly-2, clone 53–6.7) (BD Pharmingen, CA, USA), rat serum IgG2a (Serotec, UK), and biotin-conjugated donkey anti-rat IgG (Jackson ImmunoResearch Laboratories, Inc., PA, USA).
In vivo tumor models
On day 0, C57BL/6 or BALB/c mice were inoculated s.c. in the right flank with 105 B16 melanoma or RenCa renal cell carcinoma cells, respectively. All mice were randomized and ear-tagged prior to treatment. The tumor volume was measured as two perpendicular diameters approximately three times per week, and calculated by the following formula:
where d represents the two diameters. Treatment with IL-21 was either initiated early with ∼5 mm3 mean tumor volume or late with ∼50 mm3 mean tumor volume. Fifty μg of IL-21 or PBS in a dosing volume of 200 μL was administered either intraperitoneally (i.p.) or subcutaneously (s.c.) in the contralateral flank 1x/daily in the C57BL/6-B16 model and 3x/week in the BALB/c-RenCa model. The 50 μg dose was chosen on the basis of dose-titration experiments prior to this work (data not shown). Termination criteria were a tumor volume of 1,000 mm3 or more than 20% weight loss from time of cell inoculation.
In vivo immune cell depletion
In vivo CD8+ and NK1.1+ cell depletion was performed by using anti-mouse CD8 (clone 2.43) and anti-mouse NK1.1 (clone PK136) monoclonal antibodies, respectively, as previously reported [33]. C57BL/6 mice inoculated s.c. in the right flank with 105 B16 melanoma cells were injected with antibodies (100 μg/mouse) i.p. on day −1, 0, 6, and 12 in relation to the onset of treatment. Treatment with 50 μg IL-21 administered s.c. was initiated when mean tumor volume had reached ∼5 mm3 corresponding to early treatment. Prior to the experiment, the dose and schedule of the depleting antibodies were verified by flow cytometry analysis showing complete depletion of CD8+ and NK1.1+ cells throughout the course of the experiment (data not shown).
In vitro tumor cell proliferation assay
Potential effects of IL-21 on the growth of B16 melanoma cells and RenCa carcinoma cells in vitro were examined in a colorimetric assay using the proliferation reagent 4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3 benzene disulfonate (WST-1) (Roche diagnostics GmbH, Germany). Briefly, 3000 B16 or RenCa cells/well were incubated in 96-well plates in their appropriate media and stimulated with increasing concentrations of IL-21 protein. After 48 h cell growth, cells were incubated with WST-1 for 2 h at 37°C. Absorbance at 450 nm was used to determine the relative proliferation of cells.
IL-21 pharmacokinetics
IL-21 was administered intravenous (i.v.), i.p. and s.c. (50 μg/animal) to BALB/c mice. Mice were anesthetized with FORENE® Isoflurane (Abbot Scandinavia AB, Sweden) after 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, and 8 h post injections, blood was collected from the retro-orbital sinus, and a DuoSet sandwich ELISA kit was used to detect IL-21 protein in the serum (R&D systems, Inc., MN, USA). Briefly, goat anti-mouse IL-21 antibodies were coated overnight onto a 96-well plate at RT. Serum samples were diluted 1:10 or 1:100 in PBS with 1% BSA and IL-21 standards were likewise supplemented with normal mouse serum. A biotin-labeled goat anti-mouse IL-21 antibody was used as detection antibody, and for the color reaction streptavidin-HRP with tetramethylbenzidine (TMB) was used as substrate (Sigma–Aldrich Chemie GmbH, Germany). The result was measured as absorbance at 450 nm. The lower limit of detection was ∼0.06 ng/ml with a dynamic range of 0.06–100 ng/ml. The serum concentration-time data were analyzed by a non-compartmental pharmacokinetic analysis using WinNonlin Professional (Pharsight, Inc., CA, USA) based on a sparse blood sampling schedule, where the mean serum concentration-time profiles of blood samples from three animals per time point were performed. The area under the curve (AUC), a measure of drug exposure and the bioavailability are reported.
IL-21 biodistribution
Mice were injected i.p. with 0.05 ml of 125I-IL-21 (∼2 μCi/animal) or Na125I as control in the lower right abdominal quadrant or subcutaneously in the right foot pad in order to use the popliteal LN as an exclusive lymph drainage site. After 15 min, 30 min, 1 h, 2 h, 4 h, 6 h, and 8 h animals were sacrificed and the following tissues/organs were collected and analyzed: heart, lungs, liver, spleen, kidneys, thyroidal gland, small and large intestines, mesenteric LNs, right and left popliteal LNs, and skin at injection site after s.c. administration, i.e. the foot. Na125I solution was used to monitor how free 125I would behave contra protein bound 125I in order to detect if the 125I from the protein was detached in vivo (data not shown). The γ-radiation was measured in all samples by a Cobra Auto-Gamma gamma-counter (PerkinElmer, Inc., MA, USA) and related to the total γ-radiation of the initial injected dose of 125I-IL-21 in %.
Immunohistochemistry of tumor infiltrating T cells
Six μm cryo-sections were made from tumor biopsies taken out at termination of the therapeutic studies. Sections were immunohistochemically stained with rat anti-mouse CD4 (clone RM4-5) or rat anti-mouse CD8 (clone 53-6.7) antibodies (5 μg/ml), whereas rat serum IgG2a was used as matching isotype control. Biotin-conjugated donkey anti-rat IgG (diluted 1:3,000) was used as secondary antibody. Sections were fixed in 4% paraformaldehyde at 4°C and endogenous biotin activity was blocked using Biotin blocking system from Dako A/S, Denmark. Prior to the antibody stainings non-specific binding was blocked by incubation in TBS with 3% skim milk, 3% BSA, and 7% donkey serum. Incubation with the primary antibodies was made at 4°C over night followed by 1 h incubation at RT with the secondary antibody both diluted in TBS with 0.5% skim milk, 3% BSA, and 7% donkey serum. Streptavidin conjugated alkaline phosphatase and Liquid Permanent Red Chromogen (Dako A/S, Denmark) was used to visualize positive cells and sections were counterstained with Mayer’s hematoxylin to reveal nuclei morphology.
Quantification of tumor infiltrating T cells
A stereological method was established to quantify the density of tumor infiltrating T cells. Images from immunohistochemically stained tumor sections were analyzed via online light microscopy at 20× magnification using C.A.S.T. grid software ver. 2.3.1.3 from Olympus Denmark A/S, Denmark. In all tumor sections (one from each tumor) the density of tumor infiltrating lymphocytes was blindly scored counting all positive cells intratumorally and relating them to an area of interest (AOI), representing the total tumor area measured stereologically, excluding necrotic tumor tissue and non-tumor tissue such as peritumoral connective tissue. The C.A.S.T. grid software and a motorized stage system enabled side by side imaging to ensure that no area was evaluated twice or omitted.
Statistics
Student’s t-test (two-tailed, assuming equal variance) was used for statistical evaluations of differences between treated and control groups. Data are shown as mean ± SEM and a P value less than 0.05 was considered statistically significant.
Results
IL-21 does not inhibit B16 or RenCa tumor cell growth in vitro
To determine whether IL-21 protein had any direct inhibitory effects on B16 or RenCa tumor cells we performed a tumor cell proliferation assay with increasing concentrations of IL-21 (0–5,000 ng/ml). We found no effects on B16 or RenCa cell growth after 48 h incubation (Fig. 1). Consistent with these results, no IL-21 receptor mRNA expression in either tumor cell line examined by quantitative RT-PCR analysis was found (data not shown).
IL-21 does not inhibit proliferation of B16 and RenCa cells in vitro. B16 cells (filled square) or RenCa cells (square) (3,000/well) were grown in appropriate media and stimulated with the indicated concentrations of IL-21 for 48 h. Absorbance at 450 nm was measured after 2 h incubation with WST-1 proliferation reagent. Mean ± SEM of duplicates
Subcutaneous IL-21 protein therapy significantly inhibits B16 and RenCa tumor growth
Subcutaneous syngeneic B16 and RenCa tumors were established in their respective hosts, C57BL/6 and BALB/c mice, by injection of 105 cells/animal. In both models IL-21 treatment was initiated either early, when tumors were just palpable (∼5 mm3 mean tumor volume), or late, when tumors were more established (∼50 mm3 mean tumor volume).
Early treatment with IL-21 using either route of administration showed significant (P < 0.001) growth inhibition of B16 melanoma tumors (Fig. 2a). In the early treatment of RenCa carcinomas only s.c. administration of IL-21 showed significant growth inhibition (P < 0.001), whereas i.p. administration showed a borderline significant growth inhibition (P = 0.06) (Fig. 2b). The difference between s.c. and i.p. administration in the early treatment of RenCa carcinomas was likewise close to statistical significance (P = 0.09).
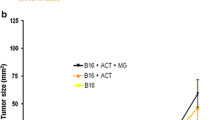
IL-21 therapy inhibits growth of syngeneic B16 melanomas and RenCa carcinomas. All animals were injected with 105 cells s.c. in the right flank and randomized prior to treatment start as indicated. Treatment was started either early (a and b) with ∼5 mm3 mean tumor volume or late (c and d) with ∼50 mm3 mean tumor volume. Fifty μg IL-21 or PBS was injected i.p. or s.c. (contralateral to the tumor site) daily in the B16 model (a and c) and 3×/week in the RenCa model (b and d). Mean ± SEM, a n = 12, b n = 15, c and d n = 10, † P = 0.06, *P < 0.05, **P < 0.01, ***P < 0.001, compared to PBS controls by Student’s t-test
In the late treatment regimens s.c. administration of IL-21 showed significant (P < 0.05) growth inhibition in both models, whereas i.p. administration was unable to significantly inhibit tumor growth (Fig 2c, d). Generally, the late treatment regimen showed less growth inhibition than the early treatment, and s.c. administration appeared to be more efficient than i.p. administration, although this difference did not reach statistical significance (Fig. 2).
Alongside these results, we monitored animal weight and general health in response to IL-21 administration and found no treatment associated effects on animal health (data not shown), suggesting that the treatment was well tolerated.
CD8+ T cells are essential for the anti-tumor activity of IL-21
In order to determine which immune effector cells were important for the anti-tumor activity of IL-21 protein in our models, C57BL/6 nude mice and wild type (WT) mice specifically depleted of CD8+ T cells or NK1.1+ cells were used. As shown in Fig. 3a, early s.c. IL-21 protein therapy was unable to inhibit B16 tumor growth in C57BL/6 nude mice. C57BL/6 nude mice are fully NK cell competent and T cell deficient, verified by flow cytometry (data not shown). This result suggests that T cells and not NK cells are essential for the anti-tumor activity of IL-21 in this model. To further examine the specific cell types involved in the anti-tumor activity, we depleted B16 tumor-bearing C57BL/6 WT mice with anti-CD8 and/or anti-NK1.1 antibodies prior to treatment start with s.c. IL-21 again initiated early. As shown in Fig. 3b, significant anti-tumor activity was still exhibited in NK.1.1+ cell-depleted animals (P < 0.01) compared to vehicle controls, whereas CD8+ T cell depletion completely abrogated the tumor growth inhibition of IL-21. Together, these results show that CD8+ T cells are the essential cells for the anti-tumor activity of IL-21 in our model.
The anti-tumor effect of IL-21 is CD8+ T cell dependent. C57BL/6 nude (a) or C57BL/6 WT mice (b) were injected with 105 B16 melanoma cells s.c. in the right flank and randomized prior to treatment start as indicated. WT animals were depleted of NK1.1+ cells, CD8+ cells or both using monoclonal Ab administrated i.p. on day −1, 0, 6 and 12 compared to treatment start. Fifty μg IL-21 or vehicle was injected s.c. (contralateral to the tumor site) daily from day 3 after tumor inoculation. Mean ± SEM, n = 10, *P < 0.05, **P < 0.01, compared to vehicle control, CD8-depleted and NK1.1 + CD8-depleted groups by Student’s t-test
IL-21 significantly increases the density of tumor infiltrating CD8+ T cells
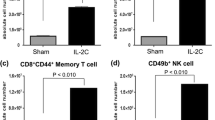
Based on the finding that CD8+ T cells were responsible for the anti-tumor activity in our models we examined whether IL-21 increased the number of tumor infiltrating CD8+ T cells as a possible mechanism of action. Immunohistochemistry was used to stain CD4+ and CD8+ T cells in tumor biopsies from our in vivo therapeutic studies. One section from each tumor biopsy obtained at the end of the late treatment experiments was stained (Fig. 2c, d). The density of TILs was scored by counting all positive cells in a stereologically defined area of interest (AOI) in which necrotic areas and peritumoral connective tissue were excluded. Thus, our results reflect the density of intratumoral infiltrating lymphocytes, which are in direct contact with tumor cells and have the potential of performing cytotoxic effects. Representative pictures of anti-CD8 stained B16 melanomas and RenCa carcinomas with and without IL-21 treatments are shown in Fig. 4. In B16 melanomas the density of tumor infiltrating CD4+ T cells was unchanged following IL-21 treatments (Fig. 5a), whereas the density of CD8+ T cells showed a significant 7–10-fold increase (P < 0.05) following i.p. and s.c. administration of IL-21 (Fig. 5b). In RenCa carcinomas the density of CD4+ T cells was likewise unchange after IL-21 treatments (Fig. 5c) and the density of CD8+ T cells was increased 3-fold after i.p. administration (P < 0.05) and 8-fold after s.c. administration (P < 0.01) of IL-21 (Fig 5d). Moreover, the difference between i.p. and s.c. IL-21 administration was significant (P < 0.05) reflecting the improved efficacy obtained in the RenCa carcinomas with s.c. administration (Fig. 2d). Together these data show that IL-21 strongly increases the density of tumor infiltrating CD8+ T cells without affecting the CD4+ T cell density, consequently increasing the CD8+/CD4+ T cell ratio in both tumor models.
Pictures of tumor infiltrating CD8+ T cells in B16 melanomas and RenCa carcinomas. Representative pictures of cryo-sections at ×20 magnification showing tumor infiltrating CD8+ T cells in B16 melanomas: PBS (a), i.p. IL-21 (b), s.c. IL-21 (c) and RenCa carcinomas: PBS (d), i.p. IL-21 (e), s.c. IL-21 (f). Tumor biopsies were obtained at the end of the experiments shown in Fig. 2c, d. CD8+ T cells were stained with liquid permanent red by immunohistochemistry according to “Materials and methods” and they are indicated by arrows. Sections have been counterstained with Mayer’s hematoxylin yielding a blue nucleus stain
IL-21 increases the density of tumor infiltrating CD8+ T cells. The bar plots show the density of tumor infiltrating CD4+ T cells (a and c) and CD8+ T cells (b and d) in B16 melanomas (a and b) and the RenCa carcinomas (c and d). Tumor biopsies were obtained at the end of the experiments shown in Fig. 2c, d and stained for CD8+ and CD4+ T cells by immunohistochemistry. All positive cells located intratumorally were counted in one section from each biopsy and related to a stereologically measured area of interest (AOI) excl. necrotic areas and non-tumor tissue, such as connective tissue. Bars represent mean ± SEM, n = 7–10, *P < 0.05, **P < 0.01, Student’s t-test. AOI area of interest
Subcutaneous administration of IL-21 results in a slow release from the injection site and significant draining to regional lymph nodes
In order to examine whether differences in the biodistribution of IL-21 could account for the observed efficacy after s.c. versus i.p administration, the kinetics of the two different routes of administration was compared. Particularly, we wanted to investigate the degree of regional LN drainage of IL-21 after s.c. administration, since IL-21 in this compartment could be relevant during the generation of immune responses. 125I labeled IL-21 was used to measure the kinetics of IL-21 distribution in several major organs and tissues as listed in Materials and methods. Figure 6 shows the distribution over time at the s.c. injection site, i.e the right foot (Fig. 6a), and in the popliteal LNs (Fig. 6b). The results show that upon s.c. injection 125I-IL-21 is released slowly from the injection site and a considerable amount is drained through the regional LN (C max ∼ 4.7% of injected dose). In contrast, very low levels was detected in the popliteal LN node after i.p. injection and in the contralateral LN after s.c. injection (C max < 0.1% of injected dose). We found no specific retention or major differences in the biodistribution between i.p. and s.c. over time in the major organs: heart, lungs, liver, spleen, kidneys, and small and large intestines (data not shown). The thyroid gland generally showed an increased radioactivity over time after both routes of administration due to its retention of free 125I.
125I-IL-21 shows slow release and significant lymph drainage following s.c. administration. 125I-IL-21 was injected either i.p. or s.c. in the right footpad in BALB/c mice, and animals were sacrificed at indicated time points and γ-radiation at the subcutaneous injection site (a) and in popliteal LNs (b) was measured. Each data point represents the mean ± SEM of triplicate mice
IL-21 has a greater bioavailability and higher peak serum concentration after subcutaneous administration
Next, we examined the serum concentration-time profiles of IL-21 after i.v., i.p. and s.c. administration to examine whether the pharmacokinetics of IL-21 contributes to the observed efficacy differences. IL-21 was detectable in serum after 5 min with all administration routes. A peak serum concentration of 113 ng/ml of IL-21 was reached 1 h post i.p administration, whereas s.c. administration provided a higher peak IL-21 concentration of 230 ng/ml 2 h post injection (Fig. 7). S.c. administration resulted in a more sustained concentration of IL-21 in serum and 2.5-fold higher AUC compared to i.p administration (665 h×ng/ml vs. 242 h×ng/ml after s.c. and i.p. administration, respectively). The AUC after i.v. administration was even higher (1,010 h×ng/ml) due to the very high initial IL-21 concentration that within 1 h decreased to a level approximately 10-fold lower than both i.p. and s.c administrations (Fig. 7). The bioavailability was 24% and 66% for i.p. and s.c. administration, respectively. These results indicate that IL-21 has a prolonged pharmacokinetic profile following s.c. administration with a higher peak serum concentration and greater bioavailability compared to i.p. administration.
Greater bioavailability and peak serum conc. of IL-21 after s.c. administration. Fifty μg IL-21 was injected i.p. in the lower right abdominal quadrant, s.c. in the right flank or i.v. in the tail vein of BALB/c mice. Blood samples were drawn at indicated time points and the serum concentration of IL-21 was measured by ELISA. Each data point represents mean ± SEM of triplicate mice
Discussion
Previous studies of IL-21 anti-tumor activity have been conducted with IL-21 secreting tumors [7,8,19,37], IL-21 expression plasmids [38], in model systems immunogenically enhanced with foreign antigens, or by the use of adoptive transfer of antigen specific lymphocytes [13,20,26,39]. Although these studies support the concept that IL-21 aids in the destruction of cancer, these methods of IL-21 administration are not clinically applicable. More recently, a number of studies have shown anti-tumor effects of intraperitoneal administration of recombinant IL-21 protein [13,32,35]. In these studies IL-21 therapy was given either in combination with other therapies, as prophylactic therapy at or before tumor inoculation, and only for very few days duration. Here, we have used intraperitoneal and subcutaneous administration of IL-21 protein to mice bearing established syngeneic tumors in order to study the effects of IL-21 alone under more therapeutically relevant conditions, and IL-21 was administered throughout the experiments to maximize the treatment effect. The use of s.c. administration of cytokines for cancer therapy has previously been applied successfully, as s.c. administration of IL-2 resulted in less adverse events yet maintaining efficacy and enabling outpatient treatments [11]. In addition, s.c. administration is believed to be more convenient for the patients. We report that IL-21 protein therapy significantly inhibits tumor growth in two syngeneic tumor models. The effect was not due to a direct inhibition of tumor cell proliferation, as also shown previously with B16 tumor cells [38]. Our data indicate that s.c. administration of IL-21 in the RenCa model is at least as effective as or perhaps more effective than i.p. administration with early as well as late therapy initiation. This might be due to prolonged availability of IL-21, since s.c. administration of IL-21 resulted in a more prolonged presence of the protein in serum and higher bioavailability compared to i.p. administration. Furthermore s.c. administration also resulted in a significant passage of IL-21 through regional lymphatics, which may also have contributed to an improved anti-tumor immune response. IL-21 has been shown to expand antigen stimulated CD8+ T cells [17,39] and this effect might be further improved by the direct stimulation of CD8+ T cells in LNs during an immune response. This notion is supported by our finding that CD8+ T cells were indispensable for the reduced tumor growth observed after IL-21 treatment. Interestingly, it has also been shown that the site of immunization produces a subsequent site-specific homing of T cells and accompanying anti-tumor activity [5]. Thus, s.c. administration of IL-21 might also mount an immune response targeted more specifically against the s.c. compartment, where the tumors in this study were located. In the B16 model the efficacy difference between s.c. and i.p. was less pronounced compared to the more immunogenic RenCa model. It is not clear at this point whether this difference is caused by differences between the two tumor cell lines or differences between their hosts (BALB/c and C57BL/6 mice for RenCa and B16 tumors, respectively). It is possible that different drug delivery methods may affect tumors with different immunogenicity differently, as recently described [4]. Altogether, our results suggest that s.c. administration of IL-21 could be advantageous and deserves clinical evaluation.
In our experiments we found that depletion of NK1.1+ cells did not reduce the anti-tumor effect of IL-21, whereas depletion of CD8+ cells or growth of tumors in athymic mice completely abrogated the effect of IL-21. Taken together, these data show that CD8+ T cells are required for the anti-tumor activity in our model, whereas NK cells played an insignificant role under these settings. In the literature either CD8+ T cells, NK cells, or both cell types have been described as required for the IL-21 anti-tumor activity depending on the models used [16]. Most of the studies demonstrating NK cell-mediated anti-tumor activity of IL-21 have been performed in i.v. metastasis models where IL-21 was administered at the time of tumor establishment or in models using IL-21-expressing tumors [2,19,34,35,37]. Two major differences might explain these different effector mechanisms. First, we have treated subcutaneous tumors, which are less accessible to NK cells compared to intravenous tumors. Second, in our experiments treatment was initiated only after the tumors became established. The larger tumor burden in this setting might overwhelm the effect of NK cells as they are unable to clonally expand. In the i.v. metastasis model where IL-21 therapy is given at the time of tumor inoculation, NK-mediated killing of the tumor cells may eradicate the tumor cells almost completely before an adaptive immune response can evolve, consistent with the notion that the efficacy in this model was sustained in Rag-1-/- mice [2]. In a study by Ma et al. [19] rejection of IL-21 secreting B16F1 tumor cells inoculated subcutaneously required both NK cells and CD8+ T cells. In this model, NK cell-depletion resulted in rapid growth of the tumors, suggesting an early role of NK cells, whereas CD8+ T cell-depletion resulted in delayed tumor growth, suggesting that adaptive immunity might have played a role later on to kill remaining tumor cells, as suggested by the authors [19]. By contrast Wang et al. showed that depletion of NK cells completely abrogated the anti-tumor effect of IL-21 expressing plasmids administered day 5 and 12 post tumor inoculation with MCA205 fibrosarcomas [38]. Although these results apparently contradict the hypothesis suggested by us and others [4,19] that NK cells primarily play a role early in the anti-tumor response, it is possible that the much slower growth rate of MCA205 cells compared to B16 cells allows NK cells to kill MCA205 cells before the tumor burden becomes too large, even though treatment is initiated day 5. Also, differences in immunogenicity and expression of ligands for activating and inhibitory NK cell receptors between the two tumor lines might contribute to these different results.
Several studies have shown that IL-21 can stimulate CD8+ T cells to proliferate, increase their cytotoxicity, and sustain survival [1,14,17,20,39]. In order to elicit tumor cytotoxicity, CD8+ T cells must infiltrate the tumor to come in close contact with the tumor cells. The benefit of TILs and specifically CD8+ TILs and the CD8+/CD4+ TIL ratio have also been shown in several different human cancers where they were predictive of improved survival [9,12,21,22,28,30]. In this study, we demonstrate that IL-21 protein given therapeutically significantly increased the density of tumor infiltrating CD8+ T cells without changing the CD4+ T cell density, consequently increasing the CD8+/CD4+ T cell ratio in both models. Interestingly, the density of CD8+ TILs was significantly higher after s.c. administration of IL-21 compared to i.p. administration in the RenCa carcinomas, supporting our notion that s.c. administration might be favorable. Generally, the RenCa carcinomas also showed approximately 10-fold higher density of infiltrating T cells compared to B16 tumors reflecting the higher inherent immunogenicity of RenCa as previously reported [15,29]. In a recently published article a similar increase in CD8+ T cell infiltration was observed in a small number of tumors following challenge with an IL-21 expressing mouse bladder cancer [10].
There are several possible explanations for the increased density of CD8+ TILs after IL-21 stimulation. First, IL-21 has been shown to increase the proliferation of antigen-stimulated CD8+ T cells [17,20,39], which in turn could yield a greater pool of tumor specific T cells infiltrating the tumor. Another possibility is that IL-21 sustains the survival of CD8+ T cells infiltrating the tumor, which has been supported by both in vitro and in vivo data [1,20]. Particularly, it has been shown that IL-21 is able to sustain CD28 expression on CD8+ T cells and increase their IL-2 production [1], both of which could enhance their survival in a challenging tumor environment [25]. In this context, it should be noted that our results represent the conditions about 8–10 days after the first IL-21 dose, and one day after the last dose, thus the effects of IL-21 have been sustained for more than a week from the initial stimulation. Finally, it is possible that IL-21 increases the specific homing of CD8+ T cells into tumors. IL-21 has been shown to increase CXC chemokines such as IP-10, MIG and I-TAC in IL-21-secreting tumors [8], which could work as T cell attractants [27], but to date there are no data demonstrating that systemic IL-21 delivery improves chemokine-mediated homing of CD8+ T cells to tumors. Presently, the mechanism of IL-21 anti-tumor activity remains to be fully elucidated, but the finding that IL-21 increased tumor infiltration of CD8+ T cells is a step forward that encourages the use of IL-21 in oncology.
In conclusion, we have shown that IL-21 protein mono-therapy inhibited established syngeneic tumor growth in two preclinical models of melanoma and renal cell carcinoma. Furthermore, we have shown that IL-21 markedly increased the density of tumor-infiltrating CD8+ T cells, which are essential for the anti-tumor effect. These findings support the use of IL-21 as a promising new anti-cancer drug.
Abbreviations
- IL-21:
-
Interleukin 21
- i.v.:
-
Intravenous
- i.p.:
-
Intraperitoneal
- s.c.:
-
Subcutaneous
- TILs:
-
Tumor infiltrating lymphocytes
- NK cells:
-
Natural killer cells
- CTLs:
-
Cytotoxic T lymphocytes
- AOI:
-
Area of interest
- AUC:
-
Area under the curve
- WT:
-
Wild type
- LN:
-
Lymph node
- IP-10:
-
Interferon-inducible protein 10
- MIG:
-
Monokine induced by interferon gamma
- I-TAC:
-
Interferon-inducible T cell alpha chemoattractant
References
Alves NL, Arosa FA, van Lier RA (2005) IL-21 sustains CD28 expression on IL-15-activated human naive CD8+ T cells. J Immunol 175:755–762
Brady J, Hayakawa Y, Smyth MJ, Nutt SL (2004) IL-21 induces the functional maturation of murine NK cells. J Immunol 172:2048–2058
Brandt K, Bulfone-Paus S, Jenckel A, Foster DC, Paus R, Ruckert R (2003) Interleukin-21 inhibits dendritic cell-mediated T cell activation and induction of contact hypersensitivity in vivo. J Invest Dermatol 121:1379–1382
Cappuccio A, Elishmereni M, Agur Z (2006) Cancer immunotherapy by interleukin-21: potential treatment strategies evaluated in a mathematical model. Cancer Res 66:7293–7300
Chang CJ, Tai KF, Roffler S, Hwang LH (2004) The immunization site of cytokine-secreting tumor cell vaccines influences the trafficking of tumor-specific T lymphocytes and antitumor efficacy against regional tumors. J Immunol 173:6025–6032
Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N (1996) Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77:1303–1310
Comes A, Rosso O, Orengo AM, Di Carlo E, Sorrentino C, Meazza R, Piazza T, Valzasina B, Nanni P, Colombo MP, Ferrini S (2006) CD25+ regulatory T cell depletion augments immunotherapy of micrometastases by an IL-21-secreting cellular vaccine. J Immunol 176:1750–1758
Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, Colombo MP, Ferrini S (2004) IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol 172:1540–1547
Diederichsen AC, Hjelmborg JB, Christensen PB, Zeuthen J, Fenger C (2003) Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother 52:423–428
Furukawa J, Hara I, Nagai H, Yao A, Oniki S, Fujisawa M (2006) Interleukin-21 gene transfection into mouse bladder cancer cells results in tumor rejection through the cytotoxic T lymphocyte response. J Urol 176:1198–1203
Geertsen PF, Gore ME, Negrier S, Tourani JM, von der MH (2004) Safety and efficacy of subcutaneous and continuous intravenous infusion rIL-2 in patients with metastatic renal cell carcinoma. Br J Cancer 90:1156–1162
Haanen JB, Baars A, Gomez R, Weder P, Smits M, de Gruijl TD, von Blomberg BM, Bloemena E, Scheper RJ, van Ham SM, Pinedo HM, van den Eertwegh AJ (2006) Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol Immunother 55:451–458
He H, Wisner P, Yang G, Hu HM, Haley D, Miller W, O’hara A, Alvord WG, Clegg CH, Fox BA, Urba WJ, Walker EB (2006) Combined IL-21 and Low-Dose IL-2 therapy induces anti-tumor immunity and long-term curative effects in a murine melanoma tumor model. J Transl Med 4:24
Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF, Wurster AL, Donaldson DD, Collins M, Young DA, Grusby MJ (2002) IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 16:559–569
Krup OC, Kroll I, Bose G, Falkenberg FW (1999) Cytokine depot formulations as adjuvants for tumor vaccines. I. Liposome-encapsulated IL-2 as a depot formulation. J Immunother 22:525–538
Leonard WJ, Spolski R (2005) Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol 5:688–698
Li Y, Bleakley M, Yee C (2005) IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol 175:2261–2269
Lipponen PK, Eskelinen MJ, Jauhiainen K, Harju E, Terho R (1992) Tumour infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. Eur J Cancer 29A:69–75
Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, Collins M, Dunussi-Joannopoulos K (2003) IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol 171:608–615
Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA (2004) IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol 173:900–999
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353:2654–2666
Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D (2000) Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408:57–63
Parrish-Novak J, Foster DC, Holly RD, Clegg CH (2002) Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol 72:856–863
Petrulio CA, Kim-Schulze S, Kaufman HL (2006) The tumour microenvironment and implications for cancer immunotherapy. Expert Opin Biol Ther 6:671–684
Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE III (2006) Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol 177:120–129
Romagnani P, Annunziato F, Lazzeri E, Cosmi L, Beltrame C, Lasagni L, Galli G, Francalanci M, Manetti R, Marra F, Vanini V, Maggi E, Romagnani S (2001) Interferon-inducible protein 10, monokine induced by interferon gamma, and interferon-inducible T-cell alpha chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) alphabeta+ CD8+ single-positive T cells, TCRgammadelta+ T cells, and natural killer-type cells in human thymus. Blood 97:601–607
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102:18538–18543
Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, Manns MP, Greten TF (2003) Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer 103:205–211
Schumacher K, Haensch W, Roefzaad C, Schlag PM (2001) Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 61:3932–3936
Sivori S, Cantoni C, Parolini S, Marcenaro E, Conte R, Moretta L, Moretta A (2003) IL-21 induces both rapid maturation of human CD34+ cell precursors towards NK cells and acquisition of surface killer Ig-like receptors. Eur J Immunol 33:3439–3447
Smyth MJ, Crowe NY, Godfrey DI (2001) NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol 13:459–463
Smyth MJ, Kelly JM, Baxter AG, Korner H, Sedgwick JD (1998) An essential role for tumor necrosis factor in natural killer cell-mediated tumor rejection in the peritoneum. J Exp Med 188:1611–1619
Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y (2005) Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med 201:1973–1985
Takaki R, Hayakawa Y, Nelson A, Sivakumar PV, Hughes S, Smyth MJ, Lanier LL (2005) IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J Immunol 175:2167–2173
Toomey JA, Gays F, Foster D, Brooks CG (2003) Cytokine requirements for the growth and development of mouse NK cells in vitro. J Leukoc Biol 74:233–242
Ugai S, Shimozato O, Kawamura K, Wang YQ, Yamaguchi T, Saisho H, Sakiyama S, Tagawa M (2003) Expression of the interleukin-21 gene in murine colon carcinoma cells generates systemic immunity in the inoculated hosts. Cancer Gene Ther 10:187–192
Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, Kim G, Leonard WJ, Hwu P (2003) In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res 63:9016–9022
Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ (2005) Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med 201:139–148
Acknowledgement
We would like to thank Heidi Winther, Bodil Andreasen, Birte Jørgensen and Kirsten Meeske for technical assistance with the experiments, and Mark Smyth for valuable discussion of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Søndergaard, H., Frederiksen, K.S., Thygesen, P. et al. Interleukin 21 therapy increases the density of tumor infiltrating CD8+ T cells and inhibits the growth of syngeneic tumors. Cancer Immunol Immunother 56, 1417–1428 (2007). https://doi.org/10.1007/s00262-007-0285-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-007-0285-4