Abstract
Exosomes are small membrane vesicles originating from late endosomes and secreted by hematopoietic and epithelial cells in culture. Exosome proteic and lipid composition is unique and might shed some light into exosome biogenesis and function. Exosomes secreted from professional antigen-presenting cells (i.e., B lymphocytes and dendritic cells) are enriched in MHC class I and II complexes, costimulatory molecules, and hsp70–90 chaperones, and have therefore been more extensively studied for their immunomodulatory capacities in vitro and in vivo. This review will present the main biological features pertaining to tumor or DC-derived exosomes, will emphasize their immunostimulatory function, and will discuss their implementation in cancer immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biology of small vesicles [1, 2, 3, 4] secreted from antigen-presenting cells (APCs) recently raised a great deal of interest with the demonstration of their potent immunostimulatory functions in tumor models [5, 6]. The origin of vesicle secretion was first described by Pan and Johnstone [7], in differentiating red blood cells where multivesicular bodies (MVBs) fused with plasma membrane in an exocytic manner. This exocytic pathway was later shown to occur in a wide variety of cell types such as B lymphocytes, mastocytes, immature DCs, platelets, cytotoxic T lymphocytes, fibroblasts, epithelial cells, and tumor cells [6, 8, 9, 10, 11, 12, 13, 14, 15]. Vesicles exocytosed from MVBs into the extracellular medium are referred to as “exosomes.” Exosomes are unilamellar vesicles of 50–100 nm diameter that sequester particular proteins and lipids [9]. Exosomes secreted from professional antigen-presenting cells (i.e., B lymphocytes and dendritic cells) are enriched in MHC class I and II complexes and costimulatory molecules, and have therefore been more extensively studied for their immunomodulatory capacities in vitro and in vivo. Tumor-derived exosomes are enriched in hsp70–90 chaperones and appear to be novel sources of tumor rejection antigens for cross-presentation. This review will present the main biological features pertaining to DC-derived exosomes (DEXs) or tumor-derived exosomes (TEXs), with emphasis on their immunostimulatory properties and implementation in the immunotherapy of cancer.
Composition of exosomes
The common procedure to purify exosomes from cell culture supernatants involves a series of centrifugations designed to remove dead cells and large debris, followed by a final high-speed ultracentrifugation to pellet exosomes. Such a procedure, however, does not discriminate between exosomes as such and other small vesicular structures, or large protein aggregates. Other criteria must be used to identify exosomes.
As do all lipid vesicles, exosomes float on sucrose gradients, and their density ranges from 1.13 g/ml (for B cell–derived exosomes) to 1.19 g/ml (for intestinal cell–derived exosomes). Contaminating material, such as protein aggregates or nucleosomal fragments released by apoptotic cells, are readily separated from exosomes by flotation on sucrose gradients [16].
As analyzed by electron microscopy, exosomes present a characteristic “cup shaped” morphology: a flattened sphere limited by a bi-lipidic layer. Their size ranges between 30 and 100 nm in diameter—B cell–derived exosomes being the most homogeneous (60–80 nm). These characteristics are consistent with the observed size and morphology of internal vesicles in multivesicular endosomes [16].
The presence of known cellular proteins in exosome preparations from various cellular sources has been analyzed by Western blot or by FACS analysis of exosome-coated beads. More extensive analyses, involving MALDI-TOF/Q-TOF mass spectrometry to identify unknown or unexpected cellular proteins present in exosomes, have also been performed on dendritic cell–, mast cell–, and enterocyte-derived exosomes [16].
The available proteomic studies define a subset of cellular proteins specifically targeted to exosomes. We analyzed exosomes produced by dendritic cells of murine and human origin, and found that 80% of the proteins are conserved between the two species. Most of the proteins, or protein families, have also been described in exosomes produced by other cell types [16]. The function of most of these proteins in exosomes is as yet unknown. Importantly, these studies also showed that exosomes are clearly distinct from microvesicles produced by apoptotic cells and are only secreted by living cells.
Both ubiquitous and cell-specific proteins may be selectively targeted to exosomes. The former are most likely involved in exosome biogenesis and perhaps in some unknown common exosome functions. They include cytosolic proteins, such as tubulin, actin, and actin-binding proteins (i.e., cytoskeleton components), as well as annexins and rab proteins (involved in intracellular membrane fusions and transport). They also include molecules involved in signal transduction (protein kinases, 14-3-3, heterotrimeric G proteins). Various metabolic enzymes (peroxydases, pyruvate or lipid kinases, α-enolase) are found in exosomes from enterocytes and human dendritic cells. Exosomes also contain heat shock proteins, such as constitutive isoforms of hsp70 and hsp90. These ubiquitous proteins are involved in antigen presentation, as they may bind antigenic peptides and participate in loading on MHC molecules. MHC class I molecules are also present in exosomes from most cell types. Finally, one of the most abundant protein families found in exosomes are tetraspanins. Several members of this family, including CD9, CD63, CD81, CD82, are highly enriched in exosomes from virtually any cell type. Tetraspanins interact with multiple protein partners, including MHC molecules and integrins, suggesting that they are involved in the organization of large molecular complexes and membrane subdomains [16].
Exosomes also contain proteins involved in specific cell functions. Exosomes from antigen-presenting cells have been analysed in the most detail. MHC class II molecules are very abundant in exosomes from all cells that express them. Exosomes from dendritic cells also contain CD86, an important costimulatory molecule for T cells. The MHC ligand on T cells, the T-cell receptor, is also specifically enriched on T cell–derived exosomes. Exosomes contain a series of cell-specific transmembrane proteins, including α and β chains of integrins (αM on dendritic cells, β2 on dendritic and T cells, and α4β1 on reticulocytes), Ig family members (ICAM-1/CD54 on B cells, the A33 antigen on enterocytes, P-selectin on platelets), or cell surface peptidases (dipeptidylpeptidase IV/CD26 in enterocytes, aminopeptidase N/CD13 in mastocytes). MFG-E8/Lactadherin, a milk fat globule protein expressed by dendritic cells and some tumor cell lines, is also very abundant in exosomes produced by these cells. These proteins could direct exosomes to target cells [16].
As for the lipid composition of exosomes, very few data are so far available. The global composition of reticulocyte-derived exosomes (specifically, those derived from white cells) is similar to that of the plasma membrane of the producing cell. Presence of lyso-bis-phosphatidic acid, a lipid enriched in late endocytic compartments, has been reported on B cell–derived exosomes. Phosphatidylserine is also present, though at low levels, on the surface of exosomes from platelets and dendritic cells. Finally, internal vesicles of late endosomes and exosomes of EBV-transformed B cells are rich in cholesterol. Altogether, these studies analyzing the molecular composition of exosomes define this population of vesicles as a bona fide secreted subcellular compartment [16].
Exosomes and their potential application in immunotherapy
Active specific immunotherapy (ASI) is designed to efficiently prime antigen-specific effector T cells—in the presence of CD4+ T-helper cells —to selectively recognize and kill tumor cells in different anatomic sites and to elicit long-lasting memory responses. Because tumor cells are not optimal APCs, genetically modified tumor cell vaccines were progressively overlooked. To date, accumulating evidence points to the efficacy of maturing DCs in eliciting cytotoxic T-lymphocyte (CTL) responses associated with objective antitumor effects in patients [17].
DC-based immunotherapy, however, remains difficult to handle for scale up, definition of quality control parameters, and long-term storage. Indeed, many investigators have shown that one leukapheresis enables up to four to ten DC injections while a priming/boosting span over years appears to be required for long lasting antitumor protections. Maturation of DCs has been shown to be necessary for efficient DC migration and T c 1 differentiation in lymph nodes [18]. However, GMP cytokine cocktails required for efficient maturation of DCs need to be made broadly available and to gain worldwide acceptance for protocol standardization. Finally, biotechnological development and regulatory authority criteria encompass comprehensive biochemical or proteic characterization of therapeutic off-the-shelf products.
Exosomes might be helpful in overcoming some of the shortcomings related to technical development of DCs. Exosome composition can be defined, MHC class I and II content can be measured, and exosomal membranes, which are stable, can be stored frozen for at least 6 months. Good manufacturing procedures (GMPs) for exosome production and purification, and preclinical studies aimed at demonstrating exosome immunogenicity in vitro and in vivo will be presented, as well as the pioneering phase I clinical trial.
Production and purification of exosomes, and good manufacturing procedures
Since exosomes mediate MHC class I– and class II–restricted T-cell stimulatory activity [19, 20] and could be considered as attractive substitutes for whole DC cultures [5], GMP laboratory procedures for exosome harvesting and purification have been set up for clinical implementations [21]. Exosomes derived from dendritic cell and tumor cell culture supernatants can be readily purified within 4–5 h starting with 2–3 l of culture supernatant based on their physical properties. Ultrafiltration of the clarified culture supernatant through a 500-kDa hollow fiber membrane followed by ultracentrifugation on a 30% sucrose/deuterium oxide cushion (density 1.13 to 1.210 g/cm3) reduced the volume and protein concentration approximately 200-fold and 1,000-fold, respectively. Immunocapture assays assessing exosomal MHC class I and II molecules as well as detection of membrane exosomal proteins by flow cytometry have been validated for assessing the quality and calibration of therapeutic DEX preparations [21]. From 2×1011 to 2×1012 exosomal MHC class I molecules were reproducibly recovered in the supernatant pellets of 3×107 to 3×108 immature human monocyte–derived DC (MD-DC) culture per 24 h (from day 5 and 6 in AIM-V medium supplemented with IL-4 and GM-CSF). In metastatic melanoma patients [22] (Fig. 1A), 1014 to 1015 exosomal MHC class II molecules can be purified from MD-DC cultures (5×108 to 5×109 cells) propagated from a single leukapheresis. Immunostimulatory properties of DEXs were extensively evaluated next, after a loading procedure of exosomal MHC class I molecules with molecularly defined peptides [22, 23]. However, exosomal MHC class II molecules can be indirectly loaded after pulsing of class II–restricted peptides onto MD-DC cultures [20].
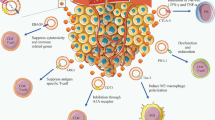
Novel approach toward immunotherapy. A Active “vaccination” using DEX vaccine: after one leukapheresis of melanoma patients, DEXs are obtained as already described. The MHC class I can be directly loaded with tumor peptides and reinjected (with or without adjuvants, such as Ampligen or ODN CpG) into patients. B Active “vaccination” using ExAs: ExAs are obtained from ascitis of patients bearing an ovarian cancer, after ultracentrifugation and purification on a sucrose/D2O cushion, and can be reinjected with adjuvant (e.g., Ampligen). Adoptive transfer of tumor-specific CTLs: ExAs pulsed onto DCs can be used in in vitro stimulation (IVS) to expand tumor-specific CTLs. Then, these CTLs can be reinfused into patients
Preclinical and clinical data
DEXs bear functional MHC class I and class II peptide complexes
In our preclinical studies, we found evidence that (1) DCs produce exosome-associated MHC class I complexes that are functional for CTL priming, (2) exosomes require mature DCs for T-cell activation in vitro and in vivo, and (3) exosomes pulsed onto mature DCs exhibit efficacy comparable to mature DCs in initiating peptide-specific CD8+ T-cell responses in vivo. Therefore, the design of an efficient exosome-based cancer vaccine should require an adjuvant that optimally mimics or substitutes for mature DCs in vivo. Indeed, we showed that exosome-mediated CD8+ T-cell priming can be triggered using ligands for TLR-3 or 9 such as ODN CpG oligomeric sequences or double stranded RNA. Most of the clinically available alternative adjuvants that do not directly activate DCs in vivo, cannot boost exosome immunogenicity in the HLA-A2 transgenic HHD2 mouse model investigating elicitation of specific A2/MART-1–restricted CTLs. We established in the melanoma B16F10 tumor model coexpressing human HLA-A2 and the gp100 tumor antigen that 1010 exosomal MHC class I–gp100 complexes admixed with CpG ODN mediate tumor rejection as efficiently as 3×105 mature DC-A2/gp100. Therefore, exosomes are valuable cell free peptide vaccines [19].
TEXs mediate tumor antigen cross-presentation by DCs to T cells
Ideally, immunotherapy strategies aimed at immunizing the host should be able to elicit T-cell–based immune responses directed against a broad repertoire of tumor rejection antigens. While mature DCs appear to be the most potent natural adjuvants, suitable protocols leading to efficient DC uptake, processing, and cross-presentation in association with MHC class I molecules, are still awaited. Several approaches involving the use of whole tumor RNA, tumor lysates, apoptotic or necrotic debris and fusion are currently under investigation. We reported that (1) melanoma cell line–derived TEXs contain tumor differentiation antigens, (2) TEXs loaded onto dendritic cells transfer shared tumor antigens generating MHC class I–restricted T clones in vitro, and (3) TEXs are a source of tumor rejection antigens since tumor exosomes promote T cell–dependent cross-protection against syngeneic and allogeneic tumors in mice [6].
Immunogenicity of peritoneal ascitis-derived exosomes (ExAs)
TEXs are not simply released in vitro by tumor cell lines in culture supernatants. We went on examining malignant effusions for the presence of exosomes and analyzed their immunogenicity on autologous peripheral T lymphocytes. Ultracentrifugation on sucrose and D2O gradients of 11 malignant effusions allowed isolation of abundant amounts of exosomal vesicules, i.e., ExAs. Malignant effusions accumulate high amounts of membrane vesicles with a mean diameter of 60–90 nm. These vesicles bear antigen-presenting molecules (MHC class I ± MHC class II, heat shock proteins) and tetraspanins (CD81), and they contain several tumor antigens (HER-2/neu, MART-1, TRP-1, gp100). Up to 2×1014 exosome-associated MHC class I molecules are recovered from malignant ascitis of 2–3 l. Exosomes from melanoma patients transport MART-1 tumor antigens to MD-DC for cross-presentation to MART-1–specific CTL clones. In 7/9 cancer patients, tumor-specific lymphocytes could be efficiently expanded from peripheral blood cells using autologous MD-DCs pulsed with autologous ExAs [4]. Although the physiopathological conditions in which exosomes accumulate abundantly in cancer patients remain unclear, ExAs represent a natural and novel source of tumor rejection antigens, opening up novel immunization avenues in advanced ovarian cancers or mesothelioma.
Exosome-based vaccines in metastatic melanoma patients
The well-defined molecular composition and unique immunogenic properties of DEXs, as well as the availability of GMP, allowed clinical trials using DEXs in metastatic tumor–bearing patients. A first feasibility and safety phase I study has been completed in France (Institut Gustave Roussy and Institut Curie, with the support of Anosys). Fifteen advanced stage III/IV melanoma patients bearing a tumor expressing the MAGE-3 antigen were included [21, 22]. DEXs were purified from the culture supernatant of day 7 autologous MD-DCs. MAGE-3 peptides (HLA-A1/B35 and –DP04-restricted) were loaded onto MD-DCs (in the first 6 patients), or directly onto DEXs (in 9 patients). Escalating doses of cryopreserved DEXs were administrated by four weekly s.c./i.d. injections for 4 weeks, and then every 3 weeks in patients who achieved stable disease or tumor regression. Feasibility of exosomes production was achieved in all patients with a range of 6–120 vaccine doses. The therapy schedule was well tolerated, without any toxicity over grade 2 (NCI-CTC). Using an indirect loading process, one stage III patient achieved stabilization and received eight additional vaccine injections with long-lasting disease control. Using direct loading but low exosome dosage, a patient achieved a regression of subcutaneous sites but lung metastasis progression also resulted. In the same group, a second patient exhibited partial response at node sites. Using direct loading and high exosome dosage, 3/6 patients exhibited objective responses (skin and lymph node lesions). It is noteworthy that 2 patients with a previous MAGE-3 vaccination were responding in this study [22, 23].
Conclusion and prospects
Exosomes are currently defined by their morphology (electronic microscopy), by their physical properties (stable at high temperature; floating at a density of 1.13 to 1.210 g/ml in a sucrose/D2O gradient), by their proteic patterns (endocytic markers, i.e., tetraspanins and hsp73), and by their enrichment in MHC class I and II, and CD86 molecules when derived from DCs. Importantly, exosomes efficiently transfer antigens from APCs to APCs, allowing initiation and amplification of antigen-specific immune responses. Extensive studies have demonstrated the relevance of exosomes in cancer immunotherapy strategies. To be able to trigger CTL responses, exosomes require mature DCs. In vivo, the use of adjuvants such as ODN CpG or double stranded RNA (Ampligen) combined with exosomes allows for the initiation of an efficient T c 1 response. These preclinical studies represent a rationale for future phase I/II trials using exosomes admixed with adjuvants, as well as novel strategies for active or adoptive immunotherapy using DEXs and TEXs (Fig. 1A, B). The identification of the mechanisms accounting for the transfer of MHC class I or class II complexes onto DCs could be probed by studies preformed in knockout mice or search for specific neutralizing Ab-recognizing exosomes. The characterization of the exosome-presenting cell in vivo might shed some light on the relevant biological functions of exosomes in vivo.
Abbreviations
- APC:
-
antigen-presenting cell
- ASI:
-
active specific immunotherapy
- CTL:
-
cytotoxic T lymphocyte
- DC:
-
dendritic cell
- FDC:
-
follicular dendritic cell
- MD-DC:
-
monocyte-derived dendritic cell
- GMP:
-
good manufacturing procedure
- HLA:
-
human leukocyte antigen
- HSP:
-
heat shock protein
- MHC:
-
major histocompatibility complex
- MVB:
-
multivesicular body
- ExAs:
-
ascitis-derived exosomes
- DEX:
-
DC-derived exosome
- TEX:
-
tumor cell–derived exosome
References
Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ (2000) Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113:3365–3374
Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ (2000) Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol 165:1259–1265
Quah B, O’Neill HC (2000) The application of dendritic cell-derived exosomes in tumour immunotherpy. Cancer Biother Radiopharm 15:185–194
André F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L (2002) Malignant effusions and immunogenic tumor derived-exosomes. Lancet 360:295–305
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4:594–600
Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7:297–303
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation: association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420
Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C (1997) Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell 8:2631–2645
Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ (1998) Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 273:20121–20127
Arnold PY, Mannie MD (1999) Vesicles bearing MHC class II molecules mediate transfer of antigen-presenting cells to CD4+ cells. Eur J Immunol 29:1363–1373
Heijnen IA, van Vugt MJ, Fanger NA, Graziano RF, de Wit TP, Hofhuis FM, Guyre PM, Capel PJ, Verbeek JS, van de Winkel JG (1996) Antigen targeting to myeloid-specific human Fc gamma RI/CD64 triggers enhanced antibody responses in transgenic mice. J Clin Investig 97:331–338
Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA (1999) Ectosomes released by human neutrophils are specialized functional units. J Immunol 163:4564–4573
Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD (1999) Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J Immunol 163:5201–5210
Geminard C, Nault F, Johnstone RM, Vidal M (2001) Characteristics of the Interaction between Hsc70 and the transferrin receptor in exosomes released during reticulocyte maturation. J Biol Chem 276:9910–9916
Skokos D, Le Panse S, Villa I, Rousselle JC, Peronet R, David B, Namane A, Mecheri S (2001) Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J Immunol 166:868–876
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579
Yang S, Kittlesen D, Slingluff CL Jr, Vervaert CE, Seigler HF, Darrow TL (2000) Dendritic cells infected with a vaccinia vector carrying the human gp100 gene simultaneously present multiple specificities and elicit high-affinity T cells reactive to multiple epitopes and restricted by HLA-A2 and -A3. J Immunol 164(8):4204–4211
Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N (2001) Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 193:233–238
Chaput N, André F, Schartz NEC, Escudier B, Turz T, Angevin E, Zitvogel L (2003) Proc Am Assoc Cancer Res 44:A4746
Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S (2002) Exosomes activate naive CD4+ T cells by transfer of MHC/peptide complexes to dendritic cells. Nat Immunol 3:1156–1162
Lamparski H, Metha-Damani A, Yao J, Patel S, Hsu D, Ruegg C, Le Pecq J (2002) Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Meth 270(2):211
H. Pêche (2001) Club Francophone Des Cellules Dendritiques, p 31 (Abstract C08)
E. Angevin (2002) 7th International Symposium on Dendritic Cells, p 28 (Abstract 20)
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented at the first Cancer Immunology and Immunotherapy Summer School, 8–13 September 2003, Ionian Village, Bartholomeio, Peloponnese, Greece.
Rights and permissions
About this article
Cite this article
Chaput, N., Taïeb, J., Schartz, N.E.C. et al. Exosome-based immunotherapy. Cancer Immunol Immunother 53, 234–239 (2004). https://doi.org/10.1007/s00262-003-0472-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-003-0472-x