Abstract
Total neoadjuvant therapy (TNT), which includes chemotherapy and radiation prior to surgical resection, has been recently accepted as the new standard of care for patients with locally advanced low and mid rectal cancers. Multiple clinical trials have evaluated this approach in the last several decades and demonstrated improvement in, local control and reduced risk of recurrence. In addition, in the course of these investigations, it has been shown that between a third and a half of patients experience a clinical complete response (cCR) after being treated with the TNT approach, leading to the development of new organ preservation protocol, now known as watch-and-wait (W&W). On this protocol, cCR patients are not referred for surgery after total neoadjuvant treatment. Instead, they remain on close surveillance and, thus, avoid potential complications associated with surgical resection. Multiple clinical trials are ongoing, investigating the long-term outcomes of these new approaches and the development of less toxic and more effective TNT regimens for LARC. Improvements in technology and rectal MRI protocols position radiologists as vital members of multidisciplinary rectal cancer management teams. Rectal MRI has become a critical tool for rectal cancer initial staging, treatment response assessment, and surveillance on W&W protocols. In this review, we summarize the findings of the pivotal clinical trials that contributed to establishing the current treatment paradigms in locally advanced rectal cancer (LARC) management, with the intention of helping radiologists play more effective roles in their multidisciplinary teams.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last several decades the treatment of LARC, which broadly includes T3-T4 tumors, involved mesorectal fascia (MRF), node positive (N +) disease, or extramural vascular invasion, (EMVI) has been rapidly evolving. At present, surgical resection remains the main curative therapy for patients with LARC. Total mesorectal excision (TME) is the main surgical technique utilized in rectal cancer resection. Introduction of total neoadjuvant therapy (TNT) has brought to the forefront the possibility of non-operative management (NOM) with organ preservation, now known as watch-and-wait (W&W) approach. Numerous trials were conducted (with many are still ongoing) to elucidate optimal TNT regimens and evaluate outcomes of the W&W approach. Along with developments in clinical practice, the increasing use of rectal MRI for rectal cancer initial staging and treatment response assessment has positioned radiologists as integral members of rectal cancer management teams. In this concise review, we will summarize the findings of the key clinical trials in the last several decades (some completed, others ongoing), which shaped the current and evolving paradigms in rectal cancer treatment. It is very important for radiologists who interpret rectal MRIs in their practice to be familiar with the outcomes of these trials, as an understanding of current treatment paradigms allows radiologists to tailor their reports to the appropriate clinical context. Furthermore, rectal MRI has become critical for success of surveillance protocols in rectal cancer patients on NOM. We hope that this review will provide a useful reference for radiologists and better position them as members of multidisciplinary disease management teams. The review focuses on clinical trials pertaining to the following topics: the selective MRI-based application of neoadjuvant therapy; evolving treatment approaches to lateral pelvic nodes, the establishment of total neoadjuvant therapy; and the emergence of the watch-and-wait strategy.
Avoidance of overtreatment of rectal cancer by neoadjuvant chemotherapy: MERCURY, OCUM, & QuickSilver
The use of neoadjuvant chemoradiation (nCRT) in the treatment of rectal cancer is associated with an impressive 50% reduction of local recurrence (LR). However, nCRT has little or no impact on overall survival (OS) and is associated with compromised bowel and sexual function, along with an increased risk of a second malignancy [1,2,3]. The current guidelines for determining the need for nCRT in rectal cancer differ between the United States and Europe. In the United States, any stage II or III tumor (i.e., T3–4 N0, M0 or T1-4 N + M0) requires nCRT. In contrast, the more nuanced European approach bases treatment decisions on risk profiles derived from rectal MRI findings [4]. Although the T and N categories remain criteria for this risk categorization, there has been a shift to focusing on anatomic criteria that predict the risk of a positive surgical margin for the purposes of determining the need for nCRT. This concept was originally proposed by the MERCURY study group in 2011. In this study, 374 patients were triaged directly to surgery if categorized via rectal MRI as “good prognosis” stage II and III tumors, defined as tumor > 1 mm from the MRF, no evidence of EMVI, and < 5 mm tumor infiltration beyond the muscularis propria. The N category was completely disregarded as a criterion for determining the need for nCRT, a significant departure from the NCCN guidelines. Impressively, this study reported a 5-year LR rate of only 3% for these “good prognosis” stage II/III tumors without the use of nCRT [5]. There have been two more recent multi-institutional studies in Germany and Canada that have reproduces this approach with similar results, further challenging the NCCN guidelines [6, 7]. Note that the rectal MRI criteria for “good prognosis” stage II/III tumors were slightly different across these three studies (Table Table 1). However, the assessment of the relationship of the tumor to the anticipated surgical resection margin, a key factor in predicting LR, was a consistent theme.
It is important to recognize that the patient selection approach in all these studies relies on high-quality rectal MRI techniques and interpretations, excellent surgical technique, a standardized assessment of the pathological specimen, and multidisciplinary review of all cases prior to treatment. A significant factor in this changing approach to nCRT is the recognition of certain limitations in rectal MRI for determining the initial T stage and N stage. For example, a population-based cancer registry data review for colorectal cancer found that the proportion of patients deemed to have N + disease increased from 7 to 53% over the 10 year period, whereas the incidence of pathologic N + rectal cancer remained stable at 33% [9]. This result highlights a significant over-staging of nodal involvement that paralleled the increase in the use of rectal MRI for the preoperative staging of rectal cancer.
A recent analysis of the MRI findings of 609 patients in a multi-institutional study found correct T-staging in only 64% of patients, with over-staging in 23% patients [10]. The accuracy of nodal staging, as expected, was exceedingly low and no better than a “flip of a coin” at 57%, with no difference in accuracy between “high and low volume” centers. In contrast, the accuracy of T-staging was slightly higher at 67% at high volume centers compared to 62% at low volume, reflecting some benefit from experience. Overall, 50.3% stage II/III rectal cancers based on rectal MRI were found to have stage I disease at histopathology, reflecting significant over-staging and consequently overtreatment [10].
The multi-institutional prospective observational OCUM trial (Optimierte Chirugie Und MRT) completed recruitment in 2016 [10, 11]. The goal of the study was the avoidance of overtreatment of rectal cancer by the selective administration of nCRT to stage II and III tumors with poor prognostic features, defined as tumor < 1 mm from the MRF, cT4 tumor, or any cT3 tumor in the lower third of the rectum. Patients with these features underwent nCRT followed by surgical resection; patients without these features went directly to surgical resection. The primary endpoint of the study was LR at 3 years.
A total of 878 patients were treated according to the OCUM study protocol, with 526 patients (60%) going directly to surgery. There was no difference in 3-year LR rates between the TME alone (LR 3.1%) or TME + nCRT (LR 3.9%) treatment arms. In this study, using “good prognostic MR criteria,” 40% of clinical stage II/III rectal cancers were able to avoid nCRT with no impact on oncologic outcomes [6, 11].
QuickSilver was a 2-year phase II nonrandomized prospective study conducted by the Rectal Cancer Alliance of Canada (RCAC) that assessed whether “good prognosis” stage II and II rectal cancers (based on rectal MRI criteria) could safely avoid nCRT. The inclusion criteria were tumor > 1 mm from MRF; T2 disease or T3 disease with extension < 5 mm beyond rectal wall; and absent or equivocal EMVI. Similar to the MERCURY study, node status was not considered in patient selection in this trial due to aforementioned limitations in the accuracy of rectal MRI for rectal cancer N-staging. Only patients scheduled for low anterior resection (LAR) were included in the study. The primary end-point was a positive pathologic circumferential resection margin (CRM), defined as tumor, tumor deposit or metastatic lymph node < 1 mm from the CRM; secondary end-points were LR rates at 2 years and disease-free survival [7]. A total of 82 rectal MRI-based “good prognosis” stage II/III patients accrued to QuickSilver between 2014 and 2016. The majority were mid-rectal tumors (65%), stage T2/T3a (60%), with no suspicious nodes (63%). Most importantly, the CRM was positive on surgical pathology in only 4 of 82 cases (4.2%). [12].
The MERCURY, OCUM, and QuickSilver studies suggest that the administration of nCRT to all stage II and III rectal tumors results in significant over-treatment, largely because of T and N over-staging by rectal MRI. However, rectal MRI can be used accurately to define “good prognosis” tumors at low risk for LR due to its high negative predictive value for CRM involvement. The oncologic outcomes have proven the efficacy of this approach with CRM positivity rates of 5% in the QuickSilver study, 3% in the MERCURY study, and 2% in the OCUM study. The 5-year LR rates in OCUM and MERCURY studies were 2.7% and 3.3%, respectively. These findings support a more selective use for nCRT in stage II and III rectal tumors.
In summary, rectal MRI results in significant T and N over-staging, the consequence of which is over-treatment of rectal cancers with nCRT. However, rectal MRI has a high accuracy in excluding CRM involvement by tumor and, consequently, in determining the likelihood of LR. The lack of CRM involvement, along with a few additional rectal MRI-based criteria, have been used to define “good prognosis” stage II/III tumors that can be spared nCRT and its potential side effects. Three prospective studies have shown that this strategy can be implemented without adversely affecting oncologic outcomes.
Lateral pelvic lymph nodes (LPLNs) in rectal cancer: an evolving treatment paradigm
There has been significant debate over the optimal approach to the management of LPLN metastasis. In the east, LPLN dissection (LPLND) for all T3/4 rectal tumors below the peritoneal reflection has been the preferred approach while in the west, neoadjuvant chemoradiation has been used to sterilize metastatic LPLNs [13, 14]. However, there are limitations to both approaches, and we review recent randomized retrospective multi-institutional trials that have suggested the solution may lie in careful preselection of patients at high risk for LPLN metastases based on MRI criteria and a hybrid treatment approach combining chemoradiation and surgery [15].
Japanese Clinical Oncology Group study (JCOG 0212) Multicenter, randomized, controlled, non-inferiority trial
A multicenter randomized trial in 33 institutions across Japan for stage II/III rectal cancer with the lower edge located below the peritoneal reflection attempted to confirm the noninferiority of TME to TME + LPLND [16]. The primary end point was relapse-free survival (RFS) with secondary endpoints of overall survival (OS) and local recurrence free survival (LRFS). Patients with clinical determination of LPLN involvement were not included in the study. Inclusion criteria were based on the clinical stage and location (low rectal tumors) of the primary rectal tumor. If LPLN measures > 10 mm (short axis) on imaging or LPLNs were present on macroscopic assessment intraoperatively during TME, these patients were excluded. A total of 351 patients were randomized to the TME + LPLN dissection arm and 350 to TME alone between 2003 and 2010. The preliminary assessment of the data showed that the addition of LPLN dissection was associated with significantly longer operative times and blood loss (p < 0.0001). The incidence of postoperative complications, however, was not significantly different. Importantly in this study the yield of metastatic LPLN was only 7% as no preselection of clinically involved LPLN was attempted [15]. Analysis of the data at 5 years could not confirm the non-inferiority of TME compared with TME + LPLN dissection. The 5-year RFS were comparable in the two groups at 73.4% for TME + LPLND and 73.3% for TME alone. Similarly, 5-year OS and local recurrence (LR) free survival were 92.6% and 90.2% and 87.7% and 82.4%. However, the cumulative LR rate in the TME + LPLND group of 7.4% was lower than the 12.6% recurrence seen in TME group largely related to fewer recurrence in the lateral pelvis [16].
Recently the data at 7-year follow-up from this randomized trial was published and showed similar RFS rates between the two groups and consequently could not confirm the non-inferiority of TME compared to TME + LPLND. However, a subgroup analysis showed improved RFS with stage III particularly stage III b/c undergoing TME + LPLND but not in stage II and IIIa. In addition, although the RFS rates were comparable between the two groups the cumulative LR rate was significantly lower in the TME + LPLND group. Since this trial focused on rectal tumors without enlarged LPLNs, the results cannot be extrapolated to patients with enlarged LPLNs prior to surgery. In conclusion LPLND cannot be recommended in all stage II/III low rectal cancers, and preselection of patients with a higher incidence of LPLN metastasis is required [17].
Lateral Node Study Consortium
The use of TME + nCRT in rectal cancer has been found to have low overall LR rates (5.8%) that are similar to patients treated with TME + LPLND (overall LR rate of 6.9%) [18]. Interestingly overall, the LR rates in the lateral compartment were also similar between the two treatment approaches [18]. However, in a small subgroup of patients treated with TME + nCRT, the lateral LR rate was noted to be substantially higher with up to 50% of patients developing lateral compartment LR. These patients all had enlarged LPLNs seen on pretreatment MRI that persisted on posttreatment MRI and the incidence of lateral LR was seen to co-relate with nodal size on baseline MRI [19].
The Lateral Node Study Consortium comprising 12 hospitals in seven countries undertook a retrospective study from 2009 to 2013 that attempted to address the question of defining MRI nodal size criteria on pretreatment MRI scans to identify patients who would most benefit from LPLN dissection after nCRT. The study enrolled 1216 patients with T3/4 rectal tumors < 8 cm from the anal verge. 703 had visible nodes on the pre-treatment MRI and 192 (16%) had LPLN with a short axis of 7 mm. The study concluded that if 7 mm nodes were present on the baseline MRI then there was a significantly higher risk of lateral LR of 19.5%, (hazard ratio, 2.060; p = 0.045), after nCRT compared to patients with nodes < 7 mm. Importantly there was no difference in distant recurrence and cancer-specific survival among patients with LPLN > 7 mm or smaller LPLN [20].
Overall, the study concluded that enlarged LPLNs seen on baseline staging MRI pose a significant risk for lateral LR irrespective of the exact cutoff used. Furthermore, LPLN enlargement does not impact distant recurrence rates suggesting they represent local disease and should be addressed by targeted therapy to the pelvis. The LPLN dissection should not be limited to the enlarged node and removal of the contents of the entire nodal compartment is needed for improved outcomes [20].
Japan Clinical Oncology Group Study JCOG1310 (PRECIOUS study)
Rectal tumors with LPLN metastases are associated with a dismal 5-year OS of 40% similar to N2a mesorectal adenopathy but superior to stage IV disease [21]. The current treatment for rectal tumors with LPLN metastases in Japan relies on TME + LPLND followed by adjuvant chemotherapy with 6 cycles of FOLFOX but new treatment options are needed to improve survival. This randomized trial was based on the hypotheses that preoperative chemotherapy may prevent dissemination of micro metastasis improve compliance with chemotherapy and survival. JCOG 1310 was a multicenter randomized open label phase II/III trial in rectal tumors below the peritoneal reflection, LPLN > 10 mm in short axis, T2-T4 with no distant metastases. Eligible patients were randomized (1:1) to the post-operative or perioperative chemotherapy arms. The primary end point was OS. The study was terminated early in 2019 due to poor patient accrual. A total of 48 patients were enrolled and randomized to postoperative FOLFOX6 (n = 26) or the perioperative FOLFOX6 (n = 22) arm. The 3-year OS was 66.1% in the postoperative arm and 84.4% in the perioperative arm. However, this achieved no statistical significance due poor patient accrual. It was noted that pathologic complete response was 9.1% in the perioperative arm and more patients completed chemotherapy with higher doses. The perioperative arm also reported higher grade 3 postoperative complications. The limited conclusion of this abbreviated trial was that perioperative FOLFOX6 may be insufficient to improve survival in low rectal tumors with LPLN metastases and more intensive regimens may be required.
In conclusion, there appears to be convergence of approach to LPLN metastases in low rectal cancer. In Japan, there is a recognition that LPLND in all low rectal cancers maybe an overtreatment. While in the West, surgeons are re-examining the notion that LPLN metastases can be treated with TME and nCRT as a subgroup of these patients will recur in the lateral compartment. It is evident that selective LPLND in patients with enlarged LPLN on MRI should be the preferred approach even after nCRT. The data from the Lateral Node Study Consortium provides some insight into the imaging criteria that should be used to identify these patients at higher risk for lateral recurrence, but an international consensus is required.
Total neoadjuvant therapy: PRODIGE-23, RAPIDO, and STELLAR trials
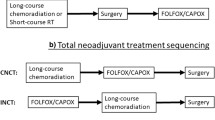
The introduction of neoadjuvant chemotherapy and radiotherapy before TME has significantly influenced treatment of rectal cancer in the last several decades. Total neoadjuvant therapy (TNT) is a relatively new treatment approach for mid to low LARC, which involves administering both systemic chemotherapy and neoadjuvant chemoradiotherapy prior to [20]surgery. A number of randomized trials, including PRODIGE-23, RAPIDO, and STELLAR TRIALS, have addressed the outcomes of this approach.
PRODIGE-23: neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer
PRODIGE 23 was a multicenter (35 hospitals in France) phase 3, randomized trial (NCT01804790) that evaluated whether administration of neoadjuvant therapy before CRT (TNT) would reduce the chances of distant metastatic disease [22]. This trial included patients with biopsy proven rectal adenocarcinoma (cT3 or cT4, M0), who were randomized to a neoadjuvant chemotherapy group (n = 231) or a standard-of-care group (n = 230) between June 5, 2012, and June 26, 2017. The regimen for the TNT group consisted of FOLFIRINOX (oxaliplatin, irinotecan, leucovorin, fluorouracil) intravenously (every 14 days for 6 cycles), chemoradiotherapy (50 Gy over 5 weeks with oral capecitabine twice daily for 5 days per week), and TME. After TME, the TNT group received adjuvant chemotherapy, consisting of 3 months of modified FOLFOX6 (oxaliplatin, leucovorin, fluorouracil, or capecitabine). The standard-of-care group was treated with chemoradiotherapy (50 Gy over 5 weeks with oral capecitabine twice daily for 5 days per week), TME, and adjuvant chemotherapy (modified FOLFOX6) for 6 months. Disease-free survival (DFS) was the primary endpoint. 3-year DFS rates were 76% and 69% in the TNT group and in the standard-of-care group, respectively (p = 0.03). Longer follow-up may reveal improved OS with TNT, but that updated analysis is scheduled for a median of 5 years follow-up. Safety analyses were done on treated patients. Among the documented side effects, one of the more significant findings was decreased peripheral sensory neuropathy to 12% with TNT versus 21% with the standard of-care regime, respectively. PRODIGE 23 demonstrated that induction chemotherapy with FOLFIRINOX before CRT and surgery significantly improved DFS in patients with cT3-4M0 rectal cancer and was better tolerated than the conventional adjuvant chemotherapy regimen. Equally importantly, pathological findings following TME demonstrated increased rates of pathological complete response in the TNT group (28%) relative to the standard-of-care group (12%). Thus, PRODIGE 23 paved the way for systemic chemotherapy to earlier in the treatment sequence, facilitating acceptance of TNT into clinical practice. This study also laid the groundwork for future studies addressing NOM with organ preservation in patients treated with TNT.
RAPIDO: rectal cancer and preoperative induction therapy followed by dedicated operation
The RAPIDO trial was a multicenter, open-label, randomized, controlled, phase 3 trial in Europe and the United States designed to reduce distant metastases without compromising locoregional control by using short-course radiotherapy followed by chemotherapy and delayed surgery [23]. Patients with newly diagnosed LARC classified as high-risk on rectal MRI (stage cT4a-b, EMVI, stage cN2, MRF involvement, or enlarged lateral lymph nodes) were randomly assigned (1:1) to either the experimental (n = 462) or standard-of-care group (n = 450). The experimental group received short-course radiotherapy (25 Gy over a maximum of 8 days), followed by six cycles of CAPOX or nine cycles of FOLFOX4 and TME. Patients in the standard-of-care group received 25–28 fractions up to 50.0–50.4 Gy, with concomitant oral capecitabine followed by TME and adjuvant chemotherapy with eight cycles of CAPOX or 12 cycles of FOLFOX4. 3-year disease-related treatment failure was the primary endpoint. Treatment failure was defined as the first occurrence of locoregional failure, distant metastases, new primary colorectal tumor, or treatment-related death. At three years, the cumulative probability of disease-related treatment failure was 23.7% in the experimental group and 30.4% in the standard-of-care group. This finding is interesting in that pathological complete response rate twice as high in the experimental group (28% vs 14%), but locoregional failures were not significantly different between the two groups, implying that the experimental regimen increased response rates. Further, cumulative probability of distant metastases at 3 years was significantly reduced in the experimental group (20% versus 26.8%, p = 0.0048) [23].
Serious adverse events occurred in 38% of the experimental group and in 34% of the standard-of-care group. Treatment-related deaths happened in four patients in each of the groups. The trial concluded that the decreased probability of disease-related treatment failure in the experimental group suggests increased efficacy of preoperative chemotherapy compared with adjuvant chemotherapy, such that preoperative chemotherapy can be considered a new standard-of-care in high-risk LARC. In addition, patient follow-up is still ongoing to evaluate the validity of the initial premise that micrometastases are more effectively treated with the neoadjuvant approach, with a resultant increase in disease-free survival.
STELLAR: short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer
Driven by the results of the PRODIGE 23 and RAPIDO trials, with the intention of improving patient compliance and reducing the side-effects of radiation therapy without compromising outcomes, the STELLAR trial was designed as a multicenter, open-label, randomized phase III study in China to determine how TNT using short-course preoperative radiotherapy followed by chemotherapy compares to standard long-course chemoradiotherapy (CRT) in patients with LARC [24]. Rectal MRI was used as a standard assessment tool for all patients to establish the degree of locoregional involvement. Patients with LARC stage T3-4 (distal or middle-third rectum) and/or regional lymph node-positive rectal cancer patients were randomly assigned (1:1) to the total neoadjuvant therapy (TNT) group or conventional chemoradiotherapy group (CRT). The TNT group received short-course radiotherapy consisting of 25 Gy in five fractions over one week, followed by four cycles of chemotherapy (n = 302). The CRT group received 50 Gy in 25 fractions over five weeks, concurrently with capecitabine (n = 297). After preoperative treatment, TME was performed at 6–8 weeks. Patients in the TNT group received two adjuvant cycles of CAPOX, whereas patients in the CRT group received six adjuvant cycles of CAPOX. 3-year DFS was the primary endpoint with 64.5% in TNT group and 62.3% in CRT groups, with no significant difference in metastasis-free survival or locoregional recurrence. 3-year overall survival was better in the TNT group than the CRT group (86.5% v 75.1%; P = 0.033). The prevalence of acute toxicities during preoperative treatment was 26.5% in the TNT group and 12.6% in the CRT group (p = 0.001). Moreover, the total rates of pathologic complete response (pCR) and sustained cCR were 21.8% and 12.3% for the TNT and CRT groups, respectively (p = 0.002). The study concluded that short-course radiotherapy with preoperative chemotherapy followed by TME was effective with acceptable toxicity and could be used as an alternative to conventional CRT for LARC.
Watch-and-wait trials: OPRA, CAO/ARO/AIO-12, Cercek et al., NORWAIT, STAR-TREC, IWWD
OPRA: organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy
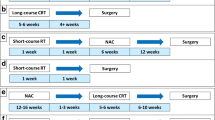
OPRA was a prospective randomized phase II clinical trial of 324 patients with stage II or III rectal adenocarcinoma (staged with rectal MRI; full colonoscopy; and CT of the chest, abdomen, and pelvis) were treated with one of two different TNT regimens and then directed either to TME or a W&W protocol based on the tumor response (NCT02008656) [25]. Patients received either induction chemotherapy followed by CRT (INCT-CRT) or CRT followed by consolidation chemotherapy (CRT-CNCT). Chemoradiotherapy in both groups consisted of 4 months of fluorouracil-leucovorin-oxaliplatin or capecitabine-oxaliplatin and 50–56 Gy of radiation, combined with either continuous infusion fluorouracil or capecitabine during radiotherapy. Patients achieving a cCR were offered a W&W protocol which included digital rectal examination, flexible sigmoidoscopy at scheduled intervals and rectal MRI every 6 months for the first 2 years and yearly for the next 3 years. Patients not achieving a cCR were offered surgery. No adjuvant chemotherapy was administered after surgical resection. DFS was the primary endpoint, and TME-free survival was the secondary endpoint. There was no difference in DFS (76%) between the two treatment groups at the median follow-up of 3 years. Organ preservation or TME-free survival at 3 years was 41% for the INCT-CRT group and 53% for the CRT-CNCT group. The higher rate of tumor regrowth in the INCT-CRT group (40%) versus the CRT-CNCT group (27%) might be at least partially attributable to differences in organ preservation between the two groups. Additional analyses demonstrated no differences in LR-free survival, distant metastases-free survival, or overall survival. Importantly, similar DFS rates were observed between the patients who underwent TME after restaging (due to lack of cCR) and patients who underwent TME after regrowth.
CAO/ARO/AIO-12
Based on the above-described PRODIGE 23 and RAPIDO trials, two main TNT regimens have been established: (1) induction chemotherapy followed by CRT and (2) CRT followed by consolidation chemotherapy (with differences in the duration of radiation therapy, as well). However, the optimal sequence of chemotherapy and CRT remained unclear, although in the OPRA trial there was no difference 3-year DFS among patients treated with induction versus consolidation chemotherapy [26]. The CAO/ARO/AIO-12 was a multicenter, randomized, phase II trial designed to determine the superiority of induction versus consolidation TNT regimens [27, 28]. Patients were randomized to either the induction group (n = 156; neoadjuvant chemotherapy prior to CRT) or to the consolidation group (n = 150; neoadjuvant chemotherapy after CRT). For both groups, chemotherapy consisted of three cycles of fluorouracil, leucovorin, and oxaliplatin, and CRT consisted of fluorouracil/oxaliplatin concurrent with radiation therapy (50.4 Gy). No increase in surgical morbidity was observed in the consolidation group, despite a longer interval between CRT completion and surgery.
In the original analysis, pathologic complete response was 17% in the induction group versus 25% in the consolidation group [27]. At the long-term follow-up analysis with median follow-up of 43 months (range, 35–60 months), there was no difference in DFS (73% in both groups) [28]. Additional metrics, including locoregional recurrence at 3 years (6% vs 5%, p = 0.67) and distant metastases (18% vs 16%, p = 0.52), did not demonstrate any significant difference between groups. Thus, the dilemma of induction versus consolidation chemotherapy in TNT regimens has not been conclusively resolved as based on this trial both approaches were essentially equivalent.
Adoption of total neoadjuvant therapy for locally advanced rectal cancer.
In a large cohort study of 811 patients, Cercek et al. examined potential benefits of TNT instead of conventional neoadjuvant CRT with planned adjuvant chemotherapy [29]. Patients in the TNT group received greater percentages of the planned oxaliplatin and fluorouracil dose than patients in the neoadjuvant CRT with planned adjuvant chemotherapy group, supporting the notion that patient compliance rates are higher in TNT regimens. The CR rate (including pathologic CR for patients who underwent surgery and cCR at least 12 months post-treatment in patients who did not undergo surgery) was 36% in the TNT group, compared with 21% in the neoadjuvant CRT with planned adjuvant chemotherapy cohort. These findings supported TNT as a viable treatment approach in rectal cancer and provided an additional pillar of support for the subsequent acceptance of W&W strategy. Furthermore, this study has led investigators to address several other questions via clinical trials. Should we use TNT to enhance the likelihood of organ preservation and include early disease, such as in the GRECCAR 12 trial, which proposed TNT for T2-3 N0-1 tumors (size less than 4 cm) [30]? Should we use TNT to stratify patients like in GRECCAR 4 and in the ongoing GRECCAR 16 trials [31, 32]? In both later trials, rectal MRI has played a critical role for early assessment of tumor response after chemotherapy alone prior to CRT. Patients with good response were referred to immediate surgery to decrease use of CRT and its attendant morbidities.
Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results.
In 2004, Habr-Gama et al. published the first long-term outcomes study of NOM for rectal cancer patients with cCRs following nCRT [33]. This study included 265 patients (with adenocarcinoma of the distal rectum, 0–7 cm from the anal verge) who underwent response assessment at 8 weeks following nCRT via clinical, radiological (abdomen/pelvic CTs, chest radiographs), and endoscopic examinations. 71 patients (26.7%) achieved a cCR and entered a strict W&W surveillance program. At a median follow-up of 57.3 months, only 2 patients had local tumor regrowth, and only 3 patients developed distant metastases. This landmark study introduced a new organ preservation paradigm in rectal cancer management. Subsequent studies carried out by other groups supported this was approach, and W&W protocols gradually entered the realm of clinical practice. Imaging wise, this has further pushed rectal MRI to the forefront of rectal cancer management and firmly established its role as an integral component in the evaluation of treatment response assessment and surveillance during NOM.
NORWAIT: watch and wait after neoadjuvant chemoradiotherapy for primary locally advanced rectal cancer
Initiated by a consortium of hospitals in Norway in 2018 (NCT03402477), this observational prospective cohort study is still ongoing, with expected completion in 2028 [34]. The study aims to enroll 100 patients with stage I-III rectal cancer who achieve a cCR, as assessed 6–12 weeks after the completion of neoadjuvant chemoradiation. Subjects enter a formal W&W program and are followed at regular intervals over a 5-year study period. The study’s primary endpoint is the rate of tumor regrowth after an initial cCR. There are multiple secondary endpoints, including the cCR rate after neoadjuvant therapy in an unselected national cohort, the rate of distant metastatic disease after initial cCR, and the overall and cancer-specific survival rates following initial cCR. The particular takeaways of this study for radiologists remain to be determined.
Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicenter registry study
In 2018, the IWWD study reported results from an analysis of pooled individual patient data from an international consortium of 47 centers representing 15 countries [35]. The purpose of this study was to describe outcomes of a W&W management strategy among rectal cancer patients achieving a cCR after neoadjuvant therapy. This registry included 880 patients who achieved a cCR after neoadjuvant therapy, the most common form of which was chemoradiation (91%). The means of defining a cCR varied among patients, depending on local institutional practices, but generally consisted of endoscopy (90%), rectal MRI (71%), or both endoscopy and rectal MRI (64%). The median follow-up period was 3.3 years.
The primary endpoint of the study was the rate of local regrowth, which occurred in 213 of 880 patients (24%). 97% of these local regrowths occurred in the bowel wall, with only 3% occurring in the regional lymph nodes only. Among the 213 patients with local regrowths, 64% were identified within the first year of W&W, and 88% were identified within the second year of W&W. Data on surgical management of regrowths were available for 148 of 213 patients, of whom 46 (31%) underwent local excision and 115 (78%) underwent TME (either primarily or after initial local excision). 88% of these TME patients had negative surgical margins.
The major takeaways from this study for radiologists are that tumor regrowth occurs in roughly a quarter of rectal cancer patients on W&W protocols. The vast majority of these local regrowths will occur in the rectal wall rather than the regional lymph nodes. Furthermore, these local regrowths become less likely over time. Therefore, careful inspection of the rectal wall on rectal MRIs for tumor recurrence is critical, particularly in the first two years of W&W.
Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy
In 2019, investigators from Memorial Sloan Kettering Cancer Center published a single-center retrospective study describing outcomes for 113 patients on a W&W management strategy after achieving a cCR to neoadjuvant therapy [36]. Following neoadjuvant therapy, patients underwent surveillance digital rectal examination and lower endoscopy at regular intervals for 5 years. Rectal MRI was not uniformly used for surveillance in this cohort due to the wide range of dates from which patients were identified.
Local regrowth occurred in 22 patients (20%) on W&W, with a median follow-up period of 33 months. The median time to local regrowth was 11.2 months, with 72% of local regrowths occurring within the first 12 months. 3 local regrowths were apparent on imaging only, with imaging findings meaningfully supplementing the endoscopic findings in 3 additional cases. All local regrowths were salvaged with TME (20 patients) or local excision (2 patients). 20 of 22 patients (91%) were free of disease at last follow-up. In the full W&W cohort, 9 patients developed distant metastases, with a higher rate (36% vs. 1%, P < 0.001) among the subset of patients with local regrowth.
The major takeaways from this study for radiologists are that rectal MRI, when utilized for surveillance in a W&W program, adds value to endoscopy and physical examination for detecting local regrowth and that patients with local regrowth should be carefully evaluated for distant metastatic disease on imaging.
STAR-TREC: Can we save the rectum by watchful waiting or transanal surgery following (chemo)radiotherapy versus TME for early rectal cancer?
STAR-TREC is an ongoing European international three-arm multicenter, rolling phase II/III partially randomized, patient preference-controlled trial (NCT02945566), led by University of Birmingham, UK, with additional sites in the Netherlands, Denmark, Belgium, and Sweden. The goal of this trial is to evaluate long-course concurrent chemoradiotherapy (LCCRT) versus short-course radiotherapy for organ preservation in early rectal cancer. Patients with cT1-3b N0 M0, rectal adenocarcinomas ≤ 4 cm in diameter (based on rectal MRI or endorectal ultrasound) are included in this trial. If patients prefer organ preservation, they are randomized 1:1 to LCCRT versus SCRT with selective transanal microsurgery. TME without neoadjuvant radiotherapy is offered to patients choosing radical surgery. The plan is to recruit 380 patients to the organ preservation arm and 120 patients to the TME arm. The primary endpoint is the rate of successful organ preservation at 30 months from the start date of treatment. Secondary endpoints include treatment-related toxicity, rate of NOM, local control at 36 months, disease-free survival at 36 months, and overall survival at 60 months. An exploratory aim is to evaluate the role of circulating tumor DNA for primary response assessment and at follow-up for early prediction of tumor relapse.
NEO: neoadjuvant chemotherapy, excision, and observation for early rectal cancer
NEO is a multicenter trial (NCT03259035) designed to evaluate clinical outcomes and organ-sparing rates in early stage rectal cancer treated with neoadjuvant chemotherapy followed by transanal excision surgery (TES) [37]. 58 patients with T1-3b N0 M0 low/mid rectal invasive well-moderately differentiated adenocarcinoma who were eligible for TES and were treated with 3 months of neoadjuvant chemotherapy (FOLFOX6/CAPOX) were accrued at seven institutions in the USA and Canada. All patients had proctoscopy, rectal MRI, and CT of the chest, abdomen, and pelvis. Patients who demonstrated response to chemotherapy based on post-treatment rectal MRI and proctoscopy were treated with TES within 2–6 weeks. Downstaging to ypT0/T1 cN0 was seen in 33 (57%) of 58 enrolled patients on TES. Among the remaining 23patients, 13 opted for observation instead of the recommended TME. Thus, 79% of patients achieved organ preservation. Locoregional relapse was seen in two patients during a 15.4-month follow-up period, resulting in 1-year and 2-year locoregional relapse-free survival rates of 98% (95% CI: 86–100%) and 90% (95% CI: 58–98%), respectively. No distant metastases or deaths were reported. These early results suggest that 3 months of induction chemotherapy may successfully downstage majority of early rectal cancer patients. Therefore, organ-preserving surgery can be offered in these cases. Further investigation and longer follow-up are warranted based on this data. Rectal MRI will continue to be an integral part of the surveillance in these patients with the goal of early detection of possible locoregional recurrences.
WoW: watch and wait as treatment for patients with rectal cancer
The WoW trial is an open label non-randomized Swedish clinical trial (NCT03125343) that aims to evaluate if it is possible to avoid surgery in patients with palpable low/mid rectal cancers who achieve cCR to neoadjuvant (chemo)radiotherapy, with similar or better outcomes relative to patients who did not achieve cCR and were treated with surgery [38]. Patients with cT4b NX disease; patients with clinical MRF involvement or positive pelvic side-wall nodes (regardless of T/N stage); and patients that have been offered short-course radiotherapy with delayed (6–8 weeks) surgery will be eligible for this trial. Patients with cCR by rectal MRI (performed 8–10 weeks after completion of CRT) will undergo endoscopy and digital rectal exam to for confirmation. All patients with confirmed cCR will be offered a W&W approach and will be followed every 3 months for the first 2 years with clinical examination, endoscopy, CEA levels, rectal MRI, and optional PET/CT. After 2 years, follow-up will decrease to every 6 months. After 5 years, follow-up with decrease to yearly for 5 additional years (i.e., 10 years of total follow-up). The accrual goal is 200 patients with cCR. Patients without cCR will be offered surgery. Biopsy and blood collection will be performed for the exploratory goal of identifying tumor and plasma markers of complete response. The primary endpoint of the trial is 3-year DFS. Additional secondary endpoints are re-growth rates during 10-year follow-up; local recurrence after salvage surgery; overall survival at 10 years); results after surgery for re-growth (complications and mortality); number of patients with complete response, partial response, and no response; and quality of life / health economic analysis.
Conclusion
The introduction of neoadjuvant therapies before TME has substantially altered the LARC treatment landscape. Multiple trials, including the RAPIDO, CAO/ARO/AIO-12, OPRA, andPRODIGE-23 trials, reported data in favor of TNT for LARC [22, 23, 26, 39, 40]. This review focused on a select group of impactful trials, with the goal of highlighting important themes that have emerged and will continue to shape future trials and retrospective analyses. Although we have already achieved a very effective treatment paradigm for LARC that has reduced LR rates significantly, the NCCN guidelines likely continue to result in the overtreatment of rectal cancers with nCRT due to T and N over-staging with rectal MRI. The approach proposed by MERCURY, OCUM, and QuickSilver relies predominantly on the tumor relationship to the anticipated CRM and the distance from the anal verge, while completely disregarding the N stage as assessed by rectal MRI. The results of all three trials have validated this approach, which merits the attention of surgeon, radiologists, and oncologists in the United States. Furthermore, multiple trials discussed in this review support the notion that compliance rates in TNT regimens are higher than in adjuvant chemotherapy regimens. Furthermore, given the high pCR rates with TNT, more patients can be potentially steered to NOM and organ preservation.
Several key questions remain. Can we improve our regimens to minimize toxicity further, such as by avoiding nCRT in specific patient populations? Does the W&W approach increase the rate of distant metastases? If so, what can we do to prevent that outcome? How long can W&W last? Will we finally see an improvement in overall survival on TNT regimens once the long-term follow-up data are available from recent trials? For now, these and many other questions are being addressed in the ongoing and upcoming trials. Radiologists should remain up to date on these developments, as this knowledge allows us to provide more thoughtful and accurate interpretation of rectal MRI at the initial staging, treatment response assessment, and surveillance time points.
References
Little, R.G., 2nd, L.A. Ebertowski, and C.S. David, Inhibition of alloantigen presentation by cyclosporine. Transplantation, 1990. 49(5): p. 937–44.
Peeters, K.C., et al., Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol, 2005. 23(25): p. 6199-206.
Birgisson, H., et al., Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol, 2005. 23(25): p. 6126-31.
Glynne-Jones, R., et al., Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2017. 28(suppl_4): p. iv22-iv40.
Taylor, F.G., et al., Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg, 2011. 253(4): p. 711-9.
Ruppert, R., et al., Avoidance of Overtreatment of Rectal Cancer by Selective Chemoradiotherapy: Results of the Optimized Surgery and MRI-Based Multimodal Therapy Trial. J Am Coll Surg, 2020. 231(4): p. 413-425.e2.
Shahida Ahmed, N.B., Alexandre Bouchard, James Brierley, Carl Brown, Gina Brown, Selliah Kanthan, Zane Cohen, Bernard Cummings, Ray Deobald, Sébastien Drolet, Stan Feinberg, Darlene Fenech, Dan Gill, David Hochman, Kartik Jhaveri, Erin Kennedy, Richard Kirsch, Neil Kopek, Vijayananda Kundapur, Eric Leung, Sender Liberman, Tony MacLean, Victoria Marcus, Alex Mathieson, Robin McLeod, Stanislas Morin, Catherine O'Brien, Michael Ott, Nikhilesh Patil, Anat Ravid, Marko Simunovic, Peter Stotland, Seng Thipphavong, Lara Williams, QuickSilver: A Phase II Study Using Magnetic Resonance Imaging Criteria to Identify "Good Prognosis" Rectal Cancer Patients Eligible for Primary Surgery. JMIR Res Protoc, 2015. 4(2): p. e41.
Kaur, H., et al., MRI Staging in an Evolving Management Paradigm for Rectal Cancer, From the AJR Special Series on Cancer Staging. American Journal of Roentgenology, 2021. 217(6): p. 1282-1293.
Brouwer, N.P.M., et al., Clinical lymph node staging in colorectal cancer; a flip of the coin? European Journal of Surgical Oncology, 2018. 44(8): p. 1241-1246.
Stelzner, S., et al., Selection of patients with rectal cancer for neoadjuvant therapy using pre-therapeutic MRI – Results from OCUM trial. European Journal of Radiology, 2022. 147.
The Ocum Group, Oncological outcome after MRI-based selection for neoadjuvant chemoradiotherapy in the OCUM Rectal Cancer Trial. British Journal of Surgery, 2018. 105(11): p. 1519-1529.
Kennedy, E.D., et al., Safety and Feasibility of Using Magnetic Resonance Imaging Criteria to Identify Patients With "Good Prognosis" Rectal Cancer Eligible for Primary Surgery: The Phase 2 Nonrandomized QuickSilver Clinical Trial. JAMA Oncol, 2019. 5(7): p. 961-966.
Sauer, R., et al., Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med, 2004. 351(17): p. 1731-40.
Watanabe, T., et al., Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol, 2018. 23(1): p. 1-34.
Fujita, S., et al., Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol, 2012. 13(6): p. 616-21.
Fujita, S., et al., Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg, 2017. 266(2): p. 201-207.
Tsukamoto, S., et al., Long-term follow-up of the randomized trial of mesorectal excision with or without lateral lymph node dissection in rectal cancer (JCOG0212). Br J Surg, 2020. 107(5): p. 586-594.
Kusters, M., et al., A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence. Ann Surg, 2009. 249(2): p. 229-35.
Kim, T.H., et al., Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol, 2008. 15(3): p. 729-37.
Ogura, A., et al., Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol, 2019. 37(1): p. 33-43.
Ohue, M., et al., A Phase II/III randomized controlled trial comparing perioperative versus postoperative chemotherapy with mFOLFOX6 for lower rectal cancer with suspected lateral pelvic node metastasis: Japan Clinical Oncology Group Study JCOG1310 (PRECIOUS study). Jpn J Clin Oncol, 2017. 47(1): p. 84-87.
Conroy, T., et al., Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol, 2021. 22(5): p. 702-715.
Bahadoer, R.R., et al., Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol, 2021. 22(1): p. 29-42.
Jin, J., et al., Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J Clin Oncol, 2022. 40(15): p. 1681-1692.
Garcia-Aguilar, J., et al., Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol, 2022. 40(23): p. 2546-2556.
Goffredo, P., et al., Non-Operative Management of Patients with Rectal Cancer: Lessons Learnt from the OPRA Trial. Cancers (Basel), 2022. 14(13).
Fokas, E., et al., Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol, 2019. 37(34): p. 3212-3222.
Fokas, E., et al., Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol, 2022. 8(1): p. e215445.
Cercek, A., et al., Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol, 2018. 4(6): p. e180071.
Rullier, E., et al., Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol Hepatol, 2020. 5(5): p. 465-474.
Rouanet, P., et al., Tailored Strategy for Locally Advanced Rectal Carcinoma (GRECCAR 4): Long-term Results From a Multicenter, Randomized, Open-Label, Phase II Trial. Dis Colon Rectum, 2022. 65(8): p. 986-995.
Brouquet, A., et al., NORAD01-GRECCAR16 multicenter phase III non-inferiority randomized trial comparing preoperative modified FOLFIRINOX without irradiation to radiochemotherapy for resectable locally advanced rectal cancer (intergroup FRENCH-GRECCAR- PRODIGE trial). BMC Cancer, 2020. 20(1): p. 485.
Habr-Gama, A., et al., Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg, 2004. 240(4): p. 711–7; discussion 717–8.
"Watch and Wait" After Neo-adjuvant Chemoradiotherapy for Primary Locally Advanced Rectal Cancer. (NORWAIT). 2020 January 7, 2023]; Available from: https://www.clinicaltrials.gov/ct2/show/NCT03402477?term=NORWAIT&draw=2&rank=1.
van der Valk, M.J.M., et al., Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet, 2018. 391(10139): p. 2537-2545.
Smith, J.J., et al., Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol, 2019. 5(4): p. e185896.
Kennecke, H.F., et al., Neoadjuvant Chemotherapy, Excision, and Observation for Early Rectal Cancer: The Phase II NEO Trial (CCTG CO.28) Primary End Point Results. Journal of Clinical Oncology, 2022. 41(2): p. 233–242.
WoW Watch and wait: Clinical complete response after (chemo)radiotherapy in advanced rectal cancer: A multicentre prospective national cohort study. 2017; Available from: http://www.ssorg.net/files/6815/7062/6019/Studieprotokoll_WoW_190207.pdf.
Bahadoer, R.R., et al., Interpreting the RAPIDO trial: factors to consider - Authors' reply. Lancet Oncol, 2021. 22(3): p. e90-e91.
Karoui, M., et al., Perioperative FOLFOX 4 Versus FOLFOX 4 Plus Cetuximab Versus Immediate Surgery for High-Risk Stage II and III Colon Cancers: A Phase II Multicenter Randomized Controlled Trial (PRODIGE 22). Ann Surg, 2020. 271(4): p. 637-645.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paroder, V., Fraum, T.J., Nougaret, S. et al. Key clinical trials in rectal cancer shaping the current treatment paradigms: reference guide for radiologists. Abdom Radiol 48, 2825–2835 (2023). https://doi.org/10.1007/s00261-023-03931-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-03931-z