Abstract
Purpose of Review
This review summarizes the relevant literature on the use of total neoadjuvant therapy (TNT) for patients with locally advanced rectal cancer. It highlights the most notable literature published and briefly discusses future directions.
Recent Findings
Recent randomized trials evaluating TNT show improved rates of pathologic complete response and patient treatment tolerance with this approach.
Summary
The rationale for TNT includes the poor patient tolerance of adjuvant chemotherapy and the persistent risk of distant disease in patients with locally advanced rectal cancer, despite improvements in local control. Randomized trials have focused on short-term pathologic endpoints. Ongoing phase 3 trials are evaluating long-term disease-related outcomes, allowing for a more thorough evaluation of this treatment paradigm. TNT may also facilitate organ preservation in appropriately selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rectal cancer is a common malignancy in both men and women, with an estimated 43,030 new cases diagnosed in the USA in 2018 [1]. Over the past several decades, local recurrence rates have declined dramatically due to improved surgical techniques and the use of neoadjuvant therapy [2,3,4,5,6,7]. As a result, the focus of clinical research has shifted to reducing the risk of distant metastatic disease, a significant source of morbidity and mortality for these patients [2, 4,5,6, 8]. The most common treatment paradigm in the USA employs neoadjuvant chemoradiation, surgery, and adjuvant chemotherapy for patients diagnosed with stage II–III disease. The use of adjuvant chemotherapy in rectal cancer is primarily an extrapolation from its use in patients with colon cancer [9, 10] and has been widely implemented in clinical practice despite limited data. An emerging and increasingly utilized approach is described as “total neoadjuvant therapy” or TNT. This approach transitions the use of systemically dosed chemotherapy to the neoadjuvant setting and has shown significant promise in multiple clinical trials. Here, we highlight the prominent literature evaluating this approach, including a review of its merits and potential shortcomings.
Historical Context
Recurrence patterns for rectal cancer have shifted over the past 30 years with improved local control rates attributable to implementation of total mesorectal excision (TME) and chemoradiation or short-course radiation therapy in the neoadjuvant setting. TME was first described in the 1930s but gained widespread attention in the late 1970s and early 1980s. With TME, a precise, sharp dissection between the visceral and parietal layers of the endopelvic fascia is performed. This includes en bloc removal of the mesorectal envelope as a single packet of tissue, with the surrounding lymphatics, vascular and perineural tumor deposits, with the primary tumor encompassed inside. Additionally, this approach is more likely to preserve autonomic nerve function in the pelvis and reduces the risk of post-operative bleeding. This technique replaced the historical blunt dissection, which often violated the mesorectal envelope leaving residual disease in the pelvis [13, 14]. TME has reduced the risk of both involved surgical margins and local recurrences in the pelvis [3, 4].
For patients with locally advanced rectal cancer, including those with T3-T4 and/or node-positive disease, the use of neoadjuvant therapy improves both local control and disease-free survival [5, 15]. Multiple trials support the current treatment paradigm in the USA [5, 15,16,17,18,19], which includes the use of 5-fluoropyrimidine (5-FU)-based chemotherapy given concurrently with neoadjuvant radiation therapy utilizing a “long-course” approach (treating to approximately 50.4 Gy over 5.5 weeks). Alternatively, “short-course” radiation therapy alone (treating to 25 Gy in 5 fractions) is more widely used in other parts of the world, including Northern Europe [20, 21].
In the past, local failure was a significant source of morbidity and mortality for patients with rectal cancer. However, with the aforementioned enhancements in locoregional therapy, the risk of developing a local recurrence has decreased from > 25% to approximately 5–10% [2,3,4,5,6,7]. This has shifted the leading cause of morbidity and mortality for these patients to distant metastases, which occurs in approximately 30% of patients [2, 4,5,6, 8]. Historical trials of the 1970s and 1980s demonstrated an improvement in overall survival in patients with rectal cancer receiving adjuvant therapy, including the use of adjuvant chemotherapy [22]. There are also prospective, randomized trial data supporting the use of adjuvant chemotherapy in patients with colon cancer, demonstrating an improvement in overall survival in those with node-positive disease [9, 10]. However, trials specifically evaluating the utility of adjuvant chemotherapy in patients with rectal cancer have largely failed to demonstrate benefit [2, 11•, 12, 13, 23, 24]. For example, one of the larger trials from the European Organization for Research and Treatment of Cancer (EORTC 22921) did not demonstrate a disease-free or overall survival benefit of adjuvant 5-FU therapy for patients with locally advanced disease. The risk of distant metastases remained approximately 30% for these patients at 10 years [2, 11•, 12]. Some of these trials were likely underpowered to detect a difference in survival due to their slow accrual and early closure [23, 24], although the EORTC and an Italian trial did not demonstrate benefit despite adequate patient sample size [2, 13]. Additionally, these trials primarily utilized 5-FU alone in the adjuvant setting, as opposed to oxaliplatin-based adjuvant therapy.
Rationale for TNT
One of the most prominent challenges in assessing the efficacy of adjuvant chemotherapy is poor patient tolerance. For example, in the EORTC 22921 trial, only 73% of patients randomized to receive adjuvant chemotherapy actually received any and only 43% received the majority of planned doses [2]. The previously mentioned Italian trial also showed poor patient compliance with adjuvant therapy with only 58% of patients receiving at least half of the six planned cycles of 5-FU-based chemotherapy [13]. The most common reasons for not completing intended therapy in these trials include disease progression, patient refusal, and post-operative morbidity [2, 13]. The need to reduce the risk of distant progression and poor compliance with adjuvant therapy necessitates the development of new treatment strategies to address this clinical scenario.
Investigators have touted the use of systemically dosed chemotherapy in the neoadjuvant setting, with the goal of avoiding some of the pitfalls associated with adjuvant therapy. It would seem likely that patient compliance would improve in the neoadjuvant setting, as patients would not be handicapped by post-operative morbidity and lower performance status. Given the risk for mortality associated with distant metastatic disease, there is potential benefit in treating micrometastatic disease earlier in a patient’s disease course. Additionally, less treatment-related toxicity in the post-operative setting may allow appropriate patients to undergo ostomy reversal at an earlier time point following low anterior resection (LAR) [8]. For those patients with locally advanced disease (T3-T4 and/or node-positive), chemotherapy in the upfront setting may allow for enhanced tumor response, potential downstaging, and higher rates of complete (R0) resection. Tumor downstaging may permit more patients the opportunity for organ preservation, both from a surgical perspective (low anterior resection (LAR) in lieu of abdominoperineal resection (APR)) and utilization of the emerging “watch-and-wait” approach. The watch-and-wait approach seeks to identify those patients who are good candidates for omission of surgery, in favor of a strict surveillance program [25].
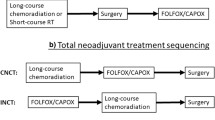
Figure 1 depicts representative timelines for the treatment paradigms utilized in the management of rectal cancer.
Example timelines for each of the following treatment paradigms. a Total neoadjuvant therapy (TNT) with long-course chemoradiation (CRT) followed by neoadjuvant chemotherapy (NAC). b Long-course CRT. c TNT with short-course radiation followed by NAC. d Short-course radiation. e TNT with NAC followed by long-course CRT. f long-course CRT with adjuvant chemotherapy
Potential Shortcomings
While there are many potential benefits of TNT, rigorous evaluation of disease-related outcomes is needed before its widespread adoption. One potential disadvantage to its use is the delay to definitive surgical resection. In those patients who do not adequately respond to neoadjuvant chemotherapy, local progression is possible. Upfront systemic therapy and the associated toxicities also have the potential to impact a patient’s ability to undergo a definitive surgical procedure by negatively impacting performance status.
Clinical Outcomes: Is TNT Beneficial?
In recent years, multiple trials have published on the outcomes of patients treated with TNT. Two general approaches have been used: [1] neoadjuvant radiotherapy (±concurrent chemotherapy) followed by chemotherapy or [2] neoadjuvant chemotherapy followed by chemoradiation. The primary endpoints of these prospective trials have focused almost exclusively on short-term endpoints, including pathologic complete response (pCR) and R0 resection rates. This allows for early assessment of patient outcomes and may be a surrogate for long-term outcomes. However, the efficacy of TNT on long-term patient outcomes needs confirmation through prospective phase 3 trials, as it has yet to be conclusively demonstrated that pCR following neoadjuvant therapy for rectal cancer meets formal criteria to act as a surrogate for local control and/or overall survival [26, 27].
Radiotherapy (±Concurrent Chemotherapy) Followed by Chemotherapy
Garcia-Aguilar et al. published a multi-institutional phase 2 trial in which patients were assigned to receive chemoradiation with 5-FU followed by increasing cycles of neoadjuvant chemotherapy prior to surgical resection. Four treatment arms were included based on the number of neoadjuvant chemotherapy cycles given prior to TME, including 0, 2, 4, or 6 cycles of modified FOLFOX (5-FU/oxaliplatin/leucovorin). The primary endpoint was pCR, which rose significantly with increasing cycles of FOLFOX. For example, the pCR rate was 38% for those patients that received 6 cycles compared to 18% for those that did not receive any neoadjuvant chemotherapy. Importantly, none of the patients in the trial experienced disease progression during neoadjuvant treatment, regardless of treatment arm [28••]. A potential confounder when interpreting the results of this trial is the interaction between rate of pCR and time from neoadjuvant treatment to surgical resection. Multiple series have suggested that delaying the time to surgery to more than 7–8 weeks after completion of chemoradiation improves the rate of pCR [29, 30], with all patients in the Garcia-Aguilar trial having a median interval between chemoradiation and definitive surgery of > 8 weeks [28••]. It should be noted, however, that other randomized trials and retrospective series have failed to show an impact on pCR for intervals > 12 weeks [31, 32].
The Polish II trial also provided useful information on long-term patient outcomes with this approach. In this trial, patients received either short-course radiation therapy and neoadjuvant chemotherapy versus long-course chemoradiation. Following neoadjuvant treatment patients proceeded to surgery after a median of 12 weeks. The authors found no difference in rate of R0 resection, local control, or disease-free survival. Interestingly, patients who received short-course radiation plus neoadjuvant chemotherapy had improved overall survival at 3 years compared to the long-course arm (73 vs. 65%, p = 0.046); however, this improvement in overall survival is discordant with the similar rates of disease control in each arm. The authors speculate that the higher dose per fraction in the short-course arm may result in a more prevalent antitumor immune response [33••].
Neoadjuvant Chemotherapy Followed by Chemoradiation
In contrast to the trials evaluating radiation first followed by chemotherapy, studies evaluating the converse have been less fruitful. The CONTRE study most closely mirrors the trial by Garcia-Aguilar et al. In this single arm, prospective trial, patients received 8 cycles of modified FOLFOX prior to chemoradiation, followed by surgery 6–10 weeks later with a 33% pCR rate [36].
Analogous to the Polish II trial, the Spanish Grupo Cancer de Recto 3 (GCR-3) trial has published 5-year outcomes. This phase 2 trial randomized patients with locally advanced rectal cancer to receive 4 cycles of CAPOX (capecitabine/oxaliplatin) either before neoadjuvant chemoradiation or after surgery and reported no difference in disease-free or overall survival at 5 years. It is important to emphasize this trial was not powered to detect differences in long-term outcomes. Nonetheless, the authors did not find a difference in pCR rates, which was 13–14% regardless of treatment arm. They postulate this may be related to underlying differences in the baseline characteristics between the two treatment arms, including a higher percentage of patients with a threatened circumferential margin in the neoadjuvant chemotherapy group [34••, 38].
Patient Tolerance and Organ Preservation
As one might anticipate, the rates of therapy completion or compliance appear improved in patients who receive neoadjuvant chemotherapy versus adjuvant chemotherapy. For the Garcia-Aguilar trial, treatment compliance rates were 77–82%, depending upon the treatment arm. This compares favorably to the 43–58% noted in previous adjuvant trials [2, 13]. Similarly, rates of post-operative complications were not increased by the addition of chemotherapy prior to surgical resection. In the Polish II trial, the rate of compliance with neoadjuvant therapy and the toxicity profile was superior in patients on the short-course radiotherapy plus neoadjuvant chemotherapy arm compared to long-course chemoradiation (72 versus 64% compliance, respectively). Several smaller, single-arm prospective trials have also demonstrated > 90% compliance with a chemoradiation followed by neoadjuvant chemotherapy approach [39,40,41]. Compliance rates are similarly improved in those patients who receive neoadjuvant chemotherapy followed by chemoradiation. In the GCR-3 trial, patients in the neoadjuvant chemotherapy arm had a compliance rate of 94 versus 57% in the adjuvant arm (p = 0.0001). Importantly, patients receiving neoadjuvant therapy also had a lower risk of developing high-grade toxicity (19 versus 54%, p = 0.004) [34••, 38]. The EXPERT and EXPERT-C trials examined the role of 4 cycles of neoadjuvant CAPOX ± cetuximab followed by chemoradiation and then surgery. Study results demonstrated an 89% or greater rate of compliance with neoadjuvant therapy [42•, 43•]. A pooled analysis of these trials (PAN-EX) reported a compliance of 91% with neoadjuvant therapy [35••]. Again, several small prospective trials examining this treatment paradigm also show high rates of treatment compliance [36, 39, 44,45,46,47,48,49].
The use of TNT may also allow for increased likelihood of organ preservation in appropriately selected patients. Investigators from the Angelita and Joaquim Gama Institute at the University of São Paulo School of Medicine have championed the use of a watch-and-wait (or non-operative) management strategy in appropriately selected patients with locally advanced rectal cancer. With this approach, patients receive chemoradiation and for those who achieve a clinical complete response (cCR), surgery is deferred, and patients enter into a strict program of surveillance [25, 50]. With concurrent 5-FU and radiation therapy to 50.4 Gy, a cCR rate of 27% was reported [50]. These investigators have subsequently evaluated the addition of systemically dosed chemotherapy following chemoradiation over the 8–10-week interval before treatment response assessment, which increased the rate of cCR to 57%. Understanding the benefit of systemically dosed chemotherapy in this trial is somewhat obscured by the addition of another cycle of concurrent chemotherapy and increased radiation dose compared to their previous study [37••, 50]. Similar to other trials evaluating TNT, 97% of patients were able to complete neoadjuvant chemotherapy [51]. These investigators also reported improvement in radiographic response on positron emission tomography (PET) scan with the addition of chemotherapy following chemoradiation [52]. These data would suggest that it is not simply time to surgical resection in the trials evaluating TNT that led to improvement in pCR [28••, 37••, 52]. TNT may result in a higher percentage of patients being eligible for organ or sphincter preservation secondary to improvement in rates of downstaging Table 1 highlights selected prospective studies utilizing TNT.
Future Directions
Recent trials have focused on short-term pathologic endpoints such as pCR and R0 resection rates. Future trials will need to focus on long-term disease outcomes before TNT can be widely incorporated into treatment guidelines. The RAPIDO trial prospectively randomized patients to neoadjuvant chemoradiation, surgery, and adjuvant chemotherapy versus short-course radiation, neoadjuvant chemotherapy, and then surgery. The primary endpoint of this trial is 3-year disease-free survival [53]. The COPERNICUS trial evaluates neoadjuvant chemotherapy prior to short-course radiation therapy. Preliminary reports indicate high treatment compliance rates (95%), which is their primary endpoint [54]. The CREATE trial is a phase 3 trial evaluating the use of neoadjuvant chemotherapy prior to short-course or long-course radiation, with a primary end point of disease-free survival at 3 years [55].
With the exception of the GCR-3 trial, most TNT studies to date have not clearly specified the use or omission of adjuvant chemotherapy, often leaving its use to the discretion of the treating physician. The ongoing phase 3 trials include more specific guidelines regarding the use of adjuvant chemotherapy and should help to better define the risks and benefits of a strictly neoadjuvant approach.
Other groups are seeking to improve the efficacy of neoadjuvant therapies [8, 56, 57] or to include the use of novel radiosensitizing agents such as poly-ADP-ribose polymerase (PARP) inhibitors [58, 59]. Investigators from Memorial Sloan Kettering are currently enrolling patients on a randomized phase 2 trial evaluating the use of TNT for organ preservation. Patients will undergo chemotherapy followed by chemoradiation or chemoradiation followed by chemotherapy. Patients that achieve a cCR will proceed to a watch-and-wait or non-operative management approach with the primary endpoint being 3-year disease-free survival [60].
Finally, some have questioned the necessity of radiation in the neoadjuvant setting, speculating that the benefit demonstrated in previous trials was due to the use of non-TME surgical techniques. This topic is also an area of active investigation, including the ongoing randomized PROSPECT [61] and BACCHUS [62] trials.
Conclusions
A total neoadjuvant therapy (TNT) approach results in high rates of patient treatment compliance in locally advanced rectal cancer patients. TNT trials have focused primarily on short-term pathologic outcomes, which appear promising. However, ongoing randomized prospective trials are evaluating long-term disease-related outcomes for these patients. This will allow for a more critical assessment of this approach to determine if it should be routinely incorporated into treatment guidelines, as an alternative to current treatment paradigms. Ongoing trials are also seeking to refine the components of neoadjuvant therapy, which may allow for a higher proportion of patients being able to receive organ-preserving therapy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
SEER Cancer Statistics [Available from: https://seer.cancer.gov/statfacts/html/colorect.html.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23.
MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457–60.
Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246(5):693–701.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373(9666):811–20.
Swedish Rectal Cancer T, Cedermark B, Dahlberg M, Glimelius B, Pahlman L, Rutqvist LE, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336(14):980–7.
Gollins S, Sebag-Montefiore D. Neoadjuvant treatment strategies for locally advanced rectal cancer. Clin Oncol (R Coll Radiol). 2016;28(2):146–51.
Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(19):3109–16.
Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(16):2198–204.
• Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184–90 Randomized control trial demonstrating no benefit to adjuvant fluorouracil-based chemotherapy in patient with rectal cancer after neoadjuvant radiotherapy (+/− concurrent chemotherapy).
Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(28):4379–86.
Sainato A, Cernusco Luna Nunzia V, Valentini V, De Paoli A, Maurizi ER, Lupattelli M, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113(2):223–9.
Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93(8):583–96.
Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(31):5124–30.
Frykholm GJ, Glimelius B, Pahlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum. 1993;36(6):564–72.
Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(28):4620–5.
Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324(11):709–15.
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(16):1926–33.
Minsky BD. Short-course radiation versus long-course chemoradiation for rectal cancer: making progress. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(31):3777–8.
Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: trans-Tasman radiation oncology group trial 01.04. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(31):3827–33.
Gastrointestinal Tumor Study G. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312(23):1465–72.
Breugom AJ, van Gijn W, Muller EW, Berglund A, van den Broek CB, Fokstuen T, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26(4):696–701.
Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25(7):1356–62.
Torok JA, Palta M, Willett CG, Czito BG. Nonoperative management of rectal cancer. Cancer. 2016;122(1):34–41.
Methy N, Bedenne L, Conroy T, Bouche O, Chapet O, Ducreux M, et al. Surrogate end points for overall survival and local control in neoadjuvant rectal cancer trials: statistical evaluation based on the FFCD 9203 trial. Ann Oncol. 2010;21(3):518–24.
Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8(4):431–40.
•• Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–66 Phase 2 trial evaluating TNT (chemoradiation → chemotherapy) in patients with locally advanced rectal cancer, showing improvement in treatment compliance with neoadjuvant therapy. This trial also demonstrated increasing rates of pCR with additional cycles of neoadjuvant chemotherapy.
Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250(4):582–9.
Wolthuis AM, Penninckx F, Haustermans K, De Hertogh G, Fieuws S, Van Cutsem E, et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol. 2012;19(9):2833–41.
Habr-Gama A, Perez RO, Proscurshim I, Nunes Dos Santos RM, Kiss D, Gama-Rodrigues J, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys. 2008;71(4):1181–8.
Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(31):3773–80.
•• Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Krynski J, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 x 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27(5):834–42 Randomized phase 3 trial comparing long-course chemoradiation to TNT utilizing short-course radiation therapy, showing no difference in the rate of R0 resection, local control, or disease-free survival. There was a survival benefit noted in the TNT arm at 3 years.
•• Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trialdagger. Ann Oncol. 2015;26(8):1722–8 Phase 2 trial comparing TNT to adjuvant chemotherapy, with no difference in disease-free or overall survival at 5 years but superior treatment compliance in the TNT arm.
•• Sclafani F, Brown G, Cunningham D, Wotherspoon A, Tait D, Peckitt C, et al. PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol. 2016;27(8):1557–65 Pooled analysis of the EXPERT (42) and EXPERT-C (43) trials.
Perez K, Safran H, Sikov W, Vrees M, Klipfel A, Shah N, et al. Complete neoadjuvant treatment for rectal Cancer: the Brown University oncology group CONTRE study. Am J Clin Oncol. 2017;40(3):283–7.
•• Habr-Gama A, Sabbaga J, Gama-Rodrigues J, Sao Juliao GP, Proscurshim I, Bailao Aguilar P, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56(10):1109–17 Single arm prospective trial which showed improvement in the rate of clinical complete response with the addition of chemotherapy following chemoradiation for patients entering into a "watch-and-wait" or non-operative management approach.
Fernandez-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clini Oncol Off J Am Soc Clin Oncol. 2010;28(5):859–65.
Gao YH, An X, Sun WJ, Cai J, Cai MY, Kong LH, et al. Evaluation of capecitabine and oxaliplatin administered prior to and then concomitant to radiotherapy in high risk locally advanced rectal cancer. J Surg Oncol. 2014;109(5):478–82.
Gao YH, Lin JZ, An X, Luo JL, Cai MY, Cai PQ, et al. Neoadjuvant sandwich treatment with oxaliplatin and capecitabine administered prior to, concurrently with, and following radiation therapy in locally advanced rectal cancer: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2014;90(5):1153–60.
Zampino MG, Magni E, Leonardi MC, Petazzi E, Santoro L, Luca F, et al. Capecitabine initially concomitant to radiotherapy then perioperatively administered in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75(2):421–7.
• Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(3):241–8 Single arm prospective trial evaluating TNT in patients with locally advanced rectal cancer (neoadjuvant chemotherapy →chemoradiation → surgery → adjuvant chemotherapy), showing high rates of treatment compliance.
• Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(14):1620–7 Single arm prospective trial evaluating TNT in patients with locally advanced rectal cancer. Treatment paradigm the same as that in the EXPERT trial (42), with the addition of cetuximab. This trial also demonstrated high rates of treatment compliance.
Dipetrillo T, Pricolo V, Lagares-Garcia J, Vrees M, Klipfel A, Cataldo T, et al. Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and radiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82(1):124–9.
Dueland S, Ree AH, Groholt KK, Saelen MG, Folkvord S, Hole KH, et al. Oxaliplatin-containing preoperative therapy in locally advanced rectal Cancer: local response, toxicity and long-term outcome. Clin Oncol (R Coll Radiol). 2016;28(8):532–9.
Koeberle D, Burkhard R, von Moos R, Winterhalder R, Hess V, Heitzmann F, et al. Phase II study of capecitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advanced rectal cancer. Br J Cancer. 2008;98(7):1204–9.
Marechal R, Vos B, Polus M, Delaunoit T, Peeters M, Demetter P, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23(6):1525–30.
Nogue M, Salud A, Vicente P, Arrivi A, Roca JM, Losa F, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist. 2011;16(5):614–20.
Schou JV, Larsen FO, Rasch L, Linnemann D, Langhoff J, Hogdall E, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23(10):2627–33.
Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–7 discussion 7-8.
Habr-Gama A, Perez RO, Sabbaga J, Nadalin W, Sao Juliao GP, Gama-Rodrigues J. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum. 2009;52(12):1927–34.
Habr-Gama A, Perez RO, Sao Juliao GP, Proscurshim I, Fernandez LM, Figueiredo MN, et al. Consolidation chemotherapy during neoadjuvant chemoradiation (CRT) for distal rectal cancer leads to sustained decrease in tumor metabolism when compared to standard CRT regimen. Radiat Oncol. 2016;11:24.
Nilsson PJ, van Etten B, Hospers GA, Pahlman L, van de Velde CJ, Beets-Tan RG, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer—the RAPIDO trial. BMC Cancer. 2013;13:279.
Gollins S. A phase II single arm feasibility trial of neoadjuvant chemotherapy (NAC) with oxaliplatin/fluorouracil (OxMdG) then short-course preoperative radiotherapy (SCPRT) then immediate surgery in operable rectal cancer (ORC): COPERNICUS (NCT01263171). J Clin Oncol Off J Am Soc Clin Oncol. 2015;33 (supplement):Abstract 3609.
Ireland. TAoCoGBa. Operable rectal cancer: survey of current practice and proposed phase III trial. [Available from: http://www.acpgbi.org/uk/content/uploads/2014/07/CREATE-Survey-2014.pdf.
Borg C, Andre T, Mantion G, Boudghene F, Mornex F, Maingon P, et al. Pathological response and safety of two neoadjuvant strategies with bevacizumab in MRI-defined locally advanced T3 resectable rectal cancer: a randomized, noncomparative phase II study. Ann Oncol. 2014;25(11):2205–10.
Wong SJ, Moughan J, Meropol NJ, Anne PR, Kachnic LA, Rashid A, et al. Efficacy endpoints of radiation therapy group protocol 0247: a randomized, phase 2 study of neoadjuvant radiation therapy plus concurrent capecitabine and irinotecan or capecitabine and oxaliplatin for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2015;91(1):116–23.
Czito BG, Deming DA, Jameson GS, et al. Safety and tolerability of veliparib combined with capecitabine plus radiotherapy in patients with locally advanced rectal cancer: a phase 1b study. Lancet Gastroenterol Hepatol. 2017 Jun;2(6):418–26.
George T, Yothers G, Hong T, et al. A phase II clinical trial platform utilizing total neoadjuvant therapy (TNT) in rectal cancer: Nrg-GI002. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34 (supplement):Abstract TPS3638.
Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, et al. Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767.
Oncology AfCTi. PROSPECT: Chemotherapy alone or chemotherapy plus radiation therapy in treating patients with locally advanced rectal cancer undergoing surgery 2016 [Available from: https://clinicaltrials.gov/ct2/show/NCT01515787.
Glynne-Jones R, Hava N, Goh V, Bosompem S, Bridgewater J, Chau I, et al. Bevacizumab and combination chemotherapy in rectal cancer until surgery (BACCHUS): a phase II, multicentre, open-label, randomised study of neoadjuvant chemotherapy alone in patients with high-risk cancer of the rectum. BMC Cancer. 2015;15:764.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sarah J. Stephens declares that she has no conflict of interest.
Christopher G. Willett declares that he has no conflict of interest.
Manisha Palta has received research support from Merck, honoraria from Oakstone CME and UpToDate, and has received compensation from Navigant for service as a consultant.
Brian G. Czito declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Radiation Therapy and Radiation Therapy Innovations in Colorectal Cancer
Rights and permissions
About this article
Cite this article
Stephens, S.J., Willett, C.G., Palta, M. et al. Total Neoadjuvant Therapy (TNT) in Rectal Cancer. Curr Colorectal Cancer Rep 14, 199–206 (2018). https://doi.org/10.1007/s11888-018-0415-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-018-0415-8