Abstract
Purpose
It is recommended to drain the pancreatic fluid collections later in the course of the acute necrotizing pancreatitis (ANP). However, earlier drainage may be indicated. We compared early (≤ 2 weeks) vs. late (3rd to 4th week) percutaneous catheter drainage (PCD) of acute necrotic collections (ANC).
Materials and methods
This retrospective study comprised ANP patients who underwent PCD of ANC. The diagnosis of ANP was based on revised Atlanta classification criteria and computed tomography performed between 5 and 7 days of illness. Patients were divided into two groups [1st 2 weeks (group I) and 3rd–4th weeks (group II)] based on the interval between the onset of pain and insertion of catheter. The technical success, clinical success, complications, and clinical outcomes were compared between the two groups.
Results
One hundred forty-eight patients (74 in each group) were evaluated. The procedures were technically successful in all patients. The clinical success rate was 67.6% in group I vs. 77% in group II (p = 0.069). The incidence of complications was significantly higher in group I (n = 12, 16%) than group II (n = 4, 5.4%) (p = 0.034). These included 15 minor (11 in group I and 4 in group II) and one major complication (group I). Of the clinical outcomes, the need for surgery was significantly higher in group I than in group II (13 patients vs. 5 patients, p = 0.031).
Conclusion
Early PCD is as technically successful as late PCD in the management of ANC. However, early PCD is associated with higher surgical rate and higher incidence of complications.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute pancreatitis (AP) is a common condition presenting to the emergency room [1]. Pancreatic fluid collections represent the most common local complication of acute necrotising pancreatitis [2]. According to the revised Atlanta classification, the acute fluid collections (< 4 weeks) have been divided into acute necrotic collections (ANC) and acute peripancreatic fluid collections, depending on the presence of necrosis [3]. In the later period (> 4 weeks), the collection gets encapsulated into walled-off necrosis (WON) or pseudocyst. Infected or symptomatic necrotic fluid collections need to be managed. The recommended management strategy is step-up approach which involves initial conservative management followed by drainage procedure and minimally invasive necrosectomy, in case of non-response to drainage procedures. Drainage methods include percutaneous, endoscopic, or a combination of these techniques [4,5,6,7]. Currently, endoscopic drainage is preferred for WONs close to the stomach or duodenum. Percutaneous catheter drainage (PCD) is the standard technique for collections in the non-encapsulated stage due to potential risks associated with endoscopic methods [8]. It is recommended to perform PCD in the later course of the disease, that is, between the third and fourth week of illness (interval from onset of pain), when the collections are likely to encapsulate [9]. However, earlier drainage (in the 1st two weeks) may be indicated. PCD is also used as an adjunct to endoscopic drainage (dual modality drainage) or surgery.
A recent study showed that early PCD is safe and effective [10]. Early PCD in patients with moderately severe and severe AP may have positive impact on the prognosis [11, 12]. Early drainage in patients with severe AP and organ failure led to the reduced infection rate, operation rate, and mortality in a recent study [13]. Another study showed a shorter systemic inflammatory response syndrome period and hospitalization in patients with early PCD compared to the control group with no drainage [14].
Despite a recent recommendation to use PCD for treating infected or symptomatic ANC in the early acute period (< 2 weeks) [15], there is a lack of literature reporting the safety and efficacy of PCD in the 1st 2 weeks of illness.
The purpose of this retrospective study was to compare the safety and efficacy, and clinical outcomes of early (≤ 2 weeks) vs. late (3rd–4th weeks) PCD of ANC in patients with AP.
Materials and methods
The institutional ethics committee approved this retrospective study. The need for informed consent was waived. Consecutive patients with acute necrotizing pancreatitis who underwent PCD in the first two weeks of illness between January 2018 and September 2021 were included (group I). The diagnosis of AP was based on the revised Atlanta classification criteria (two or more of the following): typical pancreatic type abdominal pain, elevation of serum amylase or lipase levels to more than three times the upper limit of normal, and imaging findings of AP. The decision to perform PCD in the 1st two weeks was based on the evaluation by a multidisciplinary team comprising medical gastroenterologists, surgical gastroenterologists, and interventional radiologists. Indications of PCD were suspected infection, pressure symptoms, or intra-abdominal hypertension.
Infection was suspected based on the presence of gas within pancreatic or peripancreatic collection and clinical signs of infection, including non-resolving organ failure, or persistent (> 3 days) fever, leukocytosis, or elevated C-reactive protein. In all cases, the final diagnosis of infected necrosis was based on the culture of the fluid aspirated at the time of first drainage procedure.
An equal number of matched controls who underwent PCD in the third and fourth week of illness for similar clinical indications during the same period (i.e., July 2018 to September 2021) comprised the comparison group (group II). Matching was done based on the severity of AP, age, gender, modified computed tomography (CT) severity index, and organ failure using online software.
Exclusion criteria were patients with recurrent acute, acute on chronic pancreatitis, and patients with incomplete clinical details.
Patient evaluation and treatment protocol
Patients with AP were classified into moderately severe and severe AP based on the revised Atlanta classification [3]. Severe AP was defined by persistent organ failure (> 48 h, single or multiple), while patients with moderately severe AP had transient organ failure (≤ 48 h) or had local complications without persistent organ failure [3]. The modified Marshall scoring system defined organ failure [3].
All patients were managed as per standard treatment guidelines, including fluid resuscitation, oxygen, and nutritional support (enteral or parenteral). Antibiotics were given for extrapancreatic infections or infected necrosis.
Contrast-enhanced CT was performed between 5 and 7 days after the onset of pain. Contrast-enhanced CT scans of the entire abdomen (from the domes of diaphragm to the pubic symphysis) were performed on a multidetector row scanner in the portal venous phase (70 s) following intravenous injection of 80–100 ml of a non-ionic contrast media (omnipaque 300, GE Healthcare) at a rate of 2.5 ml/second using a pressure injector. In patients with suspected vascular complications (gastrointestinal bleeding, catheter bleed, or fall in hemoglobin), an arterial phase of the entire abdomen was also acquired using bolus tracking technique after injecting contrast at a rate of 3.5–4 ml/s. In all cases, the portal venous phase images were reviewed for the purpose of this study by an abdominal radiologist with 8 years of experience in reading abdominal CT scans. Modified CT severity index was based on the presence of pancreatic inflammation, detection and quantification of pancreatic necrosis (less than or more than 30% of the pancreatic parenchyma), and extra-pancreatic complications. ANC was defined on CT as an ill-defined variably loculated non-encapsulated heterogeneous collection containing both fluid and necrotic material. In contrast, acute peripancreatic fluid collection lacks the necrotic component and appears homogeneous. The number of ANCs was assessed based on the compartments involved [2]. In each of the compartments, the largest dimension was evaluated (if measurable) and added to report the size of collection.
PCD protocol
Interventional radiologists with 3–8 years of experience in non-vascular abdominal interventions performed PCD procedures. Procedures were done under ultrasound/ CT guidance. The procedures were performed with the Seldinger technique. The collections were accessed via 18-gauge needle and 0.035" stiff guidewire. Serial dilatations of the tract were done using stiff fascial dilators (8–14F) over the guidewire. Finally, a 10–14F pigtail or a malecot catheter was placed over the guidewire into the collection. The catheter was fixed with a silk suture. The drain fluid was sent for culture and antibiotic sensitivity testing. The catheter was flushed daily with 50–100 ml of normal saline. Ultrasound evaluation was performed on the 3rd day after the procedure. Earlier assessment was performed in patients when there was no significant output from the catheter. Catheter upsizing was performed between 3rd and 5th day (under ultrasound or fluoroscopic guidance, using a 2–4F larger catheter than the initial catheter) in patients who did not show signs of improvement (resolution of fever, improvement in organ function, reduction in intra-abdominal pressure) or when there was inadequate output from the catheter due to thick contents. If there was no clinical improvement within 1 week, contrast-enhanced CT scan was performed and patients with more than 50% residual collections were managed by endoscopic/surgical necrosectomy. Patients who had more than 50% reduction in the size of the collection were managed with further catheter upsizing. In case, the second catheter upsizing failed, the patients underwent necrosectomy. The catheter was removed in patients who had no fever or signs of ongoing sepsis and had complete resolution of the collection on imaging with drain output less than 10–20 mL/day for three consecutive days (Figs. 1, 2, 3).
CECT sections in a 45-year-old female with gallstone-induced acute necrotizing pancreatitis who underwent catheter drainage on day 10 of illness. A, B Axial and coronal images show acute necrotic collections (arrows). Catheter tip is denoted by double headed arrow. The drainage was performed via transperitoneal route using an initial pigtail catheter of 14F. Upsizing procedure with an 18F malecot catheter was performed on day 14. Another 14F malecot catheter was placed in the left paracolic gutter at day 12 of illness. The catheters were flushed daily with 50–100 ml of normal saline
CECT sections in a 38-year-old male with alcohol-induced acute necrotizing pancreatitis who underwent catheter drainage in 4th week of illness. A, B Axial and coronal images show intrapancreatic and peripancreatic walled off necrotic collections with catheter tip in situ (arrows). The drainage was performed via transperitoneal route using an initial pigtail catheter of 14F on day 23 of the illness. Upsizing procedure with an 18F malecot catheter was performed on day 27
CECT sections in a 56-year-old male patient with alcohol-induced acute necrotizing pancreatitis who underwent catheter drainage with a 10F catheter in 2nd week of illness. The patient complained of feculent drain output 3 days after catheter insertion. A and B show catheter tip within the colonic lumen (thick arrows). There is a large air-containing collection in the lesser sac and gastrosplenic locations (arrows). C and D Follow up CT after surgical necrosectomy. The air containing collection has mostly resolved (arrow, C). The percutaneous catheter that transgressed the colon was removed (arrow, D). The patient later succumbed to non-resolving multiple organ failure
For collections that involved more than one compartment (e.g., lesser sac collections extending to the left pararenal space and left paracolic gutter), multiple catheters were placed (either in the same session or sequentially), if there was a safe bowel/ vessel free window. The largest component of the collection was drained first. The retroperitoneal route was preferred in all cases. However, when a collection could not be drained via retroperitoneal route, transperitoneal route was employed [16]. When the lesser sac collections were completely draped by the stomach, transgastric drainage was performed. A procedure was considered technically successful if the catheter could be placed within the collection/ collections as intended. PCD was considered clinically successful if the patient recovered (collection resolved and patient discharged from the hospital) without requiring surgical intervention.
Complications were classified into major and minor based on Society of Interventional Radiology guidelines [17].
Assessment of parameters and clinical outcomes
The following baseline parameters were recorded demographic details, etiology of AP, interval from the onset of the abdominal pain to PCD (pain to PCD interval), revised Atlanta classification severity, modified CT severity index, presence of co-morbidities, organ failure, baseline C-reactive protein and procalcitonin, number, site, and a maximum dimension of collection, and presence of infected necrosis.
Intervention details documented included initial size, number of catheters, upgradations, and final catheter size. Clinical outcomes recorded were the length of hospitalization, need for intensive care unit (ICU) admission, length of ICU stay, need for surgery, and mortality.
Statistical analysis
SPSS Version 22.0 was used to analyze data. The continuous data were presented as mean with range or median with standard deviation (based on the distribution), and the categorical data were presented as percentages. The Kolmogorov–Smirnov test assessed the normality of the data. The continuous variables were compared using the student’s t test (for normally distributed data) or Mann–Whitney U test. The categorical variables were compared using the chi-square test or Fischer’s exact test. For all the tests of association, a p value of < 0.05 was considered statistically significant.
Results
Patients and baseline characteristics
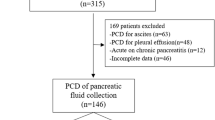
A total of 97 patients underwent PCD of ANC in the 1st two weeks. Of these, 12 patients who did not have complete baseline data and 11 patients who had recurrent AP or acute on chronic pancreatitis were excluded. Thus, 74 patients comprised group I. Additionally, over the study period, 213 patients underwent PCD of the ANC in the 3rd and 4th weeks. Of these, 74 matched controls constituted group II. Thus, 148 patients (mean age 38.9 years) were included in our study. There were 99 (66.9%) males and 49 (33.1%) females. Gallstone disease was the most common etiology (n = 65, 43.9%), followed by alcohol (n = 59, 39.8%). Severe AP and moderately severe AP were present in 135 (91.2%) and 13 (8.8%) patients, respectively. Organ failure was seen in 136 (91.8%) patients. Infected necrosis was confirmed on culture of the aspirate in 27 (36.5%) patients in group I and 35 (47.3%) patients in group II (p = 0.183). Of the patients with suspected infection (61 in group I, 63 in group II), 25 (40.9%) in group I and 32 (50.8%) in group II had positive cultures (p = 0.273). Of the patients with pressure symptoms (4 in group I, 3 in group II), one in each group had positive cultures (p = 0.809). Finally, in the IAH group (9 in group I, 8 in group II), 1 (11.1%) patient in group I and 2 (25%) patients in group II had positive cultures (p = 0.576). The most common site of the collection was the lesser sac (n = 68, 45.9%), followed by the lesser sac with paracolic extension (n = 50, 33.7%), with the mean collection size being 10.8 cm. Table 1 shows the comparison of baseline parameters between the two groups.
Clinical and biochemical features
There was no significant difference in age, sex, etiology, revised Atlanta classification severity, organ failure, and presence of co-morbidities between the groups. Baseline C-reactive protein and procalcitonin levels were not significantly different between the groups. Mean interval between onset of pain and PCD was 10.1 ± 2.9 days (9–14 days) in group I vs. 21.6 ± 4.5 days (15–30 days) in group II.
Percutaneous catheter drainage
There was no significant difference in the size and number of collections between the groups. Similarly, there was no significant difference in the initial and final catheter size and the catheter upgradations. However, the total number of catheters was significantly higher in group I (mean, 2.1 vs. 1.7, p = 0.015) (Table 2).
Technical success, clinical success, and complications
The technical success rate was 100% in both groups. The clinical success rate was 67.6% in group I vs. 77% in group II (p = 0.069). There were 16 catheter-related complications. The incidence of complications was significantly higher in group I (n = 12, 16%) than group II (n = 4, 5.4%) (p = 0.034). These included 15 minor complications (11 in group I and 4 in group II) and one major complication (only in group I). The minor complications included catheter blockage (n = 6, 4 in group I and 2 in group II), slippage (n = 2, both in group I), and pericatheter leak (n = 7, 5 in group I and 2 in group II). There was one major complication, an iatrogenic colonic perforation in one patient in the early PCD group. This patient underwent diversion colostomy with open necrosectomy. This patient died after 18 days of ICU stay.
Clinical outcomes
There was no difference in length of hospitalization, ICU stay, length of ICU stay, and mortality. However, the need for surgery was significantly higher in group I patients. Surgery was needed in 13 patients in group I compared to 5 patients who underwent surgical procedures in group II (p = 0.031). Surgical procedures performed in group I included necrosectomy (n = 8), necrosectomy with diversion ileostomy (n = 3), and diversion colostomy (n = 2). On the other hand, all five patients in group II underwent necrosectomy alone. The cause of death was multisystem organ failure.
Sub-group analysis
Patients were further divided into sub-groups based on the revised Atlanta classification severity and presence of infected necrosis.
Severe disease (n = 135)
The mean number of catheters (2.2 vs. 1.76) and complications associated with PCD (n = 16 vs. n = 4) were significantly higher in group I (p = 0.012 and 0.033, respectively). The need for surgery was also higher in group I (n = 13) compared to group II (n = 5) (p = 0.031) (Table 3).
Infected necrosis (n = 63)
Group I (n = 27) had a significantly higher mean number of catheters than group II (n = 36) (2.5 vs. 1.9, p = 0.020). PCD-related complications were also higher in group I (n = 6) than in group II (n = 2) but the difference was not statistically significant (p = 0.055) (Table 3).
Discussion
This retrospective study evaluated the safety and efficacy of early PCD of ANC in patients with acute necrotizing pancreatitis. Although, it is generally recommended to perform PCD later in the course of the disease, that is, between the third and fourth week of illness, earlier drainage (in the 1st two weeks) may be indicated. In the present study, the patients who underwent PCD in the first two weeks of illness were comparable in baseline characteristics to those who underwent PCD between the 3rd and 4th weeks. There was 100% technical success in both groups. There was no significant difference in the clinical success between the two groups. However, early PCD group needed significantly more catheters and had a significantly higher surgical rate than group II. The overall complication rate was also significantly higher in the early PCD group. However, majority of the complications were minor. Finally, early PCD group had greater need for ICU admission, longer mean ICU stays, and higher mortality than late PCD group, the differences were not statistically significant. These results suggest that despite technical feasibility, there is no potential benefit of PCD of ANC within 2 weeks of AP. Hence, early PCD within 2 weeks should not be attempted unless there is clear evidence of infected necrosis.
Patients with AP often require a multi-modality approach to managing pancreatic fluid collections, including a combination of endoscopic, percutaneous, and surgical procedures [18, 19]. The standard approach is the step-up approach, with the percutaneous/endoscopic drainage as the initial step [20]. Surgical/endoscopic necrosectomy is performed in patients who do not respond to drainage alone. Despite evolving evidence supporting the feasibility of endoscopic drainage in the first four weeks, the current recommendation is to perform endoscopic drainage after four weeks [21]. In the first four weeks, PCD is the standard procedure for draining necrotic collection. The timing of PCD has long been in debate. While most studies favor catheter drainage in the 3rd to 4th weeks, recent literature suggests the potential benefits of early drainage [10,11,12,13,14]. Early drainage has been thought to decrease the inflammatory mediators and thus ameliorate systemic inflammatory response syndrome in the early phase of the disease [18, 22]. It is, therefore, helpful in patients who deteriorate clinically in the first two weeks of illness, either secondary to systemic inflammatory response or sepsis [15]. Wang et al. reported that early-stage (less than 2 weeks) intra-abdominal PCD helped decrease the intra-abdominal pressure and the incidence of infection in patients with acute necrotizing pancreatitis [23]. Also, the most recent American Gastroenterology Association clinical practice update recommends percutaneous drainage in infected or symptomatic necrotic collections as early as 2 weeks [15]. Despite this, there is a lack of literature on the feasibility, safety, and outcomes of early PCD within two weeks.
A randomized control trial (POINTER trial) compared the outcomes after immediate drainage (within 24 h of diagnosis of infected necrosis) vs. those undergoing postponed drainage (after walled off stage) for infected pancreatic necrosis [24]. The median pain to PCD interval was 24 days (IQR 2–30 days) in the immediate group and 29 days (IQR 24–40 days) in the postponed group. The pain to PCD interval in the immediate group in the POINTER trial is considerably longer than in our study's early group. The other major difference between our cohort and the patients enrolled in POINTER trial is that all patients in POINTER trial underwent drainage for suspected infection and substantially greater number of patients were confirmed to have infected necrosis by positive culture (93% in the immediate group and 87% in the postponed group) than our cohort.
The challenges to PCD in the first two weeks of illness are technical difficulty in adequately placing catheter due to lack of encapsulation and inability to evacuate the contents due to the greater degrees of solid debris [4]. In our study and the study by Mukund et al., all the procedures were technically successful [10]. However, in our study as well as the previously published studies, the volume of the evacuated material has not been reported. The clinical success rate in the early PCD group was comparable to that reported by Mukund et al. [10]. The clinical success rate was not reported in the POINTER trial. The mean number of catheters per patient was 2.1 in our study's early PCD group, which was significantly higher than in the late PCD group. The mean number of catheters per patient (n = 3) was higher in the study by Mukund et al. [10]. This is because most of the patients in their study underwent catheter-directed necrosectomy that necessitates placement of larger catheters and hence more procedures. POINTER trial also reported a significantly greater number of catheters in the immediate group (3.1 vs. 1.9 in the postponed group), although the mean difference was not statistically significant [24].
The comprehensive complication index in the POINTER trial was comparable in the immediate and postponed groups [24]. Five (9%) patients had a perforation of visceral organs or enterocutaneous fistulae in the immediate group. In our study, one patient in the early group had bowel complication directly related to the transgression by the percutaneous catheter. We found a higher rate of minor complication in the early group compared with the late group. In the study by Mukund et al., only one major complication (hydropneumothorax due to pleural transgression by the percutaneous catheter) was reported [10].
The greater necrosectomy rate in the early group in our study is comparable to the immediate group of the POINTER trial [24]. In the early PCD group in our study, 17.5% of the patients underwent surgical necrosectomy. The higher rate of necrosectomy in the early PCD group is due to the higher necrotic contents/ solid debris that fail to drain with catheter. The necrotic contents tend to liquify over time. In the POINTER trial and Mukund et al. study, more than half of the patients underwent necrosectomy [10, 24]. In the POINTER trial, all procedures were minimally invasive (video-assisted retroperitoneal debridement (VARD)/endoscopic) [24]. In the study by Mukund et al., five patients underwent surgery, 15 VARD, and three had both VARD and surgery [10]. These differences are due to different institutional thresholds for necrosectomies.
Despite the paucity of literature, our data as well as data from the studies discussed above suggests that early PCD does not improve clinical outcomes. The complication rate is higher. Bowel perforation is a serious complication that can occur with early PCD due to lack of a well-formed posterior wall which makes it difficult to control guidewire and catheter placement. Hence, early PCD should be inserted extremely cautiously only in patients with confirmed infection of the pancreatic or peripancreatic necrosis.
There were a few limitations to our study. First, both the groups were retrospectively selected. While the early group was consecutively selected, the late group was chosen from among the patients who underwent standard drainage over the past few years. This may have led to bias. Second, there were fewer patients in the subgroup analysis. Third, comparison with patients who were managed conservatively without PCD would have added strength to the study and would have further clarified the role of early (< 2 weeks) PCD. Finally, the long-term follow-up, including the incidence of the external pancreatic fistula, was not evaluated.
Early PCD is as technically successful as late PCD in the management of ANC. The early PCD group had similar length of hospital stay, greater need for ICU admission, longer mean ICU stays, were more likely to undergo surgery, and had a higher mortality.
References
Delrue LJ, De Waele JJ, Duyck PO. Acute pancreatitis: radiologic scores in predicting severity and outcome. Abdom Imaging 2010;35:349–361.
Gupta P, Rana P, Bellam BL, et al. Site and size of extrapancreatic necrosis are associated with clinical outcomes in patients with acute necrotizing pancreatitis. Pancreatology. 2020;20:9-15.
Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111
Gupta P, Bansal A, Samanta J, et al. Larger bore percutaneous catheter in necrotic pancreatic fluid collection is associated with better outcomes. Eur Radiol. 2021;31(5):3439-3446.
Gupta P, Gupta J, Kumar C, et al. Aggressive Percutaneous Catheter Drainage Protocol for Necrotic Pancreatic Collections. Dig Dis Sci. 2020;65(12):3696-3701.
Beger HG, Büchler M, Bittner R, et al. Necrosectomy and postoperative local lavage in necrotizing pancreatitis. Br J Surg. 1988;75:207–212.
Papachristou GI, Takahashi N, Chahal P, et al. Peroral endoscopic drainage/debridement of walled-off pancreatic necrosis. Ann Surg. 2007; 245:943–951
Freeman ML, Werner J, van Santvoort HC, et al. Interventions for necrotizing pancreatitis: Summary of a multidisciplinary consensus conference. Pancreas 2012;41:1176‑94.
Choudhury SR, Manoj M, Gupta P, Samanta J, Mandavdhare H, Kochhar R. Wall maturation in necrotic collections in acute pancreatitis: a computed tomography based evaluation. Acta Gastroenterol Belg. 2022;85(3):463-467.
Mukund A, Singla N, Bhatia V, Arora A, Patidar Y, Sarin SK. Safety and efficacy of early image-guided percutaneous interventions in acute severe necrotizing pancreatitis: A single-center retrospective study. Indian J Gastroenterol. 2019;38(6):480-487
Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol. 2012;107:1146–1150
Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53(9):1340-1344.
Zhang Y, Yu WQ, Zhang J, Fu SQ, Fu QH, Liang TB. Efficacy of Early Percutaneous Catheter Drainage in Acute Pancreatitis of Varying Severity Associated With Sterile Acute Inflammatory Pancreatic Fluid Collection. Pancreas. 2020;49(9):1246-1254.
Li H, Wu Y, Xu C, An H, Guo C, Cui H. Early ultrasound-guided percutaneous catheter drainage in the treatment of severe acute pancreatitis with acute fluid accumulation. Exp Ther Med. 2018;16(3):1753-1757
Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology. 2020;158(1):67-75.e1
Verma N, Maurya M, Gupta P, et al. Retroperitoneal versus transperitoneal percutaneous catheter drainage of necrotic pancreatic collections: a comparative analysis. Abdom Radiol (NY). 2022;47(5):1899-1906.
Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199-S202
Bansal A, Gupta P, Singh AK, et al. Drainage of pancreatic fluid collections in acute pancreatitis: A comprehensive overview. World J Clin Cases. 2022;10(20):6769-6783.
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence based guidelines for the management of acute pancreatitis. Pancreatology 2013;13: 4 Suppl 2: e1-e15.
van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491-1502.
Arvanitakis M, Dumonceau J-M, Albert J, et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy 2018; 50: 524-46.
De Waele JJ. Leppaniemi AK intra-abdominal hypertension in acute pancreatitis. World J Surg. 2009;33:1128–1133.
Wang T, Liu LY, Luo H, et al. Intra-Abdominal Pressure Reduction After Percutaneous Catheter Drainage Is a Protective Factor for Severe Pancreatitis Patients With Sterile Fluid Collections. Pancreas. 2016;45(1):127-33.
Boxhoorn L, van Dijk SM, van Grinsven J, et al. Immediate versus Postponed Intervention for Infected Necrotizing Pancreatitis. N Engl J Med. 2021;385(15):1372-1381.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhatia, H., Farook, S., Bendale, C.U. et al. Early vs. late percutaneous catheter drainage of acute necrotic collections in patients with necrotizing pancreatitis. Abdom Radiol 48, 2415–2424 (2023). https://doi.org/10.1007/s00261-023-03883-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-03883-4