Abstract
Purpose
To perform a systematic review and meta-analysis of published studies to evaluate the analgesic efficacy and safety of computed tomography (CT)-guided single celiac plexus neurolysis (CPN) with the injection of a neurolytic agent into the celiac plexus in one session (CT-guided single CPN).
Methods
PubMed, the Cochrane Library, and Ichushi-Web were searched for English or Japanese articles published up to February 2022, which reported findings about patients who underwent CT-guided single CPN. The outcome measures assessed in the systematic review and meta-analysis were the pain measurement scales from 0 to 10 before and after the intervention and the rate of minor and major complications.
Results
The pooled pain measurement scales at pre-intervention and 1- or 2-, 7-, 30-, 60-, 90-, and 180-day post-intervention was 6.72 (95% confidence interval [CI], 4.77–9.46, I2 = 98%), 2.31 (95% CI 2.31–4.44, I2 = 92%), 2.84 (95% CI 1.39–5.79, I2 = 95%), 3.36 (95% CI 1.66–6.77, I2 = 98%), 3.19 (95% CI 1.44–7.08, I2 = 59%), 3.87 (95% CI 1.88–7.97, I2 = 0%), and 3.40 (95% CI 3.02–3.83, I2 = not applicable), respectively. The pooled minor complication rates of diarrhea, hypotension, nausea or vomiting, and pain associated with the procedure were 18% (95% CI 8–37%, I2 = 45%), 16% (95% CI 2–58%, I2 = 76%), 6% (95% CI 2–16%, I2 = 1%), and 7% (95% CI 2–21%, I2 = 17%), respectively. There was no major complication in the included studies.
Conclusion
CT-guided single CPN can be performed safely and provides immediate analgesic efficacy although the amount of heterogeneity is characterized as large. Further investigation of its long-term analgesic efficacy is required.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Celiac plexus neurolysis (CPN) is an intervention for ablating the neural network of the celiac plexus with the objectives of palliating chronic abdominal pain owing to malignant and benign conditions, including pancreatic cancer, inflammatory bowel disease, and chronic pancreatitis, as well as reducing the need for narcotic analgesics [1, 2]. CPN is performed using X-ray fluoroscopy [3], endoscopic ultrasound (EUS) [4], magnetic resonance imaging [5], or computed tomography (CT) [2]. Although each of these modalities has its unique advantages and disadvantages, CT-guided CPN is adopted by interventional radiologists as it is particularly advantageous for clearly visualizing retroperitoneal structures and tumor involvement, locating the needle tip, and avoiding damage to vital organs and vessels [2, 6].
Several variants of CT-guided CPN have been reported, such as single CPN with the injection of neurolytic agents into the celiac plexus in one session (CT-guided single CPN), consecutive CPN with multiple injections of neurolytic agents into the celiac plexus through an indwelling catheter, or cryoablation of the celiac plexus [7]. Among them, CT-guided single CPN is the most widely used technique. Although the role of this technique had already been established, its analgesic efficacy and safety have not been assessed in any meta-analysis. The purpose of the present study is to perform a systematic review and meta-analysis of published studies to evaluate the analgesic efficacy and safety of CT-guided single CPN.
Materials and methods
The systematic review and meta-analysis were performed in accordance with the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA). No formal approval was required at our institution for this type of study.
Literature search and study selection criteria
A literature search of PubMed, the Cochrane Library, and Ichushi-Web (Igaku Chuo Zasshi; Japan Medical Abstracts Society) was systematically conducted using relevant MeSH terms and keywords among the articles published up to February 2022 (Supplement Table). The literature search was carried out with the assistance of librarians. The inclusion criteria were as follows: (1) availability of full-text articles; (2) articles reporting data from CT-guided single CPN; and (3) articles written in English or Japanese. The exclusion criteria were as follows: (1) case reports; (2) review articles; (3) letters and editorials; (4) articles with a sample size of less than 10 cases; (5) articles with no extractable data; and (6) articles with data included in subsequent articles or duplicate reports.
Two authors (T.M. and R.Y.) independently conducted the literature search and article selection. If the reviewers disagreed, consensus was reached after discussion with a third reviewer (T.Y.).
Data extraction and quality assessment
For each selected article, we extracted the following data: baseline data for the article (first author; publication year; study period; countries; study design), total patient characteristics (number of patients; age; sex; malignancy or non-malignant disease), data concerning the CT-guided single CPN protocol (number of patients included in this study; type of sedation; patient position; needle gauge; local anesthetic prior to injecting neurolytic agent or not; contrast injection before CT-guided single CPN or not; local anesthetic mixed with neurolytic agent or not; neurolytic agent; amount of neurolytic agent), pain measurement scales at pre-intervention and 1- or 2-, 7-, 30-, 60-, 90-, and 180-day post-intervention, and complications. The complications were classified in accordance with the classification system of the Cardiovascular and Interventional Radiological Society of Europe, i.e., from grade 1 (no complication) to grade 6 (death) [8]. Specifically, grades 1 and 2 were defined as minor complications, whereas grades 3–6 were defined as major complications.
The quality of the included studies was assessed using the Cochrane Collaboration tool for randomized clinical trials and the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS) [9]. Both data extraction and quality assessment were performed independently by two reviewers (T.M. and R.Y.), with any disagreement resolved after discussion with a third reviewer (T.Y.).
Statistical analysis
The primary outcome was the change in the 0–10 visual analog scale (VAS) or 0–10 numeric rating scale (NRS) before-and-after CT-guided single CPN to evaluate its analgesic efficacy. The secondary outcome was the rate of major and minor complications to evaluate the safety of CT-guided single CPN. The pooled pain measurement scale and the rate of major and minor complications with their 95% confidence intervals (CIs) were computed using a random-effects model based on the DerSimonian–Laird method. Heterogeneity among studies was evaluated by testing Cochran’s Q statistic and the inconsistency index (I2) statistic. For Cochran’s Q test, values of p < 0.05 were considered significant. For the I2 statistic, values of < 25% were defined as low heterogeneity, 25–50% were defined as moderate, and > 50% were defined as high heterogeneity. Egger’s test was used to analyze publication bias; values of p < 0.1 were considered significant. Meta-regression analysis was conducted to identify the source of inter-study heterogeneity. A value of p < 0.05 identified the source of heterogeneity. Egger’s test and meta-regression analysis were performed if at least 4 articles were selected for each meta-analysis. The metapackage of R software (version 4.1.2; R Foundation for Statistical Computing) was used for statistical analyses.
Results
Article selection and quality assessment
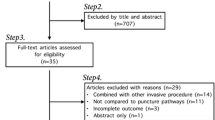
From a total of 66 articles returned by the database search, 19 articles underwent a full-text review. Then, 12 reports were excluded on the basis of the eligibility criteria [10,11,12,13,14,15,16,17,18,19,20,21]. Finally, 7 articles were selected for the systematic review and meta-analysis of the analgesic efficacy and rate of major and minor complications of CT-guided single CPN (Fig. 1) [7, 22,23,24,25,26,27]. Furthermore, 5 of the included articles were case–control studies (n = 2) and retrospective before-and-after studies (n = 3), while 2 studies were randomized control trials (RCTs). The quality of the selected RCTs (n = 2), case–control studies (n = 2), and retrospective before-and-after studies (n = 3) was assessed as some concerns, unclear, and high, respectively (Table 1).
Characteristics of the included studies
The 7 articles selected involved a total of 381 cases. The average-weighted mean age was 60 years, with 223 (59%) men and 158 (41%) women (Table 2). In the included articles, 79% had pancreatic cancer and 2% had non-malignant diseases, such as pancreatitis, persistent gastric ulceration, and median arcuate ligament syndrome.
In 3 of the 7 included articles, diazepam or midazolam was administered as sedation and the other articles were not clearly described (Table 3). Anterior or posterior techniques were described to access the celiac plexus with 18-to-23-gauge needles in the included articles (Table 3). Ethanol was used as a neurolytic agent in all the included articles. The average-weighted ethanol amount was 26 mL (range: 10– 40 mL). In 3 of the 7 articles, a local anesthetic (lidocaine) was injected immediately before ethanol injection and the other articles were not clearly described (Table 3). Iodinated contrast media were injected before injecting ethanol in all the included articles (Table 3). In 2 of the 7 articles, a local anesthetic (bupivacaine or lidocaine) was mixed with ethanol and the other articles were not clearly described (Table 3).
In 2 of the 7 articles, the numeric rating scale was used and in the remaining 5 articles, the visual analog scale was used for the assessment of pain. The number of articles for which the mean and standard deviation data of the pain measurement scales at pre-intervention and 1- or 2-, 7-, 30-, 60-, 90-, and 180-day post-intervention were provided were 5, 4, 4, 4, 2, 2, and 2, respectively (Table 4). Minor complications were described in detail in 5 articles (Table 5). There was no major complication in the included articles.
Meta-analysis
The pooled pain measurement scale at pre-intervention and 1- or 2-, 7-, 30-, 60-, 90-, and 180-day post-intervention was 6.72 (95% confidence interval [CI] 4.77–9.46, I2 = 98%, p < 0.01), 2.31 (95% CI 2.31–4.44, I2 = 92%, p < 0.01), 2.84 (95% CI 1.39–5.79, I2 = 95%, p < 0.01), 3.36 (95% CI 1.66–6.77, I2 = 98%, p < 0.01), 3.19 (95% CI 1.44–7.08, I2 = 59%, p = 0.12), 3.87 (95% CI 1.88–7.97, I2 = 0%, p = 0.35), and 3.40 (95% CI 3.02–3.83, I2 = not applicable), respectively (Figs. 2, 3). Egger’s test showed a significant publication bias for pre-intervention (Fig. 4a) (p = 0.032). There was no significant publication bias for 1- or 2-, 7-, and 30-day post-intervention (Fig. 4b, c, d) (p = 0.84, 0.99, and 0.62, respectively).
Forest plot of the pooled pain measurement scales at pre-intervention and post-intervention. a Forest plot of the overall pooled pain measurement scales at pre-intervention. b Forest plot of the overall pooled pain measurement scales at 1- or 2-day post-intervention. c Forest plot of the overall pooled pain measurement scales at 7-day post-intervention. d Forest plot of the overall pooled pain measurement scales at 30-day post-intervention. e Forest plot of the overall pooled pain measurement scales at 60-day post-intervention. f Forest plot of the overall pooled pain measurement scales at 90-day post-intervention. g Forest plot of the overall pooled pain measurement scales at 180-day post-intervention
Funnel plot of the pooled pain measurement scales at pre-intervention and post-intervention. a Funnel plot of the overall pooled pain measurement scales at pre-intervention. b Funnel plot of the overall pooled pain measurement scales at 1- or 2-day post-intervention. c Funnel plot of the overall pooled pain measurement scales at 7-day post-intervention. d Funnel plot of the overall pooled pain measurement scales at 30-day post-intervention
The pooled rates of diarrhea, hypotension, pain associated with the procedure, and nausea or vomiting were 18% (95% CI 8–37%, I2 = 45%, p = 0.12), 16% (95% CI 2–58%, I2 = 76%, p < 0.01), 7% (95% CI 2–21%, I2 = 17%, p = 0.07), and 6% (95% CI 2–16%, I2 = 1%, p = 0.40), respectively (Fig. 5). Egger’s test showed a significant publication bias for diarrhea, hypotension, pain associated with the procedure, and nausea or vomiting (Fig. 6) (p = 0.024, 0.021, 0.006, and 0.006, respectively).
Forest plot of the pooled minor complication rate. a Forest plot of the overall pooled rate of diarrhea. b Forest plot of the overall pooled rate of hypotension. c Forest plot of the overall pooled rate of pain associated with the procedure. d Forest plot of the overall pooled rate of nausea or vomiting
Funnel plot of the pooled minor complication rate. a Funnel plot of the overall pooled rate of diarrhea. b Funnel plot of the overall pooled rate of hypotension. c Funnel plot of the overall pooled rate of pain associated with the procedure. d Funnel plot of the overall pooled rate of nausea or vomiting
Meta-regression analysis
There was no evidence that the publication year, malignancy in all patients or not, total sample size, mean age, gender, type of pain measurement scale, and amount of ethanol were associated with values of the pain measurement scales, diarrhea, and hypotension in meta-regression analysis (Table 6).
Discussion
The systematic review and meta-analysis demonstrated that CT-guided single CPN immediately reduces the pain measurement scores, and the effect seems to be sustained for at least 7 and 30 days after the intervention. The effect may be sustained for 60-, 90-, and 180-day post-intervention; however, further investigation of the long-term analgesic efficacy is required owing to the small number of studies. There was no significant difference in pain control with the ethanol injection volume in this meta-regression analysis. Moreover, the demographics (age, gender), disease characteristics (malignancy in all patients or not), publication year, and total sample size were not associated with the values of pain measurement scales in this meta-analysis.
In 372 cases (98%) of a total of 381 cases in the 7 included articles, CT-guided single CPN has been performed for chronic abdominal pain associated with malignancy. In particular, chronic abdominal pain associated with pancreatic cancer accounted for 79% of all the patients in the included studies. This result is consistent with the fact that pancreatic cancer is recognized as one of the most painful malignancies with substantial suffering and is often unresponsive to typical medical management [1, 28].
The procedure of CT-guided single CPN shows that anterior or posterior techniques have been described to access the celiac plexus with 18-to-23-gauge needles in the included studies. Among them, 20 gauge or smaller needles were used in most of the included articles, which seems to be sufficient for CT-guided single CPN. Although they have not been mentioned in the included articles, lateral decubitus, posterior intradiscal, and transaortic approaches have been reported [2]. Currently, the choice of these techniques should be individualized on the basis of the operator’s preference, patient’s anatomy and comorbidities, and extent of the disease. However, because the anterior approach nearly always involves passage through the visceral organs (especially the liver and the stomach), care should be taken to minimize damage to these structures by choosing the shortest route through them, avoiding large vessels and dilated biliary ducts, and minimizing needle repositioning [6]. After iodinate contrast media were injected to determine the region of opacification and ensure the extravascular needle position, ethanol was injected as a neurolytic agent in all the included articles. These results indicate that ethanol is generally the first-choice agent for CT-guided single CPN.
Diarrhea (18% [95% CI 8–37%]) and hypotension (16% [95% CI 2–58%]) were found to be relatively frequent minor complications in this meta-analysis. These expected minor complications are due to the destruction of sympathetic signals, which causes the parasympathetic nervous system to remain unopposed. Diarrhea was transient in the included articles. However, diarrhea is rarely persistent and refractory [29]. Hypotension usually only requires an adequate intravenous bolus of normal saline during or after the procedure [30]. Other common minor complications included pain associated with the procedure (7% [95% CI 2–21%]) and nausea/vomiting (6% [95% CI 2–16%]), which was transient in the included articles. The included articles indicate that it is necessary to consider administering a local anesthetic immediately before ethanol injection or a mixture of ethanol and a local anesthetic because ethanol injection may cause severe temporary pain. There was no major complication in the included articles. Bleeding complications that require blood transfusion following fluoroscopic-guided CPN [31], lower-extremity paralysis following EUS-guided CPN [32], and thrombosis of the celiac trunk leading to hepatic, splenic, gastric, or bowel infarction following EUS-guided CPN [33] have been reported as rare major complications. Moore et al. strongly recommended CT guidance, which precisely locates the position of the needle’s point and bevel immediately before injection of the neurolytic agent, thereby avoiding complications [34]. However, publication bias was found with respect to minor complications in the meta-analysis. In other words, the minor complications reported in some studies may be underestimated. Thus, our understanding of the risks associated with CT-guided single CPN may be further limited by the underreporting of minor and severe complications.
This study has some limitations. First, the number of included studies was limited and only 7 studies that satisfied the inclusion criteria were included. In particular, the meta-analysis of the pain measurement scales 60- and 90-day post-intervention was performed in only two studies. Further, the mid-term and long-term results of CT-guided single CPN must be confirmed. Second, we could not perform subgroup analysis of the needle size, needle tip position, and access route owing to the small number of included studies. Future research should focus on these factors. Third, comparisons with other modalities, especially EUS-guided CPN, could not be made. Multicenter, large-sample, high-quality cohort studies and RCTs for CT-guided single CPN should be included in future. Despite these limitations, this systematic review and meta-analysis can provide useful information for the current clinical practice of CT-guided single CPN.
In conclusion, CT-guided single CPN can be performed safely and provides immediate analgesic efficacy although the amount of heterogeneity is characterized as large. Further investigation of its long-term analgesic efficacy is required.
References
Wyse JM, Chen YI, Sahai AV. Celiac plexus neurolysis in the management of unresectable pancreatic cancer: when and how? World journal of gastroenterology 2014;20(9):2186-2192. https://doi.org/10.3748/wjg.v20.i9.2186
Kambadakone A, Thabet A, Gervais DA, Mueller PR, Arellano RS. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics : a review publication of the Radiological Society of North America, Inc 2011;31(6):1599–1621. https://doi.org/10.1148/rg.316115526
Erdek MA, Halpert DE, González Fernández M, Cohen SP. Assessment of celiac plexus block and neurolysis outcomes and technique in the management of refractory visceral cancer pain. Pain Med 2010;11(1):92-100. https://doi.org/10.1111/j.1526-4637.2009.00756.x
Koulouris AI, Alexandre L, Hart AR, Clark A. Endoscopic ultrasound-guided celiac plexus neurolysis (EUS-CPN) technique and analgesic efficacy in patients with pancreatic cancer: A systematic review and meta-analysis. Pancreatology 2021;21(2):434-442. https://doi.org/10.1016/j.pan.2020.12.016
Liu S, Fu W, Liu Z, Liu M, Ren R, Zhai H, Li C. MRI-guided celiac plexus neurolysis for pancreatic cancer pain: Efficacy and safety. Journal of magnetic resonance imaging : JMRI 2016;44(4):923-928. https://doi.org/10.1002/jmri.25246
Cornman-Homonoff J, Holzwanger DJ, Lee KS, Madoff DC, Li D. Celiac Plexus Block and Neurolysis in the Management of Chronic Upper Abdominal Pain. Seminars in interventional radiology 2017;34(4):376-386. https://doi.org/10.1055/s-0037-1608861
Behbahani K, Chary A, Patel S, Mitchell JW, Fleishon H, Prologo JD. Percutaneous CT-Guided Cryoablation of the Celiac Plexus: A Retrospective Cohort Comparison with Ethanol. Journal of vascular and interventional radiology : JVIR 2020;31(8):1216-1220. https://doi.org/10.1016/j.jvir.2020.04.008
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovascular and interventional radiology 2017;40(8):1141-1146. https://doi.org/10.1007/s00270-017-1703-4
Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, Jang BH, Son HJ. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013;66(4):408-414. https://doi.org/10.1016/j.jclinepi.2012.09.016
Liou H, Kong MJ, Alzubaidi SJ, Knuttinen MG, Patel IJ, Kriegshauser JS. Single-Center Review of Celiac Plexus/Retrocrural Splanchnic Nerve Block for Non-Cancer Related Pain. Acad Radiol 2021;28 Suppl 1:S244-s249. https://doi.org/10.1016/j.acra.2021.03.005
Rosland JH, Geitung JT. CT guided neurolytic blockade of the coeliac plexus in patients with advanced and intractably painful pancreatic cancer. Scand J Pain 2018;18(2):247-251. https://doi.org/10.1515/sjpain-2017-0185
Osman SM, Mahmoud IH, Riad RM, Shaaban MH. Efficacy of cross-sectional imaging guided sympathetic neurolysis in abdoiniuopelvic tumors. The Egyptian Journal of Radiology and Nuclear Medicine 2018;49(3):788-796.
Edelstein MR, Gabriel RT, Elbich JD, Wolfe LG, Sydnor MK. Pain Outcomes in Patients Undergoing CT-Guided Celiac Plexus Neurolysis for Intractable Abdominal Visceral Pain. Am J Hosp Palliat Care 2017;34(2):111-114. https://doi.org/10.1177/1049909115604670
Ahmed A, Arora D. Fluoroscopy-guided Neurolytic Splanchnic Nerve Block for Intractable Pain from Upper Abdominal Malignancies in Patients with Distorted Celiac Axis Anatomy: An Effective Alternative to Celiac Plexus Neurolysis - A Retrospective Study. Indian J Palliat Care 2017;23(3):274-281. https://doi.org/10.4103/ijpc.Ijpc_28_17
Yang FR, Wu BS, Lai GH, Wang Q, Yang LQ, He MW, Ni JX. Assessment of consecutive neurolytic celiac plexus block (NCPB) technique outcomes in the management of refractory visceral cancer pain. Pain Med 2012;13(4):518-521. https://doi.org/10.1111/j.1526-4637.2012.01332.x
Kim WH, Lee CJ, Sim WS, Shin BS, Ahn HJ, Lim HY. Anatomical analysis of computed tomography images for determining the optimal oblique fluoroscope angle for percutaneous coeliac plexus block. J Int Med Res 2011;39(5):1798-1807. https://doi.org/10.1177/147323001103900522
De Cicco M, Matovic M, Bortolussi R, Coran F, Fantin D, Fabiani F, Caserta M, Santantonio C, Fracasso A. Celiac plexus block: injectate spread and pain relief in patients with regional anatomic distortions. Anesthesiology 2001;94(4):561-565. https://doi.org/10.1097/00000542-200104000-00006
Rykowski JJ, Hilgier M. Efficacy of neurolytic celiac plexus block in varying locations of pancreatic cancer: influence on pain relief. Anesthesiology 2000;92(2):347-354. https://doi.org/10.1097/00000542-200002000-00014
Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol 1999;94(4):900-905. https://doi.org/10.1111/j.1572-0241.1999.01042.x
Hilgier M, Rykowski JJ. One needle transcrural celiac plexus block. Single shot or continuous technique, or both. Reg Anesth 1994;19(4):277–283.
Endo M, Yoshida H, Fujimaki H, Yamai K. Trial of CT guided celiac nerve block. Medical Journal of Niigata Prefectural Hospital 1993(41):22-26.
Neuwersch-Sommeregger S, Köstenberger M, Stettner H, Pipam W, Breschan C, Feigl G, Likar R, Egger M. CT-Guided Coeliac Plexus Neurolysis in Patients with Intra-Abdominal Malignancy: A Retrospective Evaluation of 52 Palliative In-Patients. Pain Ther 2021;10(2):1593-1603. https://doi.org/10.1007/s40122-021-00317-1
Abdelbaser I, Shams T, El-Giedy AA, Elsedieq M, Ghanem MA. Direct intraoperative versus percutaneous computed tomographyguided celiac plexus neurolysis in non-resectable pancreatic cancer: A randomized, controlled, non-inferiority study. Rev Esp Anestesiol Reanim (Engl Ed) 2021. https://doi.org/10.1016/j.redar.2020.12.016
Arai YC, Nishihara M, Kobayashi K, Kanazawa T, Hayashi N, Tohyama Y, Nishida K, Arakawa M, Suzuki C, Kinoshita A, Kondo M, Matsubara S, Yokoe N, Hayashi R, Ohta A, Sato J, Ushida T. Neurolytic celiac plexus block reduces occurrence and duration of terminal delirium in patients with pancreatic cancer. J Anesth 2013;27(1):88-92. https://doi.org/10.1007/s00540-012-1486-3
Zhang CL, Zhang TJ, Guo YN, Yang LQ, He MW, Shi JZ, Ni JX. Effect of neurolytic celiac plexus block guided by computerized tomography on pancreatic cancer pain. Dig Dis Sci 2008;53(3):856-860. https://doi.org/10.1007/s10620-007-9905-2
Lee JM. CT-guided celiac plexus block for intractable abdominal pain. J Korean Med Sci 2000;15(2):173-178. https://doi.org/10.3346/jkms.2000.15.2.173
De Cicco M, Matovic M, Balestreri L, Fracasso A, Morassut S, Testa V. Single-needle celiac plexus block: is needle tip position critical in patients with no regional anatomic distortions? Anesthesiology 1997;87(6):1301-1308. https://doi.org/10.1097/00000542-199712000-00007
Bahn BM, Erdek MA. Celiac plexus block and neurolysis for pancreatic cancer. Curr Pain Headache Rep 2013;17(2):310. https://doi.org/10.1007/s11916-012-0310-y
Mercadante S. Celiac plexus block versus analgesics in pancreatic cancer pain. Pain 1993;52(2):187-192. https://doi.org/10.1016/0304-3959(93)90130-h
Urits I, Jones MR, Orhurhu V, Peck J, Corrigan D, Hubble A, Andrews M, Feng R, Manchikanti L, Kaye AD, Kaye RJ, Viswanath O. A Comprehensive Review of the Celiac Plexus Block for the Management of Chronic Abdominal Pain. Curr Pain Headache Rep 2020;24(8):42.https://doi.org/10.1007/s11916-020-00878-4
Warner NS, Moeschler SM, Warner MA, Hoelzer BC, Eldrige JS, Bendel MA, Mauck WD, Watson JC, Gazelka HM, Lamer TJ, Kor DJ, Hooten WM. Bleeding Complications in Patients Undergoing Celiac Plexus Block. Reg Anesth Pain Med 2016;41(4):488-493. https://doi.org/10.1097/aap.0000000000000409
Fujii L, Clain JE, Morris JM, Levy MJ. Anterior spinal cord infarction with permanent paralysis following endoscopic ultrasound celiac plexus neurolysis. Endoscopy 2012;44 Suppl 2:E265-266. https://doi.org/10.1055/s-0032-1309708
Gimeno-García AZ, Elwassief A, Paquin SC, Sahai AV. Fatal complication after endoscopic ultrasound-guided celiac plexus neurolysis. Endoscopy 2012;44 Suppl 2:E267. https://doi.org/10.1055/s-0032-1309709
Moore DC. The dreaded complications from neurolytic celiac plexus blocks are preventable! Reg Anesth Pain Med 2004;29(4):377-378. https://doi.org/10.1016/j.rapm.2004.02.001
Acknowledgements
None
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsumoto, T., Yoshimatsu, R., Osaki, M. et al. Computed tomography-guided single celiac plexus neurolysis analgesic efficacy and safety: a systematic review and meta-analysis. Abdom Radiol 47, 3892–3906 (2022). https://doi.org/10.1007/s00261-022-03670-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03670-7