Abstract
Background
An interventional procedure like celiac plexus neurolysis (CPN) has a significant role in relieving intractable pain in patients with locally advanced abdominal malignancies. Ultrasound (USG) guidance enables performance of bedside CPN by real-time visualization of the needle trajectory. The objective of the study was to perform percutaneous USG-guided CPN and to verify technical outcomes of the procedure using a post-procedure CT scan.
Methods
Eleven eligible patients of advanced upper abdominal malignancies having a pain score of >3/10 on visual analog scale (VAS) were recruited to undergo CPN. A post-procedure CT scan was performed to evaluate technical outcomes of the procedure. Patients were evaluated for pain relief. They were followed up at the 1st, 4th, and 6th weeks after CPN.
Results
Eleven patients underwent USG-guided CPN. The injected drug was visualized as an echogenic cloud in ultrasound in 7 out of 11 (64%) patients. In the remaining 4 patients, the echogenic cloud was not well formed. In the post-procedure CT scan, the spread of the drug was seen in all 11 patients. This spread was bilaterally symmetrical in 7 (64%) patients and asymmetrical or unilateral in 4 (36%) patients. All patients in the immediate post-procedure period and 91% of the patients during the 1st-, 4th-, and 6th-week follow up had improvement in their pain scores.
Conclusion
A post-procedure CT scan was useful in verifying the technical outcome of USG-guided CPN in patients with advanced upper abdominal malignancies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Visceral pain in patients with advanced upper abdominal cancers impairs quality of life and survival. In these patients, management of pain is an important aspect of palliative care provision [1]. Although oral and transdermal analgesia are the first line of treatment, they are often found to be ineffective. Celiac plexus neurolysis (CPN) is an established interventional technique, which is useful in this group of patients [2].
The celiac plexus has multiple ganglia which receive sympathetic fibers from the splanchnic nerves and parasympathetic fibers from the vagus nerves. The celiac plexus is responsible for transmitting pain sensation from the abdominal organs including the liver, pancreas, gallbladder, stomach, spleen, kidneys, adrenals, and intestine except the left colon, rectum, and pelvic organs [3]. CPN is akin to chemical splanchnicectomy of the celiac plexus causing ablation of the efferent nerve fibers.
Kappis first introduced the percutaneous technique for CPN in 1914 [4]. Since then, several modifications have been introduced to the procedure. Image guidance has made the procedure more effective and safe [5, 6]. Before CT-guided CPN, traditionally, CPN was performed under fluoroscopy. Both these guiding modalities have limitations in terms of radiation exposure and non-portability. CPN can also be performed under ultrasound guidance, either percutaneous ultrasound or endoscopic ultrasound (EUS). The success rate of EUS-guided CPN is 80% [7], whereas that of percutaneous USG-guided CPN is 91% to 93% [8, 9]. Both techniques need expertise for guided needle insertion. In obese patients and patients with gaseous abdomen, visualization of retroperitoneal structures becomes difficult in percutaneous ultrasound [7,8,9], whereas in a patient with previous surgery or a large tumor mass, it is very difficult to locate the anatomical landmark and place the needle tip correctly during EUS-guided CPN. Furthermore, cachexia can cause loss of the soft-tissue space between the gastric wall and the aorta leaving little room to place the tip of the needle. An ectatic aorta or an eccentric origin of the celiac artery may create technical difficulties as well. Celiac ganglia can be difficult to visualize in about 20% of patients, which makes direct ganglia injection impossible [9, 10]. Besides, the use of endoscopic ultrasound requires special equipment and formal training in gastroenterology [9].
Hence, we aimed to perform percutaneous USG-guided CPN using the anterior approach and verify its technical outcomes with an immediate post-procedural CT scan which is a superior and better-established imaging modality to demonstrate the technical outcome of the procedure. In this small study, we also evaluated short-term clinical outcomes of USG-guided CPN.
Methods
The prospective observational study was conducted in a tertiary care cancer hospital after approval from the institutional ethics committee. Patients with advanced upper abdominal malignancies attending the pain clinic were evaluated and assessed for feasibility of CPN and were recruited to this study depending on the availability of the required logistics in the CT suite. The procedure was explained to the patient and written informed consent was obtained. Figure 1 demonstrates the study protocol followed.
Patient selection
Patients with advanced upper abdominal malignancy complaining of abdominal pain not relieved with conservative management and satisfying the inclusion and exclusion criteria were included in the study (see Table 1). Demographic data, clinical examination findings, and biochemical details including coagulation profile were recorded (Table 2). Recent imaging with USG and CT was analyzed for feasibility of CPN.
Pain assessment
Intensity of pain was assessed using the visual analog scale (VAS) on a scale of 0–10, 0 being no pain and 10 being maximal unbearable pain [11]. Patients on weak or strong opioids with VAS >3/10 (pain of moderate to severe intensity) having intolerable opioid-related side effects or refractory to this treatment were considered for USG-guided CPN.
Pre-procedure imaging evaluation
Cross-sectional imaging like CT or magnetic resonance imaging (MRI) during the period of pain was assessed for feasibility of CPN. In case the patient did not have recent imaging or imaging prior to onset of pain, the same was repeated before deciding for the procedure. The imaging was evaluated in terms of extent of primary malignancy, especially the presence or absence of encasement or displacement of the celiac axis by a tumor or lymph nodes and preservation of fat around the celiac artery along the anatomical location of the celiac plexus. Other factors like infection or abscess in the abdominal wall or peritoneal cavity were excluded. Ascites was considered as a relative contraindication. If ascites was mild and the patient had a normal coagulation profile, then the patient was eligible for CPN. If the ascites was moderate to severe, it was first drained and the patient was reevaluated for the feasibility of CPN [12].
Technique of neurolysis (CPN)
The patients were asked to fast overnight. The procedure was discussed and planned in collaboration with the experienced radiologist before the patient was shifted to the CT suite. The patient was positioned to lie supine on the CT table (Fig. 2). Vital signs and ECG were monitored throughout the procedure. A preliminary USG was performed using a 3–5 MHz convex transducer of a portable sonography machine (SonoSite INC, Bothell, WA, USA) to decide the site and approach of needle insertion. With all aseptic precautions, following local anesthesia, a 15-cm-long 22-G Chiba needle was inserted under real-time USG guidance through the right or left route till its tip reached the pre/para-aortic location at the level of the celiac axis. The side of approach was determined on the basis of ease, comfort, and the experience of the interventionist. Once the needle was in the desired location, negative aspiration of blood was checked. Thirty milliliters of drug (14 mL 98% ethanol + 14 mL 0.5% bupivacaine + 2 mL non-ionic iodinated contrast) was injected using a glass syringe under USG guidance. As the drug was injected, an expanding echogenic area was seen on ultrasound at the site of injection (Table 3). This is caused by micro bubbles in the freshly loaded drug and it is termed as an echogenic cloud. It offers an indirect evidence of deposition and spread of the drug on ultrasound. At the end of the injection, CT was performed without removing the needle to evaluate (a) the needle location and (b) the side and spread of the drug as unilateral or bilateral, whether bilaterally equal or unequal and if there was any retrocrural or inadvertent injection of the drug. After the CT, the needle was removed while injecting saline so as to prevent alcohol spread along the needle track.
Representative pictures from different patients to show the steps of CPN: a CT during evaluation shows preserved fat (asterisk) around the celiac axis (arrows). b The patient lies down supine on the CT table, and the area under interest is cleaned and draped. c Under ultrasound guidance using a convex probe, a 22-G Chiba needle is inserted. d The tract of the needle is visualized on the USG screen (marked by arrows). e Subsequently, 30 mL of the drug including 2 mL of non-ionic iodinated contrast is injected which is seen as an echogenic cloud around the celiac axis (marked with asterisks) depicting adequate spread of the drug. f Post-procedure CT confirmed bilaterally symmetrical spread of the drug (arrows). CA celiac axis, CHA common hepatic artery, SA splenic artery
Post-procedure assessment
The patients were assessed for severity of pain immediately after the procedure and were shifted to the palliative care ward for monitoring. A modified analgesic regimen based on World Health Organization (WHO) guidelines was administered to the patient, and additional morphine was advised for any breakthrough pain [13] (http://apps.who.int/iris/bitstream/10665/37896/1/9241544821.pdf). Any complication following CPN was recorded and managed.
The patients were assessed for (a) any intra-procedural complications like injury to vessels, injury to viscera, or any persistent pain and (b) any post-procedure complications like hematoma, fever, diarrhea, symptoms of alcohol intoxication, neurological complications, vomiting, hypotension, etc.
Follow up
After discharge, the patients were followed up at the 1st, 4th, and 6th weeks, either on an outpatient basis or by telephonic interview. They were asked about the intensity of pain, analgesic requirements, adverse effects due to analgesics, concurrent treatment in the form of chemo- or radiotherapy, status of primary disease, and patient survival.
Statistical analysis
Sex, approach of CPN, and diagnosis of the patients are described in terms of percentage. Pain score and age are expressed as mean±standard deviation (SD). All these descriptive statistics, e.g. percentage (%), mean, SD, and range, were done using SPSS 16.0 software.
Results
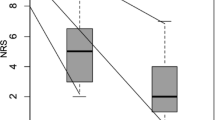
Among the 11 patients, 72.7% (8/11) were male patients and 27.2% (3/11) were female, with the age group ranging from 40 to 68 years (mean 50.8±8.9 years). All the patients had advanced abdominal malignancies not operable at the time of presentation and complained of upper abdominal and epigastric pain. 36.4% (4/11) of the patients had carcinoma of the pancreas, and 27.3% (3/11) had gallbladder cancer with enlarged retroperitoneal lymph nodes and infiltration of the adjacent structures. They presented with abdominal pain with VAS ranging from 5 to 10/10 (mean 7.7±1.4) for a duration of 1–6 months which was not getting relieved with oral analgesics or opiates. Only one patient was receiving concurrent treatment like chemotherapy for primary malignancy. On preliminary imaging, the celiac axis was free of retroperitoneal fat in every patient and, thus, USG-guided CPN using the single-needle technique was planned in each of them. Right-sided needle insertion was opted for 9 (81.8%) patients, and the left-sided approach was considered suitable in 2 (18.2%) patients. In 2 out of 11 patients, there was blood on negative aspiration after USG-guided needle placement in the desired site. Minor changes in needle placement were done in those cases. In one patient, difficulty was encountered in visualizing the needle tip. During injection of the drug under ultrasound guidance, echogenic cloud formation could not be visualized in 4 out of 11 patients. However, on post-procedure CT scans, the spread of the drug was well appreciated in all patients. The spread of the drug was seen as bilaterally symmetrical in 7 out of 11 patients, asymmetrical or unilateral in the remaining 4 patients. Retrocrural component of spread was seen in 5 patients (Fig. 3). No patient had visceral spillage of the drug. No major procedure-related complication was experienced in our study. One patient developed transient diarrhea after CPN which was managed conservatively with intravenous fluids; another patient had nausea and vomiting which was probably attributed to concurrent chemotherapy, and 1 of 11 patients had hypotension which was responded to with intravenous fluids. At the time of discharge, all the patients had significant reduction in the pain intensity with VAS 1–2/10 (mean 1.9±0.3) and, at the 1st-, 4th-, and 6th-week follow up, 91% (10/11) of the patients experienced pain with VAS ≤3/10. Only 1 patient experienced an increase in pain intensity in the follow up period due to progressive primary disease as confirmed on subsequent imaging evaluation. Only one of our patients received chemotherapy simultaneously with the procedure. It may have led to improvement in pain control from chemotherapy also. But other 10 patients had not received chemotherapy simultaneously.
Patterns of drug spread on CT: a bilaterally symmetrical around the celiac axis (arrows), b asymmetrical spread with predominant spread of the drug along the right para-aortic region (arrow), and c asymmetrical spread around the celiac axis (black arrow) as well as posteromedial to the left diaphragm (white arrow) in the retrocrural location (arrowheads). Ao abdominal aorta, asterisk celiac axis
Discussion
Chronic intractable abdominal pain is common in patients with advanced intra-abdominal malignancy, and its management is an important aspect of palliative care provision. Invasion of the celiac plexus is the most common cause of pain in patients with upper abdominal malignancy [1, 2]. The WHO analgesic step ladder is routinely followed in these patients, which consists of NSAIDs, weak opiates, and strong opiates. Interventional therapies are often reserved for patients with pain refractory to these measures. At present, strict adherence to the WHO ladder is not considered mandatory and a tailored approach to the individual patient is recommended. Although medical management of pain using opiates, steroids, etc. is common, it is often associated with various drug-related adverse effects. Celiac plexus neurolysis (CPN) has gained wide acceptance among treating physicians in achieving pain control and reducing the consumption of opioids [4, 14]. Although CPN is recommended for pain refractory to pharmacological management, it may be also considered as the first line of management of pain secondary to upper abdominal malignancy [15]. Imaging guidance for CPN is required for guiding the needle to the region of the celiac plexus. Fluoroscopy or CT guidance is considered as the modality of choice as it is standardized and reliably demonstrates the delivery and spread of the drug to the target by virtue of radiographic contrast medium mixed with the drug [4]. However, these modalities are time consuming, non-portable, unable to provide real-time visualization, and associated with risk of radiation to the patient as well as to the physician. USG on the other hand, overcomes all these disadvantages and is readily available for conducting bedside procedures [8, 9]. Although USG-guided procedures are comparatively cheaper and faster to perform, they are yet to gain acceptance among radiologists and pain physicians. Major disadvantages with ultrasound include operator dependency, requirement of expertise for guided needle insertion, and inability to visualize retroperitoneal structures in obese patients and gaseous abdomen [7,8,9]. Radiographic contrast mixed with a neurolytic agent is well seen on CT- or fluoroscopy-guided procedure and acts as a surrogate marker of accurate delivery and spread of the neurolytic agent. However, this cannot be seen on ultrasound and, hence, the operator remains unsure of the spread of the drug [4, 7,8,9]. We performed ultrasound-guided CPN using the anterior abdominal approach, and a CT scan was used to validate the technical outcomes of the procedure. USG-guided CPN showed a 100% technical success rate, as far as the post-procedure CT scan was concerned. If the spread of the iodinated contrast mixed with the neurolytic agent was seen around the celiac trunk, it was considered as successful CPN. Technical success was also confirmed by immediate post-procedure pain relief in all patients without any major procedure-related complication. High success rates of ultrasound-guided CPN were reported previously by Tadros and Elia (95.2%) [16] and Marcy et al. (93%) [8]. The latter also compared it with the technical success of CT-guided CPN and found no significant statistical difference between the two guiding modalities. In this study, USG-guided CPN is validated by CT scan images of drug spread for the first time. All the patients in the study had advanced upper abdominal malignancies and were receiving adjuvant treatment for the same. They presented to us with moderate to severe pain (VAS 7.7±1.4) not relieved with opioids and responded very well to CPN by demonstrating significant reduction in pain severity (VAS 1.9±0.3), which was maintained over a 6-week follow up period in 91% of the patients. Only one patient (9%) experienced increased pain intensity at 1 week follow up which was due to progression of the primary malignancy. Similar results were shown by Tadros et al. who conducted USG-guided CPN in 21 patients with significant reduction in pain severity from VAS 9.1±0.85 to 1.4±0.71 at 1 day post-procedure and 2±0.79 after 1 month of CPN [16]. In agreement with Bhatnagar et al. we also noted significant reduction in the opioid consumption in these patients following neurolysis [9]. Fluoroscopy- or CT-guided CPN is generally performed in the prone position. This is uncomfortable to most patients with advanced and painful abdominal malignancy. Many patients, especially those with severe abdominal distention, are unable to maintain this position for a long duration. The percutaneous anterior approach in the supine position is more comfortable to the patient and also prevents neurological complications due to inadvertent injection of neurolytic agent in the spinal artery or spinal column [17, 18]. Gastric or bowel perforation, vascular injury, hematoma, and chemical peritonitis have been reported with the anterior approach in fewer than 2% of patients [19]. These complications may occur from direct penetration by a needle or by chemical inflammation from the neurolytic agent, particularly if diffusion of the agent is not controlled. The most serious potential complication is paralysis of the lower extremities, which is extremely rare and reported to occur in less than 0.15% of patients [20]. The USG-guided anterior approach through a transhepatic or transgastric route enables real-time dynamic visualization of the needle in its entire tract. Use of color Doppler helps us to visualize and accurately localize the aorta and the celiac, hepatic, and splenic arteries during the procedure [8]. Ultrasound-guided CPN with the anterior approach using a single needle is associated with satisfactory drug delivery on both sides of the celiac trunk in most patients resulting in adequate pain control. In many studies, it is seen that there is no difference between central (single) injection and bilateral injection as far as post-procedure pain relief was concerned. As the two-injection approach is associated with severe injury to the left adrenal artery and also there is a lack of clear advantages with this technique, it is better to avoid this two-needle insertion technique [21, 22]. Ultrasound-guided CPN is a well-established procedure at our center, and most of it is performed bedside. The limitation of this study is small sample size. It is logistically not possible to perform procedures in the CT room in a busy tertiary care center of the country frequently, so a smaller number of patients are recruited for the study. In conclusion, a post-procedure CT scan was useful to verify the technical efficacy of ultrasound-guided CPN. Short-term outcomes in a small group of patients with malignancy were good.
References
Staats PS, Hekmat H, Sauter P, Lillemoe K. The effects of alcohol celiac plexus block, pain, and mood on longevity in patients with unresectable pancreatic cancer: a double-blind, randomized, placebo-controlled study. Pain Med Malden Mass. 2001;2:28–34.
Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291:1092–9.
Loukas M, Klaassen Z, Merbs W, Tubbs RS, Gielecki J, Zurada A. A review of the thoracic splanchnic nerves and celiac ganglia. Clin Anat N Y N. 2010;23:512–22.
Jain PN, Shrikhande SV, Myatra SN, Sareen R. Neurolytic celiac plexus block: a better alternative to opioid treatment in upper abdominal malignancies: an Indian experience. J Pain Palliat Care Pharmacother. 2005;19:15–20.
Hilgier M, Rykowski JJ. One needle transcrural celiac plexus block. Single shot or continuous technique, or both. Reg Anesth. 1994;19:277–83.
Worsey J, Ferson PF, Keenan RJ, Julian TB, Landreneau RJ. Thoracoscopic pancreatic denervation for pain control in irresectable pancreatic cancer. Br J Surg. 1993;80:1051–2.
Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–7.
Marcy PY, Magné N, Descamps B. Coeliac plexus block: utility of the anterior approach and the real time colour ultrasound guidance in cancer patient. Eur J Surg Oncol. 2001;27:746–9.
Bhatnagar S, Khanna S, Roshni S, et al. Early ultrasound-guided neurolysis for pain management in gastrointestinal and pelvic malignancies: an observational study in a tertiary care center of urban India. Pain Pract. 2012;12:23–32.
Gleeson FC, Levy MJ, Papachristou GI. Frequency of visualization of presumed celiac ganglia by endoscopic ultrasound. Endoscopy. 2007;39:620–4.
Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S240–52.
Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. Brit Soc Gastroenterol. Gut. 1999;45 Suppl 4:IV1–11.
Cancer pain relief and palliative care. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1990; 804:1–75.
Amr YM, Makharita MY. Comparative study between 2 protocols for management of severe pain in patients with unresectable pancreatic cancer: one-year follow-up. Clin J Pain. 2013;29:807–13.
Mercadante S, Nicosia F. Celiac plexus block: a reappraisal. Reg Anesth Pain Med. 1998;23:37–48.
Tadros MY, Elia RZ. Percutaneous ultrasound-guided celiac plexus neurolysis in advanced upper abdominal cancer pain. Egypt J Radiol Nucl Med. 2015;46:993–8.
Montero Matamala A, Vidal Lopez F, Inaraja ML. The percutaneous anterior approach to the celiac plexus using CT guidance. Pain. 1988;34:285–8.
Montero Matamala A, Vidal Lopez F, Aguilar Sanchez JL, Donoso BL. Percutaneous anterior approach to the coeliac plexus using ultrasound. Br J Anaesth. 1989;62:637–40.
Kambadakone A, Thabet A, Gervais DA, Mueller PR, Arellano RS. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics. 2011;31:1599–621.
Wang PJ, Shang MY, Qian Z, Shao CW, Wang JH, Zhao XH. CT-guided percutaneous neurolytic celiac plexus block technique. Abdom Imaging. 2006;31:710–8.
Sahai AV, Lemelin V, Lam E, Paquin SC. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326–9.
Téllez-Ávila FI, Romano-Munive AF, Herrera-Esquivel Jde J, Ramírez-Luna MA. Central is as effective as bilateral endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. Endosc Ultrasound. 2013;2:153–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SB, ST, ED, IK, RN, and GC declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 as revised in 2008. We obtained informed consent from individual patients, and institutional ethics committee clearance was obtained.
Rights and permissions
About this article
Cite this article
Bhatnagar, S., Thulkar, S., Dhamija, E. et al. Evaluation of outcomes of ultrasound guided celiac plexus neurolysis using immediate post procedure computed tomography: An observational study. Indian J Gastroenterol 36, 282–288 (2017). https://doi.org/10.1007/s12664-017-0780-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-017-0780-2