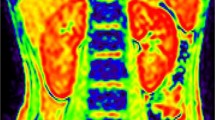
Abstract
Aim
Primary objective of this study was to compare R2* value of the post-stenotic kidney with contralateral kidney, kidneys of essential hypertensive patients, and healthy subjects using blood oxygen level-dependent magnetic resonance imaging (BOLD MRI) technique. The secondary objective was to study the effect of severity of stenosis and viability of kidneys on R2* value.
Methods
We compared 4 groups of kidneys including 92 with renal artery stenosis, 37 normal contralateral kidneys of unilateral renal artery stenosis patients, 62 kidneys of essential hypertensive patients, and 40 kidneys of healthy controls using BOLD MRI. Deoxyhemoglobin level represented by R2* was calculated before and after giving furosemide and was compared among different groups.
Results
Baseline means cortical R2* value did not differ between groups. Response to furosemide was reduced in stenotic kidneys as compared to essential hypertensive and healthy control groups (p < 0.001). The mean R2* value of the contralateral normal kidney group was not significantly different from the stenotic group. Baseline R2* value and delta R2* values did not differ between different degrees of stenosis. Higher mean cortical R2* was seen in stenotic kidneys which were small (< 7 cm) in size (24.27 ± 5.65 vs 21.7 ± 3.88; p value 0.02) or with poor corticomedullary differentiation (24.64 ± 5.8 vs 20.74 ± 3.34; p value 0.006) as compared to other stenotic kidneys. Similarly, the delta R2* value was also blunted in these small shrunken kidneys (p value < 0.001).
Conclusion
R2* values on BOLD MRI are significantly different between kidneys with and without renal artery stenosis and can potentially also predict the utility of revascularization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Renovascular hypertension (RVH) is one of the treatable causes of hypertension. Renal artery stenosis (RAS) is caused by a heterogeneous group of conditions, like atherosclerosis, vasculitis, fibromuscular dysplasia, neurofibromatosis, congenital bands, extrinsic compression, and post-radiation [1]. Decreased renal artery perfusion secondary to RAS activates pressure pathways such as renin–angiotensin–aldosterone system to restore renal artery perfusion pressures, resulting in RVH [2]. Normally renal perfusion is far beyond the basic metabolic needs of the kidney. Therefore, a decrease in renal blood flow is not associated with a simultaneous decrease in tissue oxygen content. This fact is supported by maintained renal vein oxygenation and erythropoietin levels in post-stenotic kidneys [3]. With increasing stenosis and reduction in perfusion pressure, there is a reduction in the glomerular filtration rate (GFR) and decreased Na+ reabsorption across the tubules that lead to reduced oxygen consumption and maintenance of tissue oxygen level despite a reduction in blood flow. This helps in the long-term stability of patients with renal artery stenosis on antihypertensive therapy. However, after a while, these adaptive changes are overwhelmed leading to loss of kidney function, tissue fibrosis, and end-stage renal disease [1, 4]. Whether such changes are triggered by renal hypoxia they lead to renal hypoxia is not proven yet.
Normally, most of the oxygen-dependent active processes and transports occur in the medulla as compared to the cortex. Therefore, the medulla is a metabolically highly active zone of the kidney and has high oxygen demand as compared to the cortex. High oxygen demand indirectly shows an increased amount of deoxyhemoglobin in the medulla. Recently blood oxygen level-dependent (BOLD) MRI has emerged as the only non-invasive technique of measuring regional tissue oxygenation within the kidney [5, 6]. BOLD MRI is based on the paramagnetic property of deoxyhemoglobin. The presence of deoxyhemoglobin affects the T2* relaxation time of neighboring water molecules and in turn, influences the MRI signal of the T2*-weighted gradient-echo image. Deoxyhemoglobin accelerates the rate of spin dephasing due to which there is a decrease in T2* relaxation time and an increase in apparent relaxation rate which is given by R2* (1/T2*, 1/s). Therefore R2* value is closely related to the regional concentration of deoxyhemoglobin [5,6,7,8]. It can determine local tissue oxygen, assuming that blood pO2 is in equilibrium with tissue pO2. Different methods described for analyzing BOLD images, include the ROI technique, TLCO (12 layers concentric objects) technique, compartmental method, and fractional tissue hypoxia technique. [9,10,11] ROI technique is the most frequently used method in literature but has more interobserver variability [11]. BOLD MRI has been validated in animal studies, showing that R2* correlates negatively with directly measured pO2 [12, 13]. It has been used extensively in organs, such as the brain [14]. Recently BOLD MRI is being investigated for its potential application in renal pathologies, like renal artery stenosis, renal allograft rejection, hypertension, chronic kidney disease, and diabetic nephropathy [11].
Large randomized trials such as the Angioplasty and Stenting for Renal Artery Lesions trial (ASTRAL) have not provided good results concerning renal artery angioplasty ± stenting in RAS patients [15]. Nevertheless, it is well known that some patients benefit from angioplasty and the main question remains how to predict the outcome of this procedure. In this situation, BOLD MRI can lead to early identification of kidneys that are likely to suffer from irreversible injury and their potential for salvageability by revascularization can be assessed.
The main objective of the present study was to compare intrarenal R2* value of post-stenotic kidney to contralateral kidney and with kidneys of essential hypertensive patients and normal subjects. As a secondary objective, we sought to study the effect of severity of the degree of stenosis and viability of the kidney on intrarenal R2* value.
Materials and methods
Study design
A hospital-based prospective study was conducted from February 2016 to October 2019 after approval from the institutional ethics committee. Written informed consent was taken from all the study participants.
Hundred non-diabetic patients in whom there was suspicion of renal artery stenosis based on the clinical history and Doppler findings were evaluated in our institution by MR angiography (MRA) using non-contrast MR technique as well as contrast angiography (if estimated GFR > 30 mL/min). On MR angiography renal artery stenosis was diagnosed in 65 of these patients. In 31 patients, renal arteries were normal and no other cause for hypertension could be identified, thus they were considered to be having essential hypertension. The remaining 4 patients were excluded from the further study based on the presence of other causes of hypertension (Fig. 1). Besides, 20 healthy volunteers (controls) were also enrolled in the study. Intake of loop diuretics (if any) was withheld for at least 2 days before the study. The remaining medications including ACE inhibitors and angiotensin receptor blockers were continued. Normal hydration and 4 h of fasting before the MRI study were ensured.
MRI technique
MR angiography protocol
MR imaging was done using a GE Signa 3.0 Tesla Unit (GE Healthcare, Wisconsin, USA). The coil used was Torso PA. A coronal T2 sequence of the abdomen was done initially to assess the kidney size and location. An unenhanced renal MRA sequence was done next. If there was no contraindication to Gadolinium contrast agent and patients’ GFR was more than 30 mL/min, contrast-enhanced MRA sequence was also added after doing BOLD MRI. The non-contrast-enhanced MRA sequence was performed using a 3D fat-suppressed inflow inversion recovery SSFP sequence (Inhance, GE Medical). This 3D Balanced SSFP-based application produces high-quality 3D bright blood images with a significantly increased signal-to-noise ratio. The imaging sequence was planned in the transverse imaging plane with an imaging range covering both kidneys. Free breathing was allowed with respiratory bellows used. The phase lines were acquired via respiratory triggering. Parallel imaging (array spatial sensitivity encoding technique (ASSET)) was used in the in-plane phase encoding direction with an acceleration factor of 2.
BOLD MRI protocol
BOLD MRI was performed using a GE Signa 3.0 Tesla unit (GE Healthcare, Wisconsin, USA), using an end-expiratory breath-hold 2D MPGR R2* sequence with a phased array torso coil and 16 gradient echoes. Four sections were obtained in the coronal plane covering the entire kidney (slice thickness: 5 mm). After the first BOLD acquisition, furosemide (20 mg) was administered intravenously and flushed with 20 mL of saline. BOLD measurements were taken 15 min later. Loop diuretics like furosemide inhibit ion transporters in thick ascending limb of loop of Henle, reducing the local oxygen consumption, and thus increase medullary Po2. The improvement in tissue oxygenation (or increase in BOLD signal intensity) after furosemide stimulus means that the kidney with RAS is likely to benefit from a revascularization procedure.
Image analysis
The size of the kidney was measured on coronal T2W images (Fig. 2a). MR angiography findings were used to determine as well as assess the severity of renal artery stenosis. Non-contrast (Inhance) angiography images were used for the evaluation of stenosis (Fig. 2b). Percentage of stenosis was calculated using the formula (D − d)/D (where D is the diameter of normal downstream renal artery; d is the diameter of the narrowest segment of the stenosed artery). In cases where there was diffuse stenosis, the diameter of the opposite normal renal artery was taken (as D) and in cases where there was bilateral diffuse stenosis, the normal reference range of renal arteries (as D) in the Indian population was taken into account [16]. And subsequently, stenosis kidneys were grouped as follows: (1) < 50% luminal narrowing as mild, (2) 50–70% luminal narrowing as moderate, and (3) > 70% luminal narrowing as severe [17].
a T2W MR image (coronal plane) showing bilateral normal-sized kidneys (RK 11.1 cm, LK 98.5 cm). b Non-contrast MR angiography (INHANCE) depicting bilateral normal renal arteries in axial plane. c, d Corresponding BOLD MR images of bilateral normal kidneys at baseline showing parametric gray scale and colored BOLD MR image. ROI placed in upper, mid, and lower pole regions of bilateral kidneys separately in the cortex and medulla. Mean cortical R2* and medullary R2* values were 21 and 30, respectively, for the right kidney and were 22 and 30, respectively, for the left kidney. e, f BOLD MR images of bilateral kidneys at 15-min post-furosemide. Mean cortical R2* and medullary R2* values were 20 and 24, respectively, for the right kidney and were 19 and 27, respectively, for the left kidney
R2* values and maps were generated using Functool (GE Healthcare, Wisconsin, USA) in an advanced workstation. Individual baseline anatomical T2*-weighted images were selected to define ROI within the cortex and medulla. The average area of ROI was kept between 25 and 45 mm2. Images that yielded optimal contrast between cortex and medulla were selected for placing ROI. Upper, mid, and lower pole ROI were placed in both cortex and medulla. This ROI was copied to the corresponding parametric image of R2* to determine the value of R2* within ROI (Fig. 2c, d). Mean R2* for cortex and medulla was determined. ROI for a given image included areas of cortex and medulla not obscured by artifact or any incidental lesion. The same analysis was done on both the kidneys after giving furosemide (Fig. 2e, f). The change in R2* from pre-furosemide to post-furosemide was determined as “Delta-R2*.”
Statistical analysis
Statistical package for social sciences, version 23 (SPSS-23, IBM, Chicago, USA) was used for the analysis of the data. Descriptive statistics of the continuous data were represented as mean ± SD, while categorical data were presented in frequency and percentages. Independent samples t test was used to compare the means between two independent groups, whereas a One-way ANOVA test was used to compare the means among 3 or more independent groups. Mann–Whitney U test was used to compare the continuous variables when data were nonnormally distributed. Paired samples t test was used to test the change in mean value between pre- and post-observations, whereas Wilcoxon signed-rank test was used to test the change in distribution between pre- and post-observations when the continuous variable was not normally distributed or inadequate sample size. Chi-square test/Fisher exact test was used to compare the proportions between the groups. A p value < 0.05 was considered statistically significant.
Results
After applying inclusion and exclusion criteria, 116 study participants were included in the study. Out of them, 65 patients had renal artery stenosis (Group 1) and 31 patients had essential hypertension (Group 2), while the remaining 20 were healthy volunteers (Group 3). The demographic and clinical data of these patients are described in Table 1.
Mean serum creatinine in Group 1 was 2.13 ± 1.34 mg/dL, which was higher than the other two groups (p = 0.001). Mean systolic and diastolic blood pressure were also higher in Group 1. Hemoglobin level was not significantly different between groups. Out of 65 patients with renal artery stenosis, 31 (47%) patients were having proteinuria of some degree. 12 (18.4%) patients were suffering from Takayasu arteritis. The mean size of kidneys having renal artery stenosis was less than control groups (p = 0.001).
Out of 65 patients with renal artery stenosis, 38 (58.4%) had unilateral stenosis and 27 (41.5%) had bilateral stenosis. Overall, they had 129 renal units (1 patient with unilateral renal artery stenosis underwent nephrectomy on the opposite side for an unrelated diagnosis), out of which 92 units had renal artery stenosis and 37 units were normal. Of these 92 units, 48 showed complete loss of corticomedullary differentiation (CMD), 17 had altered CMD, and 27 kidneys had normal CMD. Mild stenosis (< 50%) was found in 14% of patients, while moderate (50% to 70%) and severe (> 70%) stenosis were found in 39.5% and 46.5%, respectively. Fisher’s exact test found no significant association between the degree of stenosis and corticomedullary differentiation (p = 0.4).
The deoxyhemoglobin levels in the kidney in BOLD MRI were represented by the R2* value. In patients without renal artery stenosis, the mean R2* value in the medulla of the kidneys was higher than that of the cortex (26.47 ± 5.35 vs 22.30 ± 4.8 s−1; p < 0.001) (Table 2). A decrease in the R2* value was seen in both cortex and medulla after administration of furosemide. However, the degree of fall in the R2* value post-furosemide (delta R2*) was more in the medulla as compared to the cortex (3.38 ± 3.9 vs 1.88 ± 2.63 s−1; p value < 0.001) (Fig. 2).
On comparison of R2* values among different study groups (Table 2), baseline mean cortical R2* value was not different in the renal artery stenosis group as compared to other groups. The baseline means medullary R2* value in the renal artery stenosis group was lower than the corresponding value in the normal and essential hypertensive control group (p value < 0.001), while the difference was not significant with the contralateral study group. Fall in both cortical and medullary R2* value (delta R2*) was seen after giving furosemide in all groups; however, this response was blunted in the renal artery stenosis group as compared to hypertensive and normal control groups (p value < 0.001). Mean delta R2* in the medulla of renal artery stenosis patients was higher as compared to the cortex; however, the difference was not statistically significant. Mean delta R2* values of contralateral normal kidneys were not significantly different from that of the renal artery stenosis group (p = 0.8).
Table 3 compares the mean R2* values of stenotic kidneys with groups having a different degree of stenosis. No correlation was observed in the mean baseline as well as mean delta R2* values of both cortex and medulla among groups having a different degree of stenosis.
Results obtained on the comparison of mean R2* value with kidney size and CMD in the renal artery stenosis group are summarized in Table 4. (Figs. 3 and 4) Kidneys having renal artery stenosis were divided into two groups based on their size (< 8 cm and > = 8 cm). [18] A comparison of mean baseline and delta R2* values were done between these two groups. Mean baseline cortical R2* value was higher in small-sized stenotic kidneys as compared to normal-sized stenotic kidneys (24.27 ± 5.65 vs 21.7 ± 3.88; p value = 0.02). No such difference was seen in mean baseline medullary R2* values. Small stenotic kidneys showed less degree of change in R2* value post-furosemide (delta R2*) in both medulla and cortex as compared to normal-sized stenotic kidneys (Cortex 0.84 ± 2.28 vs 1.94 ± 2.8; p value = 0.04) (Medulla 0.81 ± 2.58 vs 3.46 ± 4.26; p value < 0.001). A similar comparison with groups having a different degree of corticomedullary differentiation of stenotic kidneys showed a higher mean baseline cortical R2* value of group with lost CMD as compared to a group with normal CMD (p value = 0.006). The blunted response was seen post-furosemide in the medulla of stenotic kidneys having partial or complete loss of corticomedullary differentiation as compared to kidneys with normal CMD (p value < 0.001). Mean baseline medullary R2* and mean delta R2* of the cortex were not significantly different among groups.
a Coronal T2W MR image showing atrophic small right kidney (5.1 cm) and normal-sized left kidney (9.9 cm). b Non-contrast MR angiography (INHANCE) in coronal plane depicting severe stenosis of right renal artery near origin (black arrow) with post-stenotic dilatation of its distal segment. c, d BOLD MR images of bilateral kidneys at baseline showing parametric gray scale and colored BOLD MR image. ROI was placed in upper, mid, and lower pole regions of bilateral kidneys. On the right side since the kidney is atrophic ROI was placed in corticomedullary region, whereas on the left side ROI was placed separately in cortex and medulla. Mean corticomedullary R2* value of right kidney was 17.6. Mean cortical R2* and medullary R2* values of left kidney were 17 and 21.6, respectively. e, f BOLD MR images of bilateral kidneys at 15-min post-furosemide. Mean corticomedullary R2* values of right kidney were 16. Mean cortical R2* and medullary R2* values of left kidney were 10.3 and 12.3, respectively
a T2W MR image (coronal plane) showing bilateral normal-sized kidneys (RK 8.6 cm, LK 10.7 cm). b Non-contrast MR angiography (INHANCE in axial plane) depicting right renal artery severe stenosis at the ostium. c, d BOLD MR images of bilateral kidneys at baseline showing parametric gray scale and colored BOLD MR image. ROI was placed in upper, mid, and lower pole regions of bilateral kidneys placed separately in the cortex and medulla. Mean cortical R2* and medullary R2* values were 18 and 27, respectively, for the right kidney and were 21 and 25, respectively, for the left kidney. e, f BOLD MR images of bilateral kidneys at 15-min post-furosemide. Mean cortical R2* and medullary R2* values were 9.6 and 13.6 of right kidney, respectively. Mean cortical R2* and medullary R2* values were 15.3 and 17.6 of left kidney, respectively
Discussion
The present study represents the application of BOLD MRI in kidneys having renal artery stenosis on large sample size. Further, these results were not only compared with the contralateral normal kidney of renal artery stenosis patients but also with kidneys of essential hypertensive patients and normal individuals.
Normal variations in intrarenal oxygenation
Our study confirms the presence of intrarenal gradient and establishes the reliability of BOLD MRI in the kidney. Absolute R2* value although is not uniform in current literature, R2* values are influenced by many factors that may be patient-related or technique-related [19, 20]. Michaeley et al. and Golvizcki et al. have shown that R2* values were higher at 3 T as compared to at 1.5 T; however, susceptibility artifacts increase and precision of measurement decreases at 3 T [21, 22]. Michaeley et al. in their study showed no significant correlation of cortical and medullary R2* values with age and sex [22]. R2* value also depends on body temperature, pH, blood hematocrit, chronic diuretic use, and antihypertensive use.
Relative change in R2* on maneuvers like diuretic use is more reliable as compared to the absolute magnitude of R2*. Administration of furosemide induces a fall in R2* value in both the medulla and cortex of normal kidneys. This fall is explained by the blockage of energy-dependent and oxygen-consuming transport of ions occurring through NaK2Cl cotransporters concentrated in the thick part of the ascending limb of the loop of Henle by furosemide, thereby decreasing deoxyhemoglobin level or R2* level. On comparing the change in R2* of the medulla with that of the cortex, we found that the mean change of R2* in the medulla with that of the cortex is more (5.25 ± 3.9 vs 3.25 ± 2.9; p value < 0.001) which is consistent with the medullary location of these cotransporters.
Comparison with control groups
In our study, there was no significant difference between the baseline cortical R2* value of post-stenotic kidneys with that of control groups. Baseline medullary R2* of stenotic kidneys was, however, lower as compared to the essential hypertensive group and normal group. Change of R2* on the administration of furosemide was reduced in post-stenotic kidneys as compared to essential hypertension and normal study groups kidneys. These observations are consistent with the study published by Golviczki et al. in 2010, where they have described that despite a reduction in renal blood flow and renal perfusion, baseline R2* value was not different between the kidneys of atherosclerotic renal artery disease and essential hypertension [23]. Similar results were seen in previous studies on stenotic swine kidneys [24]. These findings suggest that intrarenal oxygenation is well maintained in post-stenotic kidneys and is not significantly different from that of contralateral normal, essential hypertensive, and normal subject kidneys. This observation is further supported by elevated renal vein oxygenation and maintained venous erythropoietin levels in post-stenotic kidneys [25]. In response to the gradual reduction in blood supply, various compensatory mechanisms get activated to preserve the renal medullary and cortical oxygenation. One of the most important mechanisms of compensation is reduced sodium filtration and transport occurring through energy-dependent transporters predominately concentrated in the medulla thereby reducing oxygen consumption. Reduced functioning of these energy-dependent ion transporters is indirectly evident in our study in the form of improved medullary oxygenation and blunting of response in post-stenotic kidneys after giving furosemide. Other less known mechanisms like decreased GFR, collateral formation, and preglomerular AV shunting also helps in the adaptation of kidneys to reduced perfusion [23, 26].
Comparison with the degree of stenosis
A study conducted by Golviczki et al. in 2011 concluded that kidneys with severe atherosclerotic stenosis have higher cortical deoxyhemoglobin levels and blunted response to furosemide as compared to those kidneys having moderate stenosis or essential hypertensive group [27]. The severity of stenosis in their study was assessed on Doppler USG (Peak Systolic velocity > 384 cm/s across the stenosis) of the renal artery. We divided kidneys into mild, moderate, and severe degrees of stenosis based on quantitative MR angiography. When we compared baseline cortical, medullary, and delta R2* values of stenotic kidneys among the above-mentioned groups of a mild, moderate, and severe degree of stenosis, no significant difference was seen. R2* value of cortex of severely stenotic kidneys was also not different from control groups. As we determined the degree of stenosis by anatomical narrowing on MR angiography, our results could not be compared to that of the previous study. Duration and chronicity of the stenotic lesion are also important factors which when coupled with the severity of stenosis lead to the development of renal hypoxia. Therefore, the duration of stenosis is a confounding factor that should be known before deciding the influence of the degree of stenosis on renal hypoxia.
Oxygenation status in the non-viable stenotic kidney
Atrophic post-stenotic kidneys (size ≤ 8 cm) and stenotic kidneys with lost corticomedullary differentiation were having higher cortical R2* values as compared to normal-sized post-stenotic kidneys. Thus, our study further extends the observation of the development of cortical hypoxia in severely stenotic and atrophic kidneys [27]. This leads to the conclusion that non-viable atrophic post-stenotic kidneys have lost their adaptive mechanisms to maintain cortical oxygenation causing accumulation of deoxyhemoglobin in the cortex. No such difference was observed with the medullary R2* value.
In our study, kidneys that had a significant reduction in size and loss of corticomedullary differentiation also lost their response to furosemide with a marked reduction in delta R2* value as compared to viable stenotic kidneys (Figs. 3 and 4). This is consistent with a study conducted by Textor et al. where it was shown that kidneys with total arterial occlusion without any enhancement and presence of renal atrophy have minimal response to furosemide [28]. Non-functioning non-viable kidneys are associated with inactive NaK2Cl cotransporter leading to less exhaustion of available oxygen. This may be the reason for the maintenance or slightly lower value of baseline medullary R2* in non-viable kidneys in our study. These results are consistent with a study in kidney allograft where the reduction in R2* signal was observed with acute dysfunction characterized by tubulointerstitial inflammatory changes with associated impairment of tubular transport [29]. Similarly blunting of response to furosemide was obtained in studies done on more advanced CKD, but preserved blood flow and perfusion in kidneys due to the same reason [30]. The presence of active reabsorption of sodium in a viable stenotic kidney is further supported by studies done to evaluate split renal sodium excretion in the kidney using ureteral cannulation beyond stenotic lesions [31]. The results of these studies indicate that hemodynamically significant renal arterial lesion to a functioning kidney has reduced sodium delivery in the urine.
The response of contralateral kidneys
The contralateral normal kidney of unilateral renal artery stenosis cases showed significantly less change in R2* value post-furosemide (delta R2*) from that of control group kidneys (p < 0.05). The Baseline R2* value of these contralateral kidneys was not significantly different from post-stenotic kidneys. Thus, not only the stenotic kidney but the contralateral kidney also has altered intrarenal oxygenation and function. This result can be explained by the observation that unilateral RAS leads to not only hypoperfusion-dependent renin release and injury to the ipsilateral stenotic kidney, but also functional changes develop within the non-stenotic contralateral kidneys [32]. Various studies indicate structural consequences develop in both stenotic and contralateral kidneys, not easily explained by hemodynamic alone, suggesting functional interactions between the two [33].
The present study gives a better idea of fluctuations of intrarenal renal oxygenation in response to decreased blood flow in post-stenotic kidneys. We can demonstrate in our study that the human body tries its best to maintain intrarenal oxygenation despite the increasing severity of stenosis. This may be the reason why large multicentric studies like ASTRAL [15] and CORAL [34] have failed to demonstrate any significant improvement in outcome following revascularization in renal artery stenosis patients. Most of these kidneys remain stable for a long time with antihypertensive therapy because they do not experience any oxygen deficit. However, determining the threshold after which these compensatory mechanisms fail to maintain intrarenal oxygenation may help in identifying the real candidate for revascularization. Cortical hypoxia was observed in small and shrunken kidneys in our study as well as in a previous study which is a marker of failure of adaptive mechanism to maintain intrarenal oxygenation [27]. This may be a triggering point for fibrogenesis within the kidney leading to the irreversibility of the renal function [35]. Studies were done on chronic kidney disease patients which also supported that cortical hypoxia is an independent predictor of renal function decline with the more adverse renal outcome [11]. Thus, the presence of cortical hypoxia in stenotic kidneys has the potential to identify those kidneys which are progressing toward irreversibility. However, large studies to compare baseline renal BOLD findings and their long-term outcome following revascularization are required to further support and confirm this hypothesis.
Our study had some limitations. The study group consisted of patients with a wide range of duration of illness. The duration may affect the R2* value of kidneys irrespective of the severity of vascular occlusion. Moreover, the BOLD signal is also sensitive to a number of other normal and abnormal tissue features, such as transverse relaxation parameter (T2), macroscopic field inhomogeneities (susceptibility artifacts), and blood volume fraction [36]. Factors like hematocrit, water content, blood flow, blood pH, pCO2, and age of the patient may affect apparent R2* under some conditions. These factors were not uniform among patients. One of the ways to account for these influences is modification of the existing BOLD MRI technique to quantitative BOLD (qBOLD). qBOLD is based on a multicomponent approach that includes contributions from intracellular water, interstitial fluid, and intravascular blood [37]. However, tissue pO2 and T2* may not have the same correlation for all renal layers under all pathophysiological scenarios which means that it is necessary to calibrate T2*-sensitized MRI for probing renal oxygenation and perfusion [38]. Another limitation of our study is that the reproducibility of R2* determined by the ROI method was not analyzed. Our study protocol did not include follow-up of patients, type of treatment received by them, and outcome following revascularization. However, the results obtained in our study may play a crucial role in a better selection of patients for revascularization therapy. It will help in determining those patients who require medical therapy alone and the other group of patients who will be further benefited from the revascularization procedure.
Conclusion
The present study supports the validity of BOLD MRI as a rapid and non-invasive tool in the evaluation of kidneys with RAS. The R2* values on BOLD MRI are significantly different between kidneys with and without RAS. Despite a high degree of stenosis, kidneys can maintain their oxygen balance by various adaptive mechanisms until they reach that stage where their response to inhibition of sodium reabsorption is markedly blunted and cortical hypoxia develops. BOLD MRI can potentially also predict the utility of revascularization.
Data availability
Not applicable.
Code availability
Not applicable.
References
Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001 Feb 8;344(6):431–42.
Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992 Jun;262(6 Pt 1):E763-778.
Nielsen K, Rehling M, Henriksen JH. Renal vein oxygen saturation in renal artery stenosis. Clin Physiol. 1992 Mar;12(2):179–84.
Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, Grande JP. Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: clinical and histopathological correlates. Nephrology Dialysis Transplantation. 2010 Nov 1;25(11):3615–22.
Prasad PV. Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Renal Physiol. 2006 May;290(5):F958-974.
[6] Zheng Z, Shi H, Ma H, Li F, Zhang J, Zhang Y. Renal oxygenation characteristics in healthy native kidneys: assessment with blood oxygen level-dependent magnetic resonance imaging. Nephron Physiol. 2014;128(3–4):47–54.
Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9868–72.
Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996 Dec 15;94(12):3271–5.
Piskunowicz M, Hofmann L, Zuercher E, Bassi I, Milani B, Stuber M, et al. A new technique with high reproducibility to estimate renal oxygenation using BOLD-MRI in chronic kidney disease. MagnReson Imaging. 2015 Apr;33(3):253–61.
Ebrahimi B, Gloviczki M, Woollard JR, Crane JA, Textor SC, Lerman LO. Compartmental analysis of renal BOLD MRI data: introduction and validation. Invest Radiol. 2012 Mar;47(3):175–82.
Pruijm M, Mendichovszky IA, Liss P, Van der Niepen P, Textor SC, Lerman LO, et al. Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: a statement paper and systematic review. Nephrol Dial Transplant. 2018 01;33(suppl_2):ii22–8.
Pedersen M, Dissing TH, Mørkenborg J, Stødkilde-Jørgensen H, Hansen LH, Pedersen LB, et al. Validation of quantitative BOLD MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int. 2005 Jun;67(6):2305–12.
Alford SK, Sadowski EA, Unal O, Polzin JA, Consigny DW, Korosec FR, et al. Detection of acute renal ischemia in swine using blood oxygen level-dependent magnetic resonance imaging. J Magn Reson Imaging. 2005 Sep;22(3):347–53.
Forster BB, MacKay AL, Whittall KP, Kiehl KA, Smith AM, Hare RD, et al. Functional magnetic resonance imaging: the basics of blood-oxygen-level dependent (Bold) imaging. Can Assoc Radiol J. 1998 Oct;49(5):320–9.
ASTRAL Investigators, Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009 Nov 12;361(20):1953–62.
Srivastava A, Hiralal, Chopra J, Sharma PK. A computed tomography (CT) evaluation of renal artery evaluation in healthy adult north Indian population. Int J Anat Res 2018;6:5207–5212.
Yamuna J, Chandrasekharan A, Rangasami R, Ramalakshmi S, Joseph S. Unenhanced renal magnetic resonance angiography in patients with chronic kidney disease & suspected renovascular hypertension: Can it affect patient management? Indian J Med Res. 2017 Nov;146(Supplement):S22–9.
Glodny B, Unterholzner V, Taferner B, Hofmann KJ, Rehder P, Strasak A, et al. Normal kidney size and its influencing factors - a 64-slice MDCT study of 1.040 asymptomatic patients. BMC Urol. 2009 Dec 23;9:19.
Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol. 1994 Dec;267(6 Pt 2):F1059–1062.
Pruijm M, Hofmann L, Piskunowicz M, Muller M-E, Zweiacker C, Bassi I, et al. Determinants of renal tissue oxygenation as measured with BOLD-MRI in chronic kidney disease and hypertension in humans. PLoS One. 2014;9(4):e95895.
Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, et al. Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol. 2009 Sep;44(9):566–71.
Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI. Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int. 2012 Apr;81(7):684–9.
Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, et al. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010 Apr;55(4):961–6.
Gomez SI, Warner L, Haas JA, Bolterman RJ, Textor SC, Lerman LO, et al. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol. 2009 Oct;297(4):F981-986.
[25] Wiecek A, Kokot F, Kuczera M, Grzeszczak W, Kiersztejn M. Plasma erythropoietin concentrations in renal venous blood of patients with unilateral renovascular hypertension. Nephrol Dial Transplant. 1992;7(3):221–4.
Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol. 2008 Nov;295(5):F1259-1270.
Gloviczki ML, Glockner JF, Crane JA, McKusick MA, Misra S, Grande JP, et al. Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension. 2011 Dec;58(6):1066–72.
Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, et al. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008 Apr;19(4):780–8.
Djamali A, Sadowski EA, Samaniego-Picota M, Fain SB, Muehrer RJ, Alford SK, et al. Noninvasive assessment of early kidney allograft dysfunction by blood oxygen level-dependent magnetic resonance imaging. Transplantation. 2006 Sep 15;82(5):621–8.
Pruijm M, Hofmann L, Maillard M, Tremblay S, Glatz N, Wuerzner G, et al. Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension. 2010 May;55(5):1116–22.
Stamey TA, Nudelman IJ, Good PH, Schwentker FN, Hendricks F. Functional characteristics of renovascular hypertension. Medicine (Baltimore). 1961 Dec;40:347–94.
Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA. Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant. 2001 Apr;16(4):765–70.
Herrmann SMS, Saad A, Eirin A, Woollard J, Tang H, McKusick MA, et al. Differences in GFR and tissue oxygenation, and interactions between stenotic and contralateral kidneys in unilateral atherosclerotic renovascular disease. Clin J Am Soc Nephrol. 2016 Mar 7;11(3):458–69.
Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014 Jan 2;370(1):13–22.
Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007 Dec;117(12):3810–20.
Christen T, Lemasson B, Pannetier N, Farion R, Remy C, Zaharchuk G, Barbier EL. Is T2* enough to assess oxygenation? Quantitative blood oxygen level-dependent analysis in brain tumor. Radiology. 2012 Feb;262(2):495-502.
[37] He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med. 2007;57(1):115-126.
Niendorf T, Pohlmann A, Arakelyan K, Flemming B, Cantow K, Hentschel J, Grosenick D, Ladwig M, Reimann H, Klix S, Waiczies S, Seeliger E. How bold is blood oxygenation level-dependent (BOLD) magnetic resonance imaging of the kidney? Opportunities, challenges and future directions. Acta Physiol (Oxf). 2015 Jan;213(1):19-38.
Acknowledgements
None.
Funding
No funds, grants, or other support were received.
Author information
Authors and Affiliations
Contributions
Both HL and PS contributed equally to the manuscript and share the first authorship. This manuscript is a part of thesis done by PS and KP under supervision of HL. HL performed conceptualization; HL, PS, and RP contributed to methodology; PS and KP performed formal analysis and investigation; PS contributed to writing and preparation of original draft; PS, HL, RP, PY, PM, DB, SK, VA, AS, and SPS contributed to writing, reviewing, and editing of the manuscript; HL performed supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Ethics Committee of the Sanjay Gandhi Post-Graduate Institute of Medical Sciences, Lucknow, India (No. 2016–47-MD-EXP).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lal, H., Singh, P., Ponmalai, K. et al. Role of blood oxygen level-dependent magnetic resonance imaging in studying renal oxygenation changes in renal artery stenosis. Abdom Radiol 47, 1112–1123 (2022). https://doi.org/10.1007/s00261-022-03408-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03408-5