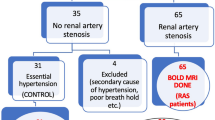
Abstract
While atherosclerotic Renal Artery Stenosis (ARAS) is a common cause of secondary hypertension and poses a threat to kidney viability, the degree to which reduced blood flow to cortical or medullary segments leads to a reduction in tissue oxygenation and/or increased overall oxygen consumption is not well understood. These studies have been limited due to the lack of an adequate method to assess tissue oxygenation in humans. BOLD (blood oxygen-level-dependent) magnetic resonance imaging detects local levels of tissue deoxyhemoglobin without requiring contrast. The normal kidney circulation consistently develops tissue oxygen gradients, leaving some areas within the deep sections of medulla relatively hypoxic, reflected by corresponding differences in cortical and medullary R2* values. Moderate reductions in renal blood flow that occur with ARAS do not invariably lead to renal hypoxia, likely due to both a surplus of oxygenated blood and a parallel decrease in GFR and tubular reabsorption of sodium that leads to decrease in Oxygen consumption. However, at some point, vascular occlusion threatens the viability of the kidney and can lead to loss of kidney function. In this chapter we will review the implementation of BOLD MRI in the diagnosis and management of renovascular disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Atherosclerotic renal artery stenosis (ARAS) remains one of the most common causes of secondary hypertension and when severe can also threaten the viability of the post-stenotic kidney. Occlusive vascular disease reduces blood flow and glomerular filtration (GFR), activates pressor systems, and ultimately leads to renal atrophy and chronic kidney disease [1, 2].
Defining the relationships between arterial blood flow and tissue oxygenation within the kidney poses a major challenge, in part due to the complexity of renal circulation and distribution of blood flow. The kidney normally is highly perfused, exhibits the highest rate of blood flow per tissue weight, and has the smallest arterio-venous differences in oxygen saturation of any organ, consistent with its filtration function and limited overall net oxygen consumption [3, 4]. By these criteria, the kidney is abundantly oxygenated as compared to other organs. A striking feature, however, is the non-uniformity of tissue oxygenation within the kidney. Under normal conditions, a gradient of oxygenation develops within the renal parenchyma from a highly perfused cortex falling to much lower levels in the deep medulla resulting from significant differences in both blood supply and oxygen consumption between the renal cortex and medulla [5, 6]. Portions of the medulla thus are functionally hypoxic under normal conditions and considered particularly susceptible to ischemic and/or other forms of acute kidney injury [6–8].
Remarkably, a decrease in RBF does not invariably lead to renal hypoxia, likely due to both a surplus of oxygenated blood and a parallel decrease in GFR and tubular reabsorption of sodium that leads to decrease in Oxygen consumption. Studies of intra-renal mechanisms of blood flow distribution emphasize that cortex and medulla can be regulated independently under some conditions [9]. However, at some point, vascular occlusion threatens the viability of the kidney and can lead to loss of kidney function, which may/may not be reversible after restoration of blood flow with revascularization [10]. Eventually, vascular occlusion leads to tissue fibrosis and can lead to end stage kidney disease (ischemic nephropathy) [11–13]. Determining when occlusive vascular lesion actually threatens kidney oxygenation and viability remains an elusive goal. Blood Oxygen Level-Dependent (BOLD) MRI is a noninvasive method developed in recent years that holds the promise of allowing improved understanding of regional tissue oxygenation within the kidney. In this chapter we will provide a review of some of the principles involved in BOLD MRI and summarize our current understanding of the implementation of BOLD MRI in renovascular hypertension.
Renal Blood Flow and Tissue Oxygenation
Renal blood flow is higher than any other organ with respect to organ weight, consistent with its primary function for blood filtration. The largest portion of renal blood flow is directed towards the cortex and filtration within cortical glomeruli. A fraction of post-glomerular blood flow is directed to the medulla and provides the basis for active tubular solute transport, preserving osmotic gradients and allowing urinary concentration [6]. The anatomical arrangement within the medulla aligns the tubules and the vasa recta in parallel fashion with a hairpin turn within deep medullary segments. This feature maximizes urine concentration by countercurrent exchange and allows diffusion of oxygen from the arterial side to venous side. In addition, the thick ascending limb is responsible for active reabsorption of chloride and sodium, an energy-dependent process that requires a large amount of oxygen. As a result, these circumstances combine to generate a hypoxic milieu that is progressively more evident in deep medullary segments. Because of nearly constant levels of oxygen depletion in this region, the kidney is particularly susceptible to ischemic injury especially in the outer medulla [5, 6].
ARAS represents a large vessel disorder that eventually obstructs the vascular lumen. Renal blood flow and perfusion pressure change only minimally until the vessel lumen cross- sectional area falls by 70–80 %. When a “critical” degree of stenosis is attained, renal hypoperfusion leads to a cascade of events from activation of the renin-angiotensin system to the rarefaction of small renal vessels, kidney fibrosis, loss of function, and atrophy [14]. Studies of oxygen delivery and consumption in anesthetized rabbits indicate that acutely reduced filtration and oxygen consumption can leave tissue oxygen levels stable even if cortical blood flow is reduced by up to 40 % [15, 16]. A smaller portion of blood flow is delivered to deeper medullary regions of the kidney via post-glomerular vasa recta; in these regions, active metabolic processes dependent upon aerobic energy pathways lead to oxygen consumption and local areas of hypoxia, even within normal kidneys, report estimates of medullary pO 2 ranging between 10 and 20 mmHg [6] but rise to 41 mmHg after administration of furosemide intravenously. Hence, low medullary pO2 reflects combined effects of reduced blood flow and increased oxygen consumption related to solute transport in the Loop of Henle [17, 18]. Local gradients of cortical and medullary oxygenation are closely regulated, sometimes independently from each other. Remarkably, these regions can tolerate reduced blood flow with compensatory changes by numerous vasoactive systems within the circulation. In a rat model, for example, angiotensin II infusion produces a 40 % decrease of cortical perfusion, but medullary perfusion can remain unchanged, apparently protected by prostaglandin E2 synthesis [19]. Quantitative changes in the degree of arteriovenous shunting as a result of changes in renal blood flow appear to maintain the oxygen tension and adjust local areas of blood supply, but also may render some focal regions more susceptible to hypoxia [15] (Fig. 17.1).
Measurements of Renal Oxygenation and Evaluation of Hypoxia
Direct measurement of renal tissue pO2 has been achieved experimentally using invasive microelectrodes [5, 20] or an advanced laser-based probe [21]. Severely hypoxic tissue can be mapped histopathologically using reduction of Pimonidazole by nicotinamide adenine dinucleotide phosphate (NADPH) in biopsy or post-mortem samples [22]. Pimonidazole mapping is limited by being sensitive only to pO2 less than 10 mmHg. Recently, Hypoxia inducible factor-α (HIF-α) has been proposed for an endogenous marker of hypoxia [23]. These and other invasive and/or post-mortem methods [24] are not readily applicable to humans.
Evaluation of Kidney Tissue Deoxyhemoglobin Using BOLD MRI
Development of Blood Oxygen Level Dependent (BOLD) MR depends upon the fact that magnetic properties of blood reflect the state of hemoglobin oxygenation. Deoxyhemoglobin is paramagnetic whereas oxyhemoglobin is diamagnetic [25]. The presence of deoxyhemoglobin affects the T2* relaxation time of neighboring water molecules and in turn influences the MRI signal of T2*-weighted (gradient echo) images. The rate of spin dephasing R2* (= 1/T2*) thereby is closely related to the tissue content of deoxyhemoglobin. Since the oxygen tension (pO2) of capillary blood generally is thought to be in equilibrium with the surrounding tissue, changes estimated by BOLD MRI are interpreted as changes in tissue pO2 [26–29]. BOLD MRI measurements of renal cortex and medulla [27] correlate with data obtained using invasive microelectrodes [5]. For example, in experiments using either microelectrodes or BOLD MRI, furosemide improves medullary oxygenation while acetazolamide, which produces diuresis, acts on the proximal tubule in the renal cortex and therefore induces little change in the medullary oxygenation [5, 27, 28].
A set of parametric images of R2* is generated from the BOLD sequence data by fitting signal intensity data from each echo on a voxel-by-voxel basis to an exponential function describing the expected signal decay as a function of echo time (TE) and solving for the unknown value of R2*. Parametric maps of R2* co-registered over the image illustrate the R2* translation of renal structures. Typically, cortex can be identified by lower R2* values, with a gradient developing to higher R2* levels in the deeper medullary sections [30, 31].
Parametric imaging can make the selection of ROIs more focused, especially when the deeper parts of the medulla are targeted, and allows the exclusion of artifacts induced by adjacent tissue anomalies from areas outside the kidneys [32, 33].
Data from swine experiments using oxygen sensing electrodes within various cortical and medullary locations within the kidney demonstrate tissue oxygen saturation consistent with deoxyhemoglobin levels identified using BOLD MRI [17, 34]. Average levels of tissue oxygen tension in the mammalian kidney range from 50 to 55 mmHg in cortex to as low as 15–20 mmHg in the deep sections of the medulla [5]. These levels coincide with a range of hemoglobin oxygen saturation, which falls steeply from 85 to 15 %, thereby making this a range favorable for detection using BOLD imaging. Further experimental studies in rats [35, 36] and other models [34, 37] using oxygen probes confirm that tissue oxygen levels fall by 45–50 % in moving from cortex to deep medullary regions.
Comparison of 1.5 and 3 T BOLD MR to Study Kidney Oxygenation
Because deoxyhemoglobin functions effectively as an imaging “contrast agent,” BOLD MR studies comparing 1.5 and 3.0 T magnetic fields indicate that BOLD MRI measurements at high field strength amplifies differences between cortical and inner medullary regions of the kidney. Maneuvers that reduce oxygen consumption related to tubular solute transport (e.g., Furosemide administration) allow functional evaluation of transport-related activity as a determinant of tissue oxygenation. Reduced response to alterations in oxygen consumption can be detected at 3 T more effectively than at 1.5 T and may provide real-time tools to examine developing parenchymal injury associated with impaired oxygenation [33].
Application of Renal BOLD MRI
BOLD MRI allows non-invasive evaluation of renal oxygenation not only in animals but also in humans. Initial studies in humans suggested that this method could identify alterations in subjects after administration of nephrotoxic contrast [38], allograft injury [39], water loading, and occlusive renal arterial disease [30, 31]. Some authors had postulated that hemodynamic injury from nephrotoxin exposure produces local hypoxia as a “final common pathway” related to kidney injury [40]. Administration of cyclosporine, for example, does in fact lead to a rise in medullary R2* levels, suggesting medullary rise in deoxyhemoglobin [41]. Surprisingly, conditions that severely limit tubular metabolic activity, such as acute interstitial inflammation associated with transplant rejection, acute tubular necrosis, or even nonfunctioning renal atrophy beyond an occluded vessel, are associated with normal or low deoxyhemoglobin in both cortical and medullary regions [31, 42]. These findings were interpreted to indicate that severe reductions in GFR and active solute transport are associated with reduced oxygen consumption, therefore leaving measured R2* levels (and thus deoxyhemoglobin) low. BOLD MRI has been used to show that Angiotensin II receptor blocker could partially ameliorate intrarenal hypoxia in chronic kidney disease patients [43]. In a recent study in diabetic subjects, they exhibited hypoxia of the renal medulla, but not much of the renal cortex. In subjects who had progressed to more advanced stages of diabetic nephropathy, renal medullary hypoxia was alleviated whereas progressive cortical hypoxia developed [44].
Hypertension
BOLD MRI has been applied to some forms of essential hypertension. Experimental studies in rats indicate that medullary blood flow (and presumably medullary oxygenation status) is reduced in hypertensive models, and more importantly, that reduced medullary blood flow itself may be sufficient to produce hypertension [45, 46]. This is supported by demonstrable reduction of nitric oxide (NO) in hypertensive rat attributed to endothelial dysfunction. All these studies were performed using invasive microelectrodes or Doppler flow probes in rat kidneys. In a rat model BOLD MRI showed that medullary R2* exhibited minimal changes to nitric oxide synthase (NOS) inhibition, substantially reduced compared to normotensive controls [47]. Studies in hypertensive African Americans demonstrate higher R2* levels associated with increased medullary volume and sodium reabsorption as compared with whites. These data support a role for increased oxygen consumption and were associated with markers of increased oxidative stress in African Americans that may accelerate hypertension and target organ injury [48].
Renovascular Disease
Has application of BOLD MRI affected our understanding of atherosclerotic renovascular disease? Renal artery stenosis (RAS) is a common cause of secondary hypertension and obviously has consequences for intrarenal oxygenation when severe. Initial studies by Juillard et al. [49] identified a progressive rise in R2* in both cortex and medulla after stepwise acute reductions in renal blood flow produced by increasing levels of stenosis in a swine model. Warner et al. showed that graded reduction in blood flow acutely decrease tissue oxygenation measured by oxygen electrodes that also appear as changes in R2* signal under similar conditions [37] (Fig. 17.2). These changes are more pronounced in the medulla than the cortex, despite proportional reductions in O2 delivery and consumption during graded ARAS. Alford et al. confirmed these data using an acute renal artery obstruction model and observed significant increase in renal R2* values. The contralateral kidney showed no such change. They also demonstrated the R2* values return to baseline upon releasing the obstruction [50].
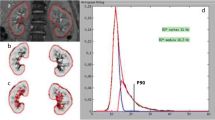
Tissue levels of oxygen tension (measured by oxygen-sensitive probes in anesthetized swine) in cortex and medulla. This figure illustrates both differences and baseline and changes in renal tissue Po2 at different levels of main renal artery blood flow. Renal cortex and medulla tissue oxygenation measured during a control period [0(BL)] and after stepwise decreases in RBF induced by a constricting clamp from baseline [(0 (AR)], by 20 % (20), 40 % (40), 60 % (60), and recovery [0 (Rec)] (Adapted with permission from Warner et al. [37])
Initial studies using 1.5 T MR were conducted in patients with a variety of renovascular lesions, some of which included total arterial occlusion. Axial slices of kidneys from 25 patients with ARAS were examined and values for R2* in cortex and medulla determined using observers selected regions of interest. These data identified heterogeneities within regions of the kidney associated with large vessel ARAS, some of which obviously followed regional distribution of segmental arterial stenosis [30]. These data confirm in human subjects the general ability to distinguish cortical and medullary oxygenation and identify the role of solute reabsorption by noting the change in medullary R2* after furosemide administration (Fig. 17.3a, b). Surprisingly, values of R2* in medullary regions of kidneys with total occlusion did not differ appreciably from cortex. These results suggested that lack of filtration and solute absorption is associated with minimal oxygen consumption, in agreement with similar data presented in renal transplant patients during episodes of acute allograft rejection with interstitial injury and reduced GFR [51].
(a, b) Examples of axial T2 images (upper row) and parametric R2* maps (lower row) outlining the kidneys in a patient with hypertension before (a) and after (b) administration of intravenous furosemide. Furosemide led to lower R2* levels, especially in the medulla within 15 min, suggesting that deoxyhemoglobin levels in this region reflected furosemide-suppressible oxygen consumption
Our group undertook further studies to examine tissue oxygenation using higher magnet strength (3 T) in humans with atherosclerotic renal artery stenosis. We studied patients with unilateral ARAS (Doppler ultrasound measurements >260 cm/s) under controlled conditions as part of an inpatient protocol; all patients were treated with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers during fixed sodium intake of 150 mEq per day. Kidney function was reasonably preserved (creatinine <1.7 mg/dL) and diuretics were limited to thiazides. Data from those individuals were compared with age-matched patients with essential hypertension undergoing the same protocol. Cortical and medullary blood flows and volumes were determined by multidetector computed tomography [31]. Results of these studies confirmed the value of higher magnet strength to distinguish differences in cortical and medullary oxygenation. The hemodynamic significance of these lesions was supported by reductions in blood flow and kidney volumes as compared both to contralateral kidneys and those from essential hypertension. Renal vein renin levels were markedly elevated compared to essential hypertension. As expected, tissue medullary (deepest section of the medulla or the most hypoxic zone) deoxyhemoglobin, as reflected by R2* values, was higher compared with the cortical R2* for both groups. Surprisingly, average levels of cortical and medullary R2* in these ARAS patients did not differ from those of the patients with essential hypertension or from those of the contralateral, normally perfused kidney. Despite reductions in blood flow in the stenotic kidneys sufficient to elevate levels of plasma renin activity, levels of R2* were similar to those in the other patients, indicating preservation of the cortical and medullary oxygenation in these patients under these conditions. Importantly, both GFR and the response of medullary R2* in the stenotic kidney to furosemide administration were reduced compared with the contralateral kidneys. These data suggest that oxygen consumption related to solute transport in the stenotic kidney is less than the contralateral kidney under these conditions. In fact the contralateral kidney may increase its metabolic activity as it assumes some of the filtration and reabsorption functions of the stenotic kidney. We interpret these data to demonstrate a remarkable intrarenal adaptation in the stenotic kidney that effectively preserves oxygenation gradients between cortex and medulla despite reduced blood flow and GFR. One explanation is that the fall in GFR reduces solute filtration, thereby reducing the “workload” and metabolic energy requirements in the medulla. These observations are supported by with similar findings in a chronic rat model of mild renovascular hypertension; they showed that after 4 weeks, no renal hypoxia could be detected in the kidney downstream to a renal artery stenosis. The fact that oxygenation is preserved despite substantial falls in blood flow partly may explain the observations in clinical trials that kidney function may remain stable during antihypertensive drug therapy, sometimes for many years [10].
The ability of the kidney to adapt reduced blood flow obviously has limits, however. We extended our human protocol studies using MDCT and BOLD MRI to patients with more advanced reductions in blood flow, defined as loss of tissue mass and (Doppler ultrasound measurements >384 cm/s) with cortical atrophy (Table 17.1). Levels of serum creatinine could be as high as 2.5 mg/dL. As compared to less severe ARAS, both kidney volume and tissue perfusion in the cortical regions were further reduced (Fig. 17.4). Not surprisingly, levels of cortical R2* were elevated in this group, suggesting that overt tissue hypoxia was developing. Deep medullary regions continued to have high R2* absolute levels that did not differ from those in otherwise normal kidneys. The fraction of axial slices medullary tissue with elevated R2* levels was considerably larger, however. These results demonstrate that severe vascular occlusion eventually overwhelms the capacity of the kidney to adapt to reduced blood flow, manifest as overt cortical hypoxia as measured by blood oxygen level–dependent MRI.
CT angiographic images (upper row) of the right kidneys illustrating three patients with (1) no renal artery stenosis (2) moderate renal artery stenosis and (3) severe renal artery stenosis. Below each in the bottom row are corresponding axial images with R2* parametric maps illustrating higher fraction with elevated deoxyhemoglobin (orange-red) evident with progressively more severe disease. Note that areas in each slice are heterogeneous, with more uniform cortical values but widely varying R2* levels at different depths and locations within each slice
Are the published results in ARAS contradictory? We believe not, but they do emphasize the complexity in this disorder. Based on initial studies, early protocols have focused on selecting focal regions of interest to identify a single value for R2* in cortex and a single value in medulla [31, 56]. This approach is problematic, particularly in subjects with heterogeneous vascular disease, as it is challenging to differentiate between cortex and medulla, moreover, the precision and reproducibility of R2* values will be affected by the size and location of ROI. Larger ROIs that include the entire medullary compartments may provide more representative and less variable mean values, but often include multiple medullary and cortico-medullary overlap zones with different hemodynamics [33]. Small, selective ROIs are less vulnerable to volume averaging, but may be skewed by fluctuations caused by spatial and temporal heterogeneity in oxygen distribution within the kidney, particularly in the medulla [57]. Recently, we suggested a new method of BOLD MR analysis that somehow avoids these problems mentioned previously, called the “fractional tissue hypoxia” method, defined as the percentage of R2* values above 30 s−1 on axial slices rather than selected cortical and medullary sites, values from this method correlated inversely with renal blood flow, tissue perfusion and glomerular filtration rate in patients with renovascular disease [58]. Studies using somewhat different methods for analyzing R2* (an average level over entire coronal image slices) indicate a slightly higher level of R2* in subjects with preserved kidney volumes that responded favorably to renal revascularization [59]. We believe these data define a level of “adaptation” that preserves tissue oxygenation both in cortex and medulla over a range of renal blood flow, analogous in some respects to the role of autoregulation in preserving stable renal blood flow and GFR over a range of renal perfusion pressures. When the level of renal blood flow falls below some threshold, oxygenation can no longer be preserved and hypoxia develops. These observations are consistent with the acute experimental data that demonstrated a rise in R2* levels only when the fall in blood flow approached 80 %.
Limitations and Future Directions
Application of BOLD MR is by no means standardized or routine. Some authors argue that effects related to spacial distribution of blood or magnetic field non-homogeneities may limit application of these tools. In a recent publication Michaely et al. [60] could not identify evident correlation between R2* (defined by ROI selection) values and renal function in patients with different stages of CKD as defined by eGFR in a large cohort with a wide variety of kidney disease. For these studies, BOLD imaging was added to MR studies undertaken for a broad range of indications without regard to sodium intake, medications (including those that alter oxygen delivery and/or consumption, such as ACEI/ARBs or diuretics) and the specific etiology of the underlying kidney disease. The authors conclude that gross measures of cortical and medullary oxygenation do not depend directly on the level of eGFR, These results directly contradict the hypoxia/common final pathway hypothesis [40] that predicts reduced renal oxygenation in CKD, at least in the early stages. However, limitations in the study protocol must be addressed. Hydration status and sodium balance were not uniform among the subjects, the use of medications that might alter oxygen delivery and/or oxygen consumption, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, antioxidants, and diuretics, was not assessed. Subjects with later stages of CKD are more likely to be taking these agents. Moreover, recent data suggest an added level of complexity in the relationship between renal tissue hypoxia and CKD. Renal tissue oxygenation may depend not only on the severity of CKD but also on the etiology of the underlying kidney disease [61]. Our studies up to now have been conducted under conditions with controlled sodium intake, standardized drug therapy, and excluded other obvious conditions that might affect tissue oxygenation (e.g., advanced renal failure, diabetes). Importantly, methods for analyzing BOLD images are not standardized. As can be seen in the previous figures, maps of R2* indicate considerable local heterogeneities within image slices. The premise that observer selected regions of interest that lead to characterization of single values of R2* that apply to the entire cortex or entire medulla represents the most valid approach merits critical study. Further studies need to address stability in renal oxygenation over time and particularly the changes in ARAS associated both with medical therapy and with renal revascularization. It is likely that developing methods that more realistically address observer selected ROIs and that acknowledge the heterogeneities of oxygenation, particularly within the medulla, will be essential to advancing this field [58]. A further objective is to define more precisely exactly how variation in tissue oxygenation is related to activation—or possibly reversal—of parenchymal tissue injury in this disorder. Our overall goal is to improve the use of BOLD MRI to allow more precise identification of kidneys at risk from vascular injury that may benefit from renal revascularization and/or adjunctive measures to repair the kidney before irreversible kidney damage develops.
Conclusion
BOLD MRI is a noninvasive technique that requires no contrast or radiation that has great potential as a functional tool to evaluate patients with ARAS. Initial studies indicate a complex relationship between changes in blood flow that allows adaptation to reduced perfusion and GFR that nonetheless preserve a normal cortical-to-medulla gradient of deoxyhemoglobin. More severe vascular compromise ultimately overwhelms these adaptive changes, leading to overt cortical hypoxia and expansion of medullary hypoxic zones. It is likely those additional processes that modify regional oxygenation in the kidney, such as diabetes, circulatory failure, and others also may affect these processes. At this point, standardized protocols and evaluation of ARAS with BOLD remain investigational. We believe that careful application and analysis of BOLD MR will provide critical insights into early disruption of renal physiology and function prior to the onset of irreversible renal injury. Clinical applications may identify patients that are free from the risks of “hypoxic” injury, or conversely, identify those most likely to gain from measures to reverse disorders of impaired tissue oxygenation.
References
Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol. 2004;15(8):1974–82.
Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation. 2005;112(9):1362–74.
O’Connor PM. Renal oxygen delivery: matching delivery to metabolic demand. Clin Exp Pharmacol Physiol. 2006;33(10):961–7.
Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol. 2008;295(5):F1259–70.
Brezis M, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol. 1994;267(6 Pt 2):F1063–8.
Brezis M, Rosen S. Hypoxia of the renal medulla – its implications for disease. N Engl J Med. 1995;332(10):647–55.
Klenzak J, Himmelfarb J. Sepsis and the kidney. Crit Care Clin. 2005;21(2):211–22.
Heyman SN, Rosenberger C, Rosen S. Regional alterations in renal haemodynamics and oxygenation: a role in contrast medium-induced nephropathy. Nephrol Dial Transplant. 2005;20 Suppl 1:i6–11.
Evans RG, Eppel GA, Anderson WP, Denton KM. Mechanisms underlying the differential control of blood flow in the renal medulla and cortex. J Hypertens. 2004;22(8):1439–51.
Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361(20):1953–62.
Alcazar JM, Rodicio JL. Ischemic nephropathy: clinical characteristics and treatment. Am J Kidney Dis. 2000;36(5):883–93.
Coen G, Manni M, Giannoni MF, Bianchini G, Calabria S, Mantella D, et al. Ischemic nephropathy in an elderly nephrologic and hypertensive population. Am J Nephrol. 1998;18(3):221–7.
Jacobson HR. Ischemic renal disease: an overlooked clinical entity? Kidney Int. 1988;34(5):729–43.
Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens. 2010;23(11):1159–69.
Evans RG, Eppel GA, Michaels S, Burke SL, Nematbakhsh M, Head GA, et al. Multiple mechanisms act to maintain kidney oxygenation during renal ischemia in anesthetized rabbits. Am J Physiol Renal Physiol. 2010;298(5):F1235–43.
O’Connor PM, Kett MM, Anderson WP, Evans RG. Renal medullary tissue oxygenation is dependent on both cortical and medullary blood flow. Am J Physiol Renal Physiol. 2006;290(3):F688–94.
Warner L, Glockner JF, Woollard J, Textor SC, Romero JC, Lerman LO. Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol. 2011;46(1):41–7.
Gomez SI, Warner L, Haas JA, Bolterman RJ, Textor SC, Lerman LO, et al. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol. 2009;297(4):F981–6.
Sadowski J, Badzynska B. Specific features and roles of renal circulation: angiotensin II revisited. J Physiol Pharmacol. 2006;57 Suppl 11:169–78.
Liss AG, Liss P. Use of a modified oxygen microelectrode and laser-Doppler flowmetry to monitor changes in oxygen tension and microcirculation in a flap. Plast Reconstr Surg. 2000;105(6):2072–8.
Braun RD, Lanzen JL, Snyder SA, Dewhirst MW. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am J Physiol Heart Circ Physiol. 2001;280(6):H2533–44.
Zhong Z, Arteel GE, Connor HD, Yin M, Frankenberg MV, Stachlewitz RF, et al. Cyclosporin A increases hypoxia and free radical production in rat kidneys: prevention by dietary glycine. Am J Physiol. 1998;275(4 Pt 2):F595–604.
Rosenberger C, Heyman SN, Rosen S, Shina A, Goldfarb M, Griethe W, et al. Up-regulation of HIF in experimental acute renal failure: evidence for a protective transcriptional response to hypoxia. Kidney Int. 2005;67(2):531–42.
Evans RG, Gardiner BS, Smith DW, O’Connor PM. Methods for studying the physiology of kidney oxygenation. Clin Exp Pharmacol Physiol. 2008;35(12):1405–12.
Pauling L, Coryell CD. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936;22(4):210–6.
Dunn JF, Swartz HM. Blood oxygenation. Heterogeneity of hypoxic tissues monitored using bold MR imaging. Adv Exp Med Biol. 1997;428:645–50.
Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94(12):3271–5.
Prasad PV, Epstein FH. Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: effects of aging and cyclooxygenase inhibition. Kidney Int. 1999;55(1):294–8.
Prasad PV. Evaluation of intra-renal oxygenation by BOLD MRI. Nephron Clin Pract. 2006;103(2):c58–65.
Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, et al. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008;19(4):780–8.
Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, et al. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55(4):961–6.
Gloviczki ML, Lerman LO, Textor SC. Blood oxygen level-dependent (BOLD) MRI in renovascular hypertension. Curr Hypertens Rep. 2011;13(5):370–7.
Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, et al. Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol. 2009;44(9):566–71.
Pedersen M, Dissing TH, Morkenborg J, Stodkilde-Jorgensen H, Hansen LH, Pedersen LB, et al. Validation of quantitative BOLD MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int. 2005;67(6):2305–12.
Li LP, Ji L, Santos EA, Dunkle E, Pierchala L, Prasad P. Effect of nitric oxide synthase inhibition on intrarenal oxygenation as evaluated by blood oxygenation level-dependent magnetic resonance imaging. Invest Radiol. 2009;44(2):67–73.
Palm F, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Angiotensin II type 2 receptors and nitric oxide sustain oxygenation in the clipped kidney of early Goldblatt hypertensive rats. Hypertension. 2008;51(2):345–51.
Warner L, Gomez SI, Bolterman R, Haas JA, Bentley MD, Lerman LO, et al. Regional decreases in renal oxygenation during graded acute renal arterial stenosis: a case for renal ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R67–71.
Hofmann L, Simon-Zoula S, Nowak A, Giger A, Vock P, Boesch C, et al. BOLD-MRI for the assessment of renal oxygenation in humans: acute effect of nephrotoxic xenobiotics. Kidney Int. 2006;70(1):144–50.
Djamali A, Sadowski EA, Muehrer RJ, Reese S, Smavatkul C, Vidyasagar A, et al. BOLD-MRI assessment of intrarenal oxygenation and oxidative stress in patients with chronic kidney allograft dysfunction. Am J Physiol Renal Physiol. 2007;292(2):F513–22.
Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17(1):17–25.
Kristensen DH, Pedersen M, Gron MC, Flyvbjerg A, Madsen M, Frokiaer J, et al. Intrarenal blood oxygenation and renal function measured by magnetic resonance imaging during long-term cyclosporine treatment. Transplant Proc. 2005;37(8):3302–4.
Park SY, Kim CK, Park BK, Huh W, Kim SJ, Kim B. Evaluation of transplanted kidneys using blood oxygenation level-dependent MRI at 3 T: a preliminary study. AJR Am J Roentgenol. 2012;198(5):1108–14.
Manotham K, Ongvilawan B, Urusopone P, Chetsurakarn S, Tanamai J, Limkuansuwan P, et al. Angiotensin II receptor blocker partially ameliorated intrarenal hypoxia in chronic kidney disease patients: a pre-/post-study. Intern Med J. 2012;42(4):e33–7.
Yin WJ, Liu F, Li XM, Yang L, Zhao S, Huang ZX, et al. Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eur J Radiol. 2012;81(7):1426–31.
Cowley AW, Mattson DL, Lu SH, Roman RJ. The renal medulla and hypertension. Hypertension. 1995;25(4):663–73.
Cowley AW, Roman RJ, Fenoy FJ, Mattson DL. Effect of renal medullary circulation on arterial-pressure. J Hypertens. 1992;10:S187–93.
Li L, Storey P, Kim D, Li W, Prasad P. Kidneys in hypertensive rats show reduced response to nitric oxide synthase inhibition as evaluated by BOLD MRI. J Magn Reson Imaging. 2003;17(6):671–5.
Textor SC, Gloviczki ML, Flessner MF, Calhoun DA, Glockner J, Grande JP, et al. Association of filtered sodium load with medullary volumes and medullary hypoxia in hypertensive African Americans as compared with whites. Am J Kidney Dis. 2012;59(2):229–37.
Juillard L, Lerman LO, Kruger DG, Haas JA, Rucker BC, Polzin JA, et al. Blood oxygen level-dependent measurement of acute intra-renal ischemia. Kidney Int. 2004;65(3):944–50.
Alford SK, Sadowski EA, Unal O, Polzin JA, Consigny DW, Korosec FR, et al. Detection of acute renal ischemia in swine using blood oxygen level-dependent magnetic resonance imaging. J Magn Reson Imaging. 2005;22(3):347–53.
Djamali A, Sadowski EA, Samaniego-Picota M, Fain SB, Muehrer RJ, Alford SK, et al. Noninvasive assessment of early kidney allograft dysfunction by blood oxygen level-dependent magnetic resonance imaging. Transplantation. 2006;82(5):621–8.
Li LP, Vu AT, Li BS, Dunkle E, Prasad PV. Evaluation of intrarenal oxygenation by BOLD MRI at 3.0 T. J Magn Reson Imaging. 2004;20(5):901–4.
Tumkur SM, Vu AT, Li LP, Pierchala L, Prasad PV. Evaluation of intra-renal oxygenation during water diuresis: a time-resolved study using BOLD MRI. Kidney Int. 2006;70(1):139–43.
Pruijm M, Hofmann L, Maillard M, Tremblay S, Glatz N, Wuerzner G, et al. Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension. 2010;55(5):1116–22.
Pei XL, Xie JX, Liu JY, Wang S, Tian XK. A preliminary study of blood-oxygen-level-dependent MRI in patients with chronic kidney disease. Magn Reson Imaging. 2012;20(3):330–5.
Simon-Zoula SC, Hofmann L, Giger A, Vogt B, Vock P, Frey FJ, et al. Non-invasive monitoring of renal oxygenation using BOLD-MRI: a reproducibility study. NMR Biomed. 2006;19(1):84–9.
Lubbers DW, Baumgartl H. Heterogeneities and profiles of oxygen pressure in brain and kidney as examples of the pO2 distribution in the living tissue. Kidney Int. 1997;51(2):372–80.
Saad A, Crane J, Glockner JF, Herrmann SMS, Friedman H, Ebrahimi B, et al. Human renovascular disease: estimating fractional tissue hypoxia to analyze blood oxygen level–dependent MR. Radiology. 2013;268(3):770–8.
Chrysochou C, Mendichovszky IA, Buckley DL, Cheung CM, Jackson A, Kalra PA. BOLD imaging: a potential predictive biomarker of renal functional outcome following revascularization in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27(3):1013–9.
Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI. Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int. 2012;81(7):684–9.
Neugarten J. Renal BOLD-MRI and assessment for renal hypoxia. Kidney Int. 2012;81(7):613–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Saad, A.F., Textor, S.C. (2014). Blood Oxygen Level Dependent (BOLD) MR Analysis of Tissue Oxygenation in Atherosclerotic Renal Artery Stenosis. In: Lerman, L., Textor, S. (eds) Renal Vascular Disease. Springer, London. https://doi.org/10.1007/978-1-4471-2810-6_17
Download citation
DOI: https://doi.org/10.1007/978-1-4471-2810-6_17
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-2809-0
Online ISBN: 978-1-4471-2810-6
eBook Packages: MedicineMedicine (R0)