Abstract
Retroperitoneal soft tissue lesions represent a wide range of disease processes with overlapping imaging findings. Familiarity with the CT and MR characteristics of these conditions is important to guide clinical evaluation. We review the tissue types, characteristic clinical, demographic, and imaging features of retroperitoneal tumors and tumor-like non-neoplastic conditions with CT and MR correlation, including anatomic and imaging clues, and provide a diagnostic approach to aide the radiologist in making a specific diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A wide range of benign and malignant pathologies arise from the retroperitoneal spaces of the abdomen and pelvis. In general, a mass or process is considered to be primary to the retroperitoneum if it originates from the soft tissues, lymphatics or neural tissue of the retroperitoneum and not from its solid organs. This heterogeneous group of diseases often poses a diagnostic challenge for radiologists.
Two Main categories of primary retroperitoneal pathologies are recognized: neoplastic and non-Neoplastic. The majority of primary retroperitoneal neoplasms are malignant (Table 1) [1,2,3,4]. Although a definitive diagnosis often cannot be established on imaging, the primary role of the radiologist is to determine whether a biopsy is necessary and/or if the mass is resectable.
Primary retroperitoneal non-neoplastic pathologies include Castleman disease, extramedullary erythropoiesis Erdheim–Chester disease and amyloidosis many of which demonstrate characteristic imaging findings that are important to recognize in order to avoid unnecessary biopsy or surgery (Table 2).
In this article, we review neoplastic and non-neoplastic primary retroperitoneal conditions, their relevant pathophysiology, clinical presentation, and characteristic CT and MRI imaging findings, particularly key features that can aid in establishing an accurate diagnosis and guide therapy. Additionally, we will provide a comprehensive diagnostic approach for the differentiation of neoplastic from non-neoplastic pathologies that can serve as a road map for narrowing the differential diagnosis and allow for appropriate and timely management.
Anatomy of the retroperitoneum at a glance
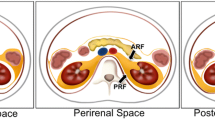
A comprehensive knowledge of retroperitoneal anatomy is invaluable to accurately characterize and diagnose its conditions. The retroperitoneum can be grossly divided into several spaces: the anterior and posterior pararenal spaces, the perirenal space, and the fat-containing great vessel space. These can be further divided by separating the anterior pararenal space into the pancreatoduodenal space (containing the pancreas and duodenum) and the pericolonic space (surrounding the ascending and descending colon). Below the level of the kidneys, the anterior and posterior pararenal spaces merge to form an infrarenal retroperitoneal space, which in turn communicates inferiorly with the prevesical space and extraperitoneal compartments of the pelvis (Fig. 1) [5]. Several signs can be used to differentiate peritoneal from retroperitoneal masses, via determination of the organ of tumor origin (Table 3) [6].
Schematic diagram of retroperitoneal anatomy with CT correlation. The anterior pararenal space a, b is delineated by the peritoneum anteriorly and the anterior pararenal facia posteriorly and contains the pancreas and transverse colon. The posterior pararenal space (a, b), primarily composed of fat, is bounded by the posterior pararenal fascia anteriorly and the transversals fascia posteriorly. The great vessel space a contains the aorta, the inferior vena cava, fat, neural tissue, and lymphatics. Many of the retroperitoneal spaces are contiguous with extraperitoneal spaces of the pelvis (c). The perirenal space b, d, e is bounded by the anterior and posterior perirenal fasciae and contains fat as well as bridging septa that extend from the renal capsule to the perirenal fascia (e)
Diagnostic imaging approach to differentiate neoplastic from non-neoplastic pathologies and suggested management
Imaging plays a major role in the detection and characterization of retroperitoneal pathologies, and recognition of key imaging features can help in narrowing the differential diagnosis. Both contrast-enhanced CT and MRI are useful for tissue characterization, accurate determination of mass origin and extent, and assessment of benign or malignant potential.
Morphology is an important clue as to whether a retroperitoneal soft tissue mass is malignant. On imaging, benign processes often have a non-mass-like appearance surrounding and encasing adjacent anatomical structures, such as mantle-like encasement of the ureters and vessels with tethering and luminal narrowing. Inflammatory processes may cause wall thickening and pseudoaneurysm formation of the aorta or mesenteric arteries. Malignant tumors present with more well-defined borders and mass-like appearances and are commonly associated with adjacent organ and/or structure displacement/distortion.
A mass centered in the retroperitoneum often indicates malignancy. For most primary retroperitoneal malignancies, the only curative treatment is complete resection with a tumor-free margin. Therefore, when a diagnosis of primary malignancy is likely, patients usually are referred for surgical evaluation.
In contrast, chemotherapy is usually the first line treatment for lymphoma and metastatic disease. When these entities are considered on imaging (specifically when a primary neoplasm is detected elsewhere), biopsy should be suggested for confirmation (also see Table 4). Individual conditions, grouped by tissue type, will be discussed in greater detail in the subsequent section.
Imaging findings that suggest a specific primary retroperitoneal malignancy: resection should be considered
Fat components
The presence of gross fat in a primary retroperitoneal mass that also has myxoid, solid, infiltrative or nodular soft tissue components strongly suggests liposarcoma (Fig. 2). Tumors not primary to the retroperitoneum, such as adrenal myelolipoma or renal angiomyolipoma, also contain gross fat intermixed with soft tissue; however, these are centered or completely contained within their respective organs of origin (Fig. 3). Retroperitoneal lipomas appear as well-defined masses with homogenous fat attenuation. Although benign, lipomas cannot be distinguished from well-differentiated liposarcomas on imaging and any purely fatty mass should be treated as a liposarcoma until confirmed otherwise by histology [7]. Other primary retroperitoneal tumors to consider when gross fat is present include teratoma (usually demonstrates calcification and heterogeneous enhancement) and extra-adrenal myelolipoma (a rare entity, most commonly in the presacral space) [1, 8].
a Liposarcoma in a middle-aged man. a Contrast-enhanced CT shows a mixed fat and soft tissue attenuation mass in the pelvis (arrow). b Axial T1W FS post-contrast MRI shows enhancement of the soft tissue components of the mass (arrowhead). b Liposarcoma in a middle-aged man. a Contrast-enhanced CT shows a mixed fat and soft tissue attenuation mass in the pelvis (arrow). b/c Axial T1W FS pre- (b) and post-contrast c MRI shows enhancement of the soft tissue components of the mass (arrowhead). c Liposarcoma in a middle-aged man. a Contrast-enhanced CT shows a mixed fat and soft tissue attenuation mass in the pelvis (arrow). b/c Axial T1W FS pre- (b) and post-contrast (c) MRI shows enhancement of the soft tissue components of the mass (arrowhead)
Intravascular extension
A primary retroperitoneal lesion with intravascular growth is characteristic of leiomyosarcoma (Fig. 4). The main differential consideration is intravascular spread of an abdominal or pelvic solid organ primary malignancy, most commonly hepatocellular carcinoma, adrenal cortical carcinoma, renal cell carcinoma, or uterine metastasizing leiomyoma. Therefore, evidence of a primary mass in any of these organs should be sought.
a Leiomyosarcoma in a middle-aged woman, endovascular growth. Contrast-enhanced CT in the portal venous phase shows a heterogeneous soft tissue mass completely filling and expanding the infrahepatic (arrow in b) and intra-hepatic (arrowheads in a) segments of the IVC. b Leiomyosarcoma in a middle-aged woman, endovascular growth. Contrast-enhanced CT in the portal venous phase shows a heterogeneous soft tissue mass completely filling and expanding the infrahepatic (arrow in b) and intra-hepatic (arrowheads in a) segments of the IVC
Myxoid stroma
Neurogenic tumors, myxoid liposarcomas, and myxofibrosarcomas are the most common retroperitoneal tumors that contain myxoid matrices, which on MRI has characteristic marked T2 hyperintensity as well as delayed contrast enhancement [2, 9]. Progressive enhancement peaking on the delayed phase distinguishes myxoid stroma from internal necrosis within a mass, which does not enhance.
Necrotic component
Necrosis is a characteristic finding of large or high-grade neoplasms. When necrosis is seen in retroperitoneal tumors leiomyosarcoma, undifferentiated pleomorphic sarcoma, PEComas, and pleomorphic liposarcomas are the main differential considerations. Necrotic tissue is heterogeneously hyperintense on T2WI and demonstrates no enhancement on post-contrast imaging [6].
Hypervascularity
Among the hypervascular lesions are paragangliomas, leiomyosarcoma, myxofibrosarcoma, other sarcomas, and arteriovenous malformations and fistulas. Hypervascularity differentiates these from entities that are usually hypovascular such as lymphoma, multiple myeloma, and low-grade sarcomas [10]. Other imaging characteristics of these lesions that further narrow the differential will be subsequently discussed.
Imaging findings for which clinical management is uncertain: Surgery versus watchful waiting
Cystic contents
Many benign neoplasms have relatively large cystic components and may pose a diagnostic challenge. Examples of cystic masses include cystic lymphangiomas cystic mesotheliomas (rare benign neoplasms of mesothelial origin), ganglioneuromas, cystic schwannomas, paragangliomas, cystadenomas, and epidermoid cysts.
Imaging findings that are nonspecific but suggest malignancy: biopsy should be considered
Lymphoma and metastatic disease typically require tissue confirmation. Imaging findings indicative of either should prompt recommendation of biopsy.
Enlarged abdominopelvic lymph nodes can indicate lymphoma or metastatic disease. Lymphoma usually produces nodes in multiple contiguous anatomic distributions. The nodes are homogenous, mildly enhancing, and surround but not occlude the vasculature. Splenomegaly with or without splenic nodules may be present. Metastatic adenopathy is typically heterogeneous. Necrosis may be present if the nodes are large and they may obstruct vasculature, bowel, or the ureters. When metastatic disease is suspected, parenchymal organs should be closely examined for evidence of a primary mass or other metastases.
Differential considerations of malignant retroperitoneal tumors based on tissue type
Primary retroperitoneal lesions with characteristics of a mass are often malignant [1,2,3,4]. These lesions are typically asymptomatic until they become large and come to clinical attention when symptoms develop from mass effect. With the exception of a few select tumor types that are discussed below, there are no laboratory or clinical abnormalities that suggest a specific tumor. For clinical and demographic characteristics of these conditions please refer to Table 1.
Primary retroperitoneal neoplasms
Mesenchymal tumors
Liposarcoma
Liposarcomas are most common primary tumors to arise in the retroperitoneum [11,12,13]. The only potentially curative treatment is resection with a tumor-free margin. Local or regional recurrence is the most common cause of death. Distant metastases are most often to the lungs [14].
Liposarcomas are divided into 5 histologic subtypes: well differentiated, dedifferentiated, myxoid, pleomorphic and not otherwise specified [15]; of these differentiated/dedifferentiated liposarcomas are the most common [16]. More than one histologic subtype can co-exist within the same tumor, in these cases, overall prognosis depends on the least differentiated subtype.
Imaging has a critical role both in diagnosis and staging. Most liposarcomas present as large well-defined masses of mixed fluid and soft tissue components that avidly enhance with intravenous contrast (Fig. 2). Gross fat is not always present, but when found in a heterogeneous retroperitoneal mass not arising from a solid organ strongly suggests liposarcoma. Differential considerations for a gross fat-containing retroperitoneal mass include lipomas, which are homogenous and entirely composed of fat with no soft tissue components, and PEComas, which have a dominant soft tissue component but may contain smaller amounts of gross fat.
The imaging appearance of liposarcomas correlates with their histologic composition [17]. A higher ratio of soft tissue compared to lipid is found in high-grade tumors [18, 19]. Well-differentiated liposarcomas typically present as well-circumscribed masses nearly completely composed of gross fat, with a small nodular, reticular, or hazy soft tissue component (Fig. 2). Dedifferentiated liposarcomas, considered intermediate grade, contain a larger proportion of internal nonlipid soft tissue [20]. Myxoid components in liposarcomas are indicative of higher grade and can be readily distinguishable on imaging, as the myxoid components are similar to fluid on non-contrast CT and show T2 hyperintense signal when compared to muscle on MRI. On post-contrast imaging, unlike fluid-containing structures, myxoid tumors show gradual reticular internal enhancement (Fig. 3) [15, 19].
Leiomyosarcoma
Leiomyosarcomas are mesenchymal tumors that show smooth muscle differentiation. The retroperitoneum is the most common site of origin of leiomyosarcomas. Treatment is surgical. Retroperitoneal leiomyosarcomas are classified into IVC and non-IVC origin tumors; although the two subtypes are resected through a different surgical approach, they are similar in their internal composition, metastatic pattern and long-term prognosis [21].
The majority of non-IVC origin leiomyosarcomas arise in the perirenal space of the retroperitoneum. Tumors that originate from the IVC wall and most commonly arise at or caudal to the level of the retrohepatic IVC, presenting at an earlier stage due to development of clinical symptoms related to IVC obstruction. Metastases are typically hematogenous, most commonly to the lungs, liver, bone, soft tissue and the peritoneum [22]. Local lymph node metastases are uncommon. This distinguishes leiomyosarcomas from angiosarcomas which also present as a soft tissue mass arising from the IVC but more commonly metastasize to local lymph node chains [23].
On imaging, leiomyosarcomas present as a large, heterogeneous, infiltrative, lipid-free soft tissue mass, similar to muscle on CT and T1WI, hyperintense to muscle on T2WI with heterogeneous enhancement. A central necrotic or avascular area is also often present [24, 25]. Although most leiomyosarcomas are extraluminal, if a mass is centered within the IVC lumen, it can be confidently diagnosed as a leiomyosarcoma of IVC origin (Fig. 4) [26]. The vessel lumen may be partially or completely occluded and is often expanded [24].
Undifferentiated pleomorphic sarcoma (UPS)
Undifferentiated pleomorphic sarcomas (UPS), previously called malignant fibrous histiocytomas, are tumors of mesenchymal origin composed of fibroblastic or myofibroblastic cells. UPS is the third most common type of primary malignancy in the retroperitoneum, following liposarcoma and leiomyosarcoma [4, 27]. Treatment is surgical.
On imaging, UPS present as large heterogeneous tumors with similar signal characteristics to muscle except for hyperintense signal on T2WI, strong arterial enhancement and delayed hypoenhancement [28]. Although less common than in leiomyosarcomas, central necrosis may occur in large tumors (Fig. 5). Approximately 20% of UPS show internal calcification, which is uncommon in leiomyosarcomas [29, 30].
A Undifferentiated pleomorphic sarcoma (formerly malignant fibrous histiocytoma) in a middle-aged man. a coronal T1W FS pre-contrast, b T1W FS post-contrast, c T2 TSE coronal images of the abdomen. T1 hyperintense signal within the mass is consistent with hemorrhage (arrow in a) and the mass also demonstrates heterogeneous enhancement (arrowhead in b). The T2W TSE image shows anatomic relationships of the mass (arrows in c) centered in the retroperitoneum. b Undifferentiated pleomorphic sarcoma (formerly malignant fibrous histiocytoma) in a middle-aged man. a coronal T1W FS pre-contrast b T1W FS post-contrast C: T2 TSE coronal images of the abdomen. T1 hyperintense signal within the mass is consistent with hemorrhage (arrow in a) and the mass also demonstrates heterogeneous enhancement (arrowhead in b). The T2W TSE image shows anatomic relationships of the mass (arrows in c) centered in the retroperitoneum. c Undifferentiated pleomorphic sarcoma (formerly malignant fibrous histiocytoma) in a middle-aged man. a coronal T1W FS pre-contrast, b T1W FS post-contrast, c T2 TSE coronal images of the abdomen. T1 hyperintense signal within the mass is consistent with hemorrhage (arrow in a) and the mass also demonstrates heterogeneous enhancement (arrowhead in b). The T2W TSE image shows anatomic relationships of the mass (arrows in c) centered in the retroperitoneum
Similar to other soft tissue malignancies, including liposarcoma and leiomyosarcoma, UPS show diffusion restriction on MRI. Although diffusion restriction is useful to distinguish retroperitoneal malignancy from benign entities, its use in determining the specific type of malignancy is limited [31].
Less common sarcomas
Other rare histologic subtypes of retroperitoneal sarcomas include rhabdomyosarcoma, angiosarcoma, chondrosarcoma, and synovial sarcoma. These tumors appear similar on imaging: a large soft tissue mass, heterogenous but similar to muscle in CT attenuation, primarily isointense to muscle on T1WI hyperintense on T2WI, with heterogenous enhancement. A few imaging characteristics that may help suggest a specific diagnosis include high flow vessels that show prominent T2 signal void within angiosarcomas, chondroid matrix with stippled, punctate and arc-like calcifications occasionally present in chondrosarcomas, and a prominent component of hemorrhage, necrosis, and calcification in synovial sarcomas [32,33,34,35].
Perivascular epithelioid cell tumor (PEComa)
PEComas are mesenchymal neoplasms composed of epithelioid and spindle cells arranged around blood vessels. Most PEComas are benign (such as angiomyolipoma and lymphangioleiomyomatosis), a minority of PEComas are malignant sarcomas that may arise in the retroperitoneum [36]. These tumors are associated with tuberous sclerosis, and have a female predominance and a poor prognosis [37]. Treatment is surgical.
PEComas do not have pathognomonic imaging findings and therefore tissue sampling is usually necessary for diagnosis. On imaging, retroperitoneal PEComas are large (> 5 cm) infiltrative heterogeneous masses that demonstrate avid arterial enhancement, areas of necrosis, hemorrhage, and occasional punctate foci of calcification [38]. Gross fat may be present, which can make these neoplasms indistinguishable from liposarcomas. Invasion of retroperitoneal organs may also be seen [39].
Lymphoma
Most lymphomas involving the retroperitoneum originate from the urinary tract; a minority are of primary retroperitoneal origin. Treatment is chemotherapy. Characteristic imaging findings include well-circumscribed nodular or lobulated soft tissue masses of homogenous CT attenuation, T1WI hypointense and T2WI hyperintense signal and homogenous enhancement that peaks in the delayed phase. Involved retroperitoneal lymph nodes typically form a confluent lobulated soft tissue mass that extends into the pelvis or the mesentery (Fig. 6, Video 1). Lymphoma-related adenopathy tends to insinuate between rather than narrow adjacent vascular structures (“sandwich sign”) [40, 41]. Necrosis, calcification, and hemorrhage are rare (Figs. 7, 8, 9, 10, 11, 12).
Lymphoma in a 40-year-old man. Contrast-enhanced CT shows confluent mass-like lymphadenopathy in the retroperitoneum (black arrow) showing homogenous low-level enhancement with lymphadenopathy also extending to the root of the mesentery (arrowhead). The nodes displace and surround in spite of the degree of adenopathy do not obstruct the vasculature (arrow). A nodal mass surrounding the vasculature has been described as the “sandwich sign”
A. Schwannoma in a 52-year-old male. a Coronal Fat Sat T2-weighted image of the pelvis demonstrating T2 hyperintense mass to left of the L4–L5 disk space (asterisk). b Coronal T1W FS post-contrast image showing heterogeneous enhancement (white arrow) of the mass (asterisk). An L4–L5 nerve appears to be entering the mass (black arrows in a, b). The mass is associated with the left L4–L5 neural foramina. b Schwannoma in a 52-year-old male. a Coronal Fat Sat T2-weighted image of the pelvis demonstrating T2 hyperintense mass to left of the L4–L5 disk space (asterisk). b Coronal T1W FS post-contrast image showing heterogeneous enhancement (white arrow) of the mass (asterisk). An L4–L5 nerve appears to be entering the mass (black arrows in a, b). The mass is associated with the left L4–L5 neural foramina
A Diffuse plexiform neurofibroma in a 23-year-old male with history of Neurofibromatosis Type 1 (NF1). a coronal T2W FS and b sagittal T2 W TSE image shows an infiltrative mass (asterisk in a and arrows and asterisk in b) extending along a nerve and surrounding the urinary bladder. Note widening of the thecal sac in this patient with sacral Tarlov’s cysts (not shown). b Diffuse plexiform neurofibroma in a 23-year-old male with history of Neurofibromatosis Type 1 (NF1). a coronal T2W FS and b sagittal T2 W TSE image shows an infiltrative mass (asterisk in a and arrows and asterisk in b) extending along a nerve and surrounding the urinary bladder. Note widening of the thecal sac in this patient with sacral Tarlov’s cysts (not shown)
A Ganglioneuroma in a young female. b Axial subtraction T1W FS image demonstrates a large (5 × 7 cm) heterogeneously enhancing, well -circumscribed pelvic, midline mass (asterisk). The mass extends/arises from the widened right S1-2 neural foramen (arrows). The mass abuts the right ovary and right iliac artery but it does not encase the vasculature and there is no evidence of right ovarian involvement (not shown, B = urinary bladder) b Sagittal T2-weighted image reveals heterogeneous hyperintensity signal within the mass. Note mass effect on the adjacent posterior wall of the urinary bladder (b). Again appreciated is the extension on the mass into the right S1-2 neural foramen (white arrows). B Ganglioneuroma in a young female. a Axial subtraction T1W FS image demonstrates a large (5 × 7 cm) heterogeneously enhancing, well-circumscribed pelvic, midline mass (asterisk). The mass extends/arises from the widened right S1-2 neural foramen (arrows). The mass abuts the right ovary and right iliac artery but it does not encase the vasculature and there is no evidence of right ovarian involvement (not shown, B = urinary bladder) b. Sagittal T2-weighted image reveals heterogeneous hyperintense signal within the mass. Note mass effect on the adjacent posterior wall of the urinary bladder (b). Again appreciated is the extension on the mass into the right S1-2 neural foramen (white arrows)
A Sacrococcygeal Primary Germ Cell Tumor (Yolk Sac type and Mature Teratoma) in a young male patient. a Axial T1W FS post-contrast image demonstrates a large 8.5 × 6 cm heterogeneously enhancing retroperitoneal bilobed mass that is centered within the presacral and precoccygeal space. The mass is composed of two main components: one is more heterogeneously enhancing presacral solid component (asterisk, representing a yolk sac tumor on pathology); and a second more anteriorly positioned minimally to none enhancing fat-containing component (star, representing mature teratoma component of the tumor). b Axial T2W TSE image demonstrates heterogeneous T2 signal of the posterior yolk sac component (asterisk) and hyperintense fat (asterisk) and hemorrhage (short white arrows) containing anterior mature teratoma component (star). b Sacrococcygeal Primary Germ Cell Tumor (Yolk Sac type and Mature Teratoma) in a young male patient. a Axial T1W FS post-contrast image demonstrates a large 8.5 × 6 cm heterogeneously enhancing retroperitoneal bilobed mass that is centered within the presacral and precoccygeal space. The mass is composed of two main components: one is more heterogeneously enhancing presacral solid component (asterisk, representing a yolk sac tumor on pathology); and a second more anteriorly positioned minimally to none enhancing fat-containing component (star, representing mature teratoma component of the tumor). b Axial T2W TSE image demonstrates heterogeneous T2 signal of the posterior yolk sac component (asterisk) and hyperintense fat (asterisk) and hemorrhage (short white arrows) containing anterior mature teratoma component (star)
a Retroperitoneal hemorrhage with active extravasation from a ruptured pseudoaneurysm (PSA) in a 52-year-old female status post catheter directed spasmolysis one day ago. a IV contrast-enhanced CT image obtained in the coronal plane demonstrates a large right retroperitoneal hemorrhage (H) with an active extravasation (black arrows in a). b Selective arteriogram of the common iliac artery demonstrates a small pseudoaneurysm of the right distal external iliac artery (R EIA) (arrow in b). Active extravasation was noted on dynamic imaging (not shown). The patient underwent successful thrombin injection of the PSA. b Retroperitoneal hemorrhage with active extravasation from a ruptured pseudoaneurysm (PSA) in a 52-year-old female status post catheter directed spasmolysis one day ago. a IV contrast-enhanced CT image obtained in the coronal plane demonstrates a large right retroperitoneal hemorrhage (H) with an active extravasation (black arrows in a). b Selective arteriogram of the common iliac artery demonstrates a small pseudoaneurysm of the right distal external iliac artery (R EIA) (arrow in b). Active extravasation was noted on dynamic imaging (not shown) The patient underwent successful thrombin injection of the PSA
The primary differential diagnosis for infiltrative, confluent retroperitoneal soft tissue is retroperitoneal fibrosis (RPF). Both RPF and lymphoma have a similar imaging appearance, and therefore these entities may be indistinguishable on imaging. Extension of a mass beyond the retroperitoneum favors a diagnosis of lymphoma, as such extension is not typical for RPF. Anterior displacement of the aorta by a confluent nodal mass is more characteristic of lymphoma rather than RPF, which usually encases the vasculature without displacing it (Fig. 13). Diffusion restriction on MRI is more pronounced in lymphoma compared to RPF [41].
Neurogenic tumors
Neurogenic tumors constitute 10–20% of all primary retroperitoneal malignancies. They are more likely to be benign and have a better prognosis. [42] In the retroperitoneum, they follow the distribution of the sympathetic ganglia and paraspinal areas, including the organs of Zuckerkandl. The main subcategories of neurogenic tumors in adults include nerve sheath tumors (schwannoma, neurofibroma), ganglionic cell tumors (ganglioneuroma), and paraganglionic cell tumors (paraganglioma).
Neurofibroma and Schwannoma
Neurofibroma (NF) and schwannoma are benign nerve sheath tumors. [43] NFs can be isolated (90%) or can occur in association with type 1 neurofibromatosis (NF1). On imaging, both tumors have a variable appearance, demonstrating solid and/or cystic components depending on the degree of degeneration within the tumor, with possible hemorrhage and calcification. Due to the presence of myxoid degeneration, these tumors tend to be centrally hyperintense on T1WI, peripherally hyperintense on T2WI, and hypoattenuating on CT. On post-contrast imaging, homogeneous or target-like enhancement can be seen. NFs are unencapsulated and result in expansion of the entire nerve, with nerve fibers coursing through the mass itself. Plexiform neurofibromas have an infiltrative appearance with multiple sites of disease that track along nerve roots (Fig. 8). Malignant transformation is more common with neurofibromas. Schwannomas are encapsulated and usually extend along the course of a nerve, resulting in nerve flattening. The entering and exiting nerve signs may be apparent. Highly cellular components demonstrate hypointense signal on both T1 and T2WI (Fig. 7) [43, 44].
Ganglioneuroma
Ganglioneuromas are benign tumors that arise from sympathetic ganglia and are most commonly located in the retroperitoneum, extending along the paravertebral sympathetic ganglia and sometimes the adrenal medulla. They carry a favorable prognosis. These tumors are most commonly asymptomatic, however, on occasion secrete catecholamines or androgenic hormones [45]. On imaging, ganglioneuromas are hypoattenuating masses that demonstrate hypointense T1 and hyperintense T2 signal, and occasionally present with a centrally located intact vessel (Fig. 9). Punctate calcifications are characteristic and seen in 20-30% of tumors [46]. Post-contrast enhancement is variable.
Paraganglioma (extra-adrenal pheochromocytoma)
Paragangliomas are tumors related to chromaffin cells that originate in an extra-adrenal location; most commonly in the organ of Zuckerkandl. Production/secretion of catecholamines and associated symptoms is a hallmark of these tumors, present in approximately 40% of cases [45]. They typically appear as a large mass with areas of hemorrhage and necrosis on cross-sectional imaging (Video 2). Large paragangliomas can be complicated by extensive retroperitoneal hemorrhage and rupture. Punctate calcifications and avid contrast enhancement are common. Metastases are seen in up to 50% of cases. [42]
Germ cell origin tumors (GCT)
Primary extragonadal GCT and sex cord stromal tumors (SCST) Primary extragonadal GCT (Video 3) are thought to arise from aberrant primordial germ cell rests, and account for approximately 1–2.5% of all germ cell tumors [47]. Although the retroperitoneum is the second most common site of presentation, the majority of retroperitoneal GCTs represent metastases from a primary gonadal lesion. Sometimes primary gonadal tumors may not be detected on imaging due to tumor regression [47]. Several subtypes of these tumors have been recognized, including seminomas and non-seminomatous GCT (embryonal carcinomas, yolk sac tumors, choriocarcinomas, teratomas, and mixed GCTs).
Retroperitoneal primary SCST arise from ectopically located sex cord stromal tissue or from sex cord-like differentiation of somatic cells. Extragonadal primary SCST are commonly seen in the pelvis, retroperitoneal location is less common. Several types of stromal tumors are recognized, including thecomas, Sertoli–Leydig cell tumors, and unclassified sex cord tumors. Elevated serum estrogen levels are typical in patients with granulosa cell tumors and thecomas.
Imaging findings of SCST are nonspecific: tumors are heterogeneous and enhancing on all cross-sectional imaging modalities. These tumors may demonstrate local invasion (Fig. 10) [48,49,50].
Teratoma
Retroperitoneal teratomas represent 10% of all teratomas and originate from ectopically located pluripotent germ cells [51, 52]. Teratomas are subdivided into three main categories: mature (cystic/solid, most are benign), immature (malignant) and monodermal (highly differentiated) [53]. Imaging may not reliably differentiate these types.
Mature teratomas (also called dermoid cysts) are predominately cystic. Calcifications and fat components are common. The large cystic components contain fatty sebaceous fluid and fat-fluid or fluid-fluid levels. Malignant transformation occurs in 2–3% of mature teratomas, specifically adenocarcinoma type [7].
Immature teratomas consist of 10% of undifferentiated tissue [50]. Elevated AFP levels may be present. On CT and MRI they appear as enhancing predominantly solid masses of variable CT attenuation and MR signal, with foci of fat, calcification, and simple cysts (Video 4). Local and vascular invasion is partly responsible for their poor prognosis. Treatment is surgical [5].
Primary non-neoplastic retroperitoneal conditions
Multiple non-neoplastic disease entities may be encountered in the retroperitoneum (Table 2), with more common entities including abscess, arteriovenous malformations (AVM), and hematoma, and less common ones including amyloidosis, retroperitoneal fibrosis, Erdheim–Chester disease, extramedullary hematopoiesis, and Castleman disease.
Retroperitoneal hemorrhage and AVM are the most common vascular lesions of the retroperitoneum. Ill-defined, infiltrative morphology, and high attenuation on unenhanced CT distinguish hemorrhage from neoplasms (Fig. 11). A blush of enhancement may be present from extravasation if there is active hemorrhage. AVM present as a tangle of enlarged vessels on CT and MRI, with prominent flow voids on T2WI. Further characteristics of these entities are listed in Table 2.
Retroperitoneal fibrosis and Erdheim–Chester disease
Retroperitoneal fibrosis (RPF) and Erdheim–Chester disease (ECD) may resemble a number of the retroperitoneal neoplasms such as lymphoma, multiple myeloma, metastatic disease and desmoid tumor. Involvement of other systems and organs or pattern of distribution may aid in differentiation of RPF and ECD from malignant processes. Both conditions demonstrate a mantle growth pattern with fibrotic changes encasing the RP structures and vessels and absence of invasion. ECD involves RP visceral organs (especially kidneys and perirenal space), long bones, skin, globes, lungs, brain, pituitary, and heart (Fig. 12). RPF develops around the aortic bifurcation and spreads upward, enveloping renal hila (Fig. 13, Video 5). Both processes may result in ureteral and vascular obstruction [54]. Tethering of vessels to adjacent vertebrae and medial displacement of the ureters are characteristic of nonmalignant RPF. Active inflammation seen in acute stage RPF shows avid enhancement on post-contrast CT and MRI, and T2 hyperintense signal on MRI. Chronic (acellular) RPF demonstrates T2 hypointense signal and delayed or no enhancement on post-contrast imaging. A malignant form of RPF also exists and carries a poor prognosis; it is characterized by large size, irregular lobular margins, variable enhancement, and significant mass effect resulting in anterior displacement of vessels and lateral displacement of the ureters [54].
Extramedullary hematopoiesis
The retroperitoneum is an uncommon site for extramedullary hematopoiesis (EMH), which on imaging presents as homogeneous bilateral soft tissue masses that extend along the paravertebral region or arise in the perirenal space, and may contain macroscopic fat, hemosiderin deposits and demonstrate variable enhancement (Fig. 14). Calcifications and bony destruction are rare [55]. Disease processes that affect marrow hematopoiesis result in EMH [7]. The presence of hepatosplenomegaly, anemia and skeletal changes may aid in diagnosis [56, 57].
Extramedullary hematopoiesis. Contrast-enhanced CT at the level of the kidneys shows bilateral mixed fat and soft tissue attenuation masses (arrows) representing hematopoetic tissue in the anterior pararenal and perirenal space in a patient with profound, chronic anemia. Although the masses are heterogeneous, calcifications and fluid density components are absent. RK = right kidney, LK = left kidney
Castleman disease
Castleman disease (CD) is a rare disease characterized by the development of benign lymphadenopathy that may be localized or systemic, often mimicking malignancy [58, 59]. On imaging, CD presents as homogeneously hyperenhancing mass(es) some with calcifications (Fig. 15) [60, 61]. Diagnosis requires tissue sampling.
Castleman’s disease in a middle-aged male. Axial contrast-enhanced CT shows an avidly enhancing retroperitoneal soft tissue mass (M) near the left iliac vessels exerting mass effect on the left psoas muscle. Soft tissue attenuation (asterisk) around the mass representing lymphoid tissue infiltrates the retroperitoneal fat around the mass
Amyloidosis
Amyloidosis is a heterogeneous group of diseases resulting from the extracellular deposition of abnormal protein either at a specific site (localized amyloidosis) or throughout the body (systemic amyloidosis) [62]. It may be a primary disease process or secondary to a variety of chronic infectious, inflammatory, and neoplastic conditions; accordingly, clinical presentation is widely variable. Isolated involvement of the retroperitoneum may occur in localized amyloidosis [63].
CT and MRI features include the replacement of retroperitoneal fat with infiltrative soft tissue representing extracellular deposits of abnormally folded protein, which encases the aorta and retroperitoneal organs (Fig. 16a) and may progressively calcify over time (Fig. 16b). This appearance is similar to RPF and lymphoma, although unlike those conditions amyloid deposits can demonstrate signal dropout on opposed phase T1WI due to the intermixing of lipid and water elements [63]. Protein deposits may coalesce into a discrete mass (amyloidoma) and mimic malignancies including lymphoma or plasmacytoma on cross-sectional imaging. Biopsy is often necessary for diagnosis [63].
a Retroperitoneal amyloidosis in a 64-year-old man. a Axial contrast-enhanced CT of the abdomen shows infiltrative soft tissue surrounding the aorta and replacing retroperitoneal fat (asterisk). b Axial contrast\-enhanced CT shows several coarse calcifications, usually seen in chronic amyloid deposits, throughout the infiltrative soft tissue (arrowheads). b Retroperitoneal amyloidosis in a 64-year-old man. a Axial contrast-enhanced CT of the abdomen shows infiltrative soft tissue surrounding the aorta and replacing retroperitoneal fat (asterisk). b Axial contrast-enhanced CT shows several coarse calcifications, usually seen in chronic amyloid deposits, throughout the infiltrative soft tissue (arrowheads)
Conclusion
Primary retroperitoneal pathologies are a diverse group of benign and malignant conditions that arise within the retroperitoneal space independent of the organs located there. Although the exact diagnosis is often difficult to establish based on imaging alone, a few specific imaging findings on CT and MRI can help in narrowing the differential. Familiarity with the epidemiology, pathogenesis, imaging features, and treatment of these retroperitoneal entities can aid radiologic diagnoses and guide appropriate patient management.
References
Mota M, Bezerra ROF, Garcia MRT (2018) Practical approach to primary retroperitoneal masses in adults. Radiol Bras 51 (6):391-400. https://doi.org/10.1590/0100-3984.2017.0179
Goenka AH, Shah SN, Remer EM (2012) Imaging of the retroperitoneum. Radiol Clin North Am 50 (2):333-355, vii. https://doi.org/10.1016/j.rcl.2012.02.004
Elsayes KM, Staveteig PT, Narra VR, Chen ZM, Moustafa YL, Brown J (2007) Retroperitoneal masses: magnetic resonance imaging findings with pathologic correlation. Curr Probl Diagn Radiol 36 (3):97-106. https://doi.org/10.1067/j.cpradiol.2006.12.003
Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD (2015) Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging 40 (6):1887-1903. https://doi.org/10.1007/s00261-014-0311-x
Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Jr., Kolokythas O (2011) Imaging of uncommon retroperitoneal masses. Radiographics 31 (4):949-976. https://doi.org/10.1148/rg.314095132
Nishino M, Hayakawa K, Minami M, Yamamoto A, Ueda H, Takasu K (2003) Primary retroperitoneal neoplasms: CT and MR imaging findings with anatomic and pathologic diagnostic clues. Radiographics 23 (1):45-57. https://doi.org/10.1148/rg.231025037
Shaaban AM, Rezvani M, Tubay M, Elsayes KM, Woodward PJ, Menias CO (2016) Fat-containing Retroperitoneal Lesions: Imaging Characteristics, Localization, and Differential Diagnosis. Radiographics 36 (3):710-734. https://doi.org/10.1148/rg.2016150149
Craig WD, Fanburg-Smith JC, Henry LR, Guerrero R, Barton JH (2009) Fat-containing lesions of the retroperitoneum: radiologic-pathologic correlation. Radiographics 29 (1):261-290. https://doi.org/10.1148/rg.291085203
Jagannathan JP, Tirumani SH, Ramaiya NH (2016) Imaging in Soft Tissue Sarcomas: Current Updates. Surg Oncol Clin N Am 25 (4):645-675. https://doi.org/10.1016/j.soc.2016.05.002
Elnemr A, Yonemura Y, Shinbo M, Nishino E (2010) Primary retroperitoneal mullerian adenocarcinoma. Rare Tumors 2 (1):e6. https://doi.org/10.4081/rt.2010.e6
Weiss SW (1996) Lipomatous tumors. Monogr Pathol 38:207-239
Fiore M, Grosso F, Lo Vullo S, Pennacchioli E, Stacchiotti S, Ferrari A, Collini P, Lozza L, Mariani L, Casali PG, Gronchi A (2007) Myxoid/round cell and pleomorphic liposarcomas: prognostic factors and survival in a series of patients treated at a single institution. Cancer 109 (12):2522-2531. https://doi.org/10.1002/cncr.22720
Hekimoglu K (2013) Giant retroperitoneal liposarcomas: diagnostic approach with multidetector computed tomography and magnetic resonance imaging. Jbr-Btr 96 (6):375-377. https://doi.org/10.5334/jbr-btr.466
Ghadimi MP, Al-Zaid T, Madewell J, Peng T, Colombo C, Hoffman A, Creighton CJ, Zhang Y, Zhang A, Lazar AJ, Pollock RE, Lev D (2011) Diagnosis, management, and outcome of patients with dedifferentiated liposarcoma systemic metastasis. Ann Surg Oncol 18 (13):3762-3770. https://doi.org/10.1245/s10434-011-1794-0
Baheti AD, O’Malley RB, Kim S, Keraliya AR, Tirumani SH, Ramaiya NH, Wang CL (2016) Soft-Tissue Sarcomas: An Update for Radiologists Based on the Revised 2013 World Health Organization Classification. AJR Am J Roentgenol 206 (5):924-932. https://doi.org/10.2214/AJR.15.15498
Levy AD, Manning MA, Al-Refaie WB, Miettinen MM (2017) Soft-Tissue Sarcomas of the Abdomen and Pelvis: Radiologic-Pathologic Features, Part 1-Common Sarcomas: From the Radiologic Pathology Archives. Radiographics 37 (2):462-483. https://doi.org/10.1148/rg.2017160157
Lahat G, Madewell JE, Anaya DA, Qiao W, Tuvin D, Benjamin RS, Lev DC, Pollock RE (2009) Computed tomography scan-driven selection of treatment for retroperitoneal liposarcoma histologic subtypes. Cancer 115 (5):1081-1090. https://doi.org/10.1002/cncr.24045
Kim EY, Kim SJ, Choi D, Lee SJ, Kim SH, Lim HK, Song SY (2008) Recurrence of retroperitoneal liposarcoma: imaging findings and growth rates at follow-up CT. AJR Am J Roentgenol 191 (6):1841-1846. https://doi.org/10.2214/AJR.07.3746
Kim T, Murakami T, Oi H, Tsuda K, Matsushita M, Tomoda K, Fukuda H, Nakamura H (1996) CT and MR imaging of abdominal liposarcoma. AJR Am J Roentgenol 166 (4):829-833. https://doi.org/10.2214/ajr.166.4.8610559
Hong SH, Kim KA, Woo OH, Park CM, Kim CH, Kim MJ, Chung JJ, Han JK, Rha SE (2010) Dedifferentiated liposarcoma of retroperitoneum: spectrum of imaging findings in 15 patients. Clin Imaging 34 (3):203-210. https://doi.org/10.1016/j.clinimag.2009.12.025
Cooley CL, Jagannathan JP, Kurra V, Tirumani SH, Saboo SS, Ramaiya NH, Shinagare AB (2014) Imaging features and metastatic pattern of non-IVC retroperitoneal leiomyosarcomas: are they different from IVC leiomyosarcomas? J Comput Assist Tomogr 38 (5):687-692. https://doi.org/10.1097/RCT.0000000000000097
Abraham JA, Weaver MJ, Hornick JL, Zurakowski D, Ready JE (2012) Outcomes and prognostic factors for a consecutive case series of 115 patients with somatic leiomyosarcoma. J Bone Joint Surg Am 94 (8):736-744. https://doi.org/10.2106/JBJS.K.00460
Ganeshalingam S, Rajeswaran G, Jones RL, Thway K, Moskovic E (2011) Leiomyosarcomas of the inferior vena cava: diagnostic features on cross-sectional imaging. Clin Radiol 66 (1):50-56. https://doi.org/10.1016/j.crad.2010.08.004
O’Sullivan PJ, Harris AC, Munk PL (2008) Radiological imaging features of non-uterine leiomyosarcoma. Br J Radiol 81 (961):73-81. https://doi.org/10.1259/bjr/18595145
Yakupoglu A, Ulus S, Cantasdemir M (2016) Leiomyosarcoma of the Inferior Vena Cava Confirmed by Aspiration Biopsy With a Catheter During Digital Subtraction Angiography. Vasc Endovascular Surg 50 (3):164-167. https://doi.org/10.1177/1538574416637445
Huang J, Liu Q, Lu JP, Wang F, Wang L, Jin AG (2011) Primary intraluminal leiomyosarcoma of the inferior vena cava: value of MRI with contrast-enhanced MR venography in diagnosis and treatment. Abdom Imaging 36 (3):337-341. https://doi.org/10.1007/s00261-010-9656-y
Brennan MF, Antonescu CR, Alektiar KM, Maki RG (2016) Undifferentiated Pleomorphic Sarcoma (UPS) (Malignant Fibrous Histiocytoma (MFH) and Myxofibrosarcoma). In: Management of Soft Tissue Sarcoma. Springer International Publishing, Cham, pp 143-152. https://doi.org/10.1007/978-3-319-41906-0_7
Cong Z, Gong J (2011) Primary malignant fibrous histiocytoma of the liver: CT findings in five histopathological proven patients. Abdom Imaging 36 (5):552-556. https://doi.org/10.1007/s00261-011-9691-3
Osman S, Lehnert BE, Elojeimy S, Cruite I, Mannelli L, Bhargava P, Moshiri M (2013) A comprehensive review of the retroperitoneal anatomy, neoplasms, and pattern of disease spread. Curr Probl Diagn Radiol 42 (5):191-208. https://doi.org/10.1067/j.cpradiol.2013.02.001
Kim KH, Lee SH, Cha SH, Kim YS, Sung DJ (2012) Malignant fibrous histiocytoma arising from a hydronephrotic kidney: a case report and review of the literature. Clin Imaging 36 (3):239-242. https://doi.org/10.1016/j.clinimag.2011.09.001
Lee SY, Jee WH, Jung JY, Park MY, Kim SK, Jung CK, Chung YG (2016) Differentiation of malignant from benign soft tissue tumours: use of additive qualitative and quantitative diffusion-weighted MR imaging to standard MR imaging at 3.0 T. Eur Radiol 26 (3):743-754. https://doi.org/10.1007/s00330-015-3878-x
Gaballah AH, Jensen CT, Palmquist S, Pickhardt PJ, Duran A, Broering G, Elsayes KM (2017) Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol 90 (1075):20170039. https://doi.org/10.1259/bjr.20170039
Sung CK, Kim B, Moon KC, Ku JH, Ha SB (2017) Retroperitoneal Tumors. In: Kim SH, Cho JY (eds) Oncologic Imaging: Urology. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 227-260. https://doi.org/10.1007/978-3-662-45218-9_6
Miao C, Luo C-H (2018) Retroperitoneal Rhabdomyosarcoma. In: Luo C-H (ed) Retroperitoneal Tumors. Springer Netherlands, Dordrecht, pp 183-184. https://doi.org/10.1007/978-94-024-1167-6_17
Miao C, Luo C-H (2018) Retroperitoneal Synovial Sarcoma. In: Luo C-H (ed) Retroperitoneal Tumors. Springer Netherlands, Dordrecht, pp 259-261. https://doi.org/10.1007/978-94-024-1167-6_33
Thway K, Fisher C (2015) PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol 19 (5):359-368. https://doi.org/10.1016/j.anndiagpath.2015.06.003
Crino PB, Nathanson KL, Henske EP (2006) The tuberous sclerosis complex. N Engl J Med 355 (13):1345-1356. https://doi.org/10.1056/NEJMra055323
Phillips CH, Keraliya AR, Shinagare AB, Ramaiya NH, Tirumani SH (2016) Update on the imaging of malignant perivascular epithelioid cell tumors (PEComas). Abdom Radiol (NY) 41 (2):368-376. https://doi.org/10.1007/s00261-015-0568-8
Liang W, Xu C, Chen F (2015) Primary retroperitoneal perivascular epithelioid cell neoplasm: A case report. Oncol Lett 10 (1):469-472. https://doi.org/10.3892/ol.2015.3210
Hedgire SS, Kudrimoti S, Oliveira IS, Nadkarni N, McDermott S, Hahn PF, Mino-Kenudson M, Harisinghani MG (2017) Extranodal lymphomas of abdomen and pelvis: imaging findings and differential diagnosis. Abdom Radiol (NY) 42 (4):1096-1112. https://doi.org/10.1007/s00261-016-0964-8
Rosenkrantz AB, Spieler B, Seuss CR, Stifelman MD, Kim S (2012) Utility of MRI features for differentiation of retroperitoneal fibrosis and lymphoma. AJR Am J Roentgenol 199 (1):118-126. https://doi.org/10.2214/AJR.11.7822
Neville A, Herts BR (2004) CT characteristics of primary retroperitoneal neoplasms. Crit Rev Comput Tomogr 45 (4):247-270
Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG, Lee JM (2003) Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics 23 (1):29-43. https://doi.org/10.1148/rg.231025050
MJ, Thomas JM, Fisher C, Moskovic EC (2005) Imaging features of retroperitoneal and pelvic schwannomas. Clin Radiol 60 (8):886-893. https://doi.org/10.1016/j.crad.2005.01.016
Erickson D, Kudva YC, Ebersold MJ, Thompson GB, Grant CS, van Heerden JA, Young WF, Jr. (2001) Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab 86 (11):5210-5216. https://doi.org/10.1210/jcem.86.11.8034
Lonergan GJ, Schwab CM, Suarez ES, Carlson CL (2002) Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. Radiographics 22 (4):911-934. https://doi.org/10.1148/radiographics.22.4.g02jl15911
Choyke PL, Hayes WS, Sesterhenn IA (1993) Primary extragonadal germ cell tumors of the retroperitoneum: differentiation of primary and secondary tumors. Radiographics 13 (6):1365-1375; quiz 1377-1368. https://doi.org/10.1148/radiographics.13.6.8290730
Keitoku M, Konishi I, Nanbu K, Yamamoto S, Mandai M, Kataoka N, Oishi T, Mori T (1997) Extraovarian sex cord-stromal tumor: case report and review of the literature. Int J Gynecol Pathol 16 (2):180-185. https://doi.org/10.1097/00004347-199704000-00017
Trabelsi A, Ben Abdelkarim S, Hadfi M, Fatnaci R, Stita W, Sriha B, Korbi S (2008) Primary mesenteric Sertoli–Leydig cell tumor: a case report and review of the literature. J Oncol 2008:619637. https://doi.org/10.1155/2008/619637
Ueno T, Tanaka YO, Nagata M, Tsunoda H, Anno I, Ishikawa S, Kawai K, Itai Y (2004) Spectrum of germ cell tumors: from head to toe. Radiographics 24 (2):387-404. https://doi.org/10.1148/rg.242035082
Gatcombe HG, Assikis V, Kooby D, Johnstone PA (2004) Primary retroperitoneal teratomas: a review of the literature. J Surg Oncol 86 (2):107-113. https://doi.org/10.1002/jso.20043
Davidson AJ, Hartman DS, Goldman SM (1989) Mature teratoma of the retroperitoneum: radiologic, pathologic, and clinical correlation. Radiology 172 (2):421-425. https://doi.org/10.1148/radiology.172.2.2664866
Mathur P, Lopez-Viego MA, Howell M (2010) Giant primary retroperitoneal teratoma in an adult: a case report. Case Rep Med 2010. https://doi.org/10.1155/2010/650424
Surabhi VR, Menias C, Prasad SR, Patel AH, Nagar A, Dalrymple NC (2008) Neoplastic and non-neoplastic proliferative disorders of the perirenal space: cross-sectional imaging findings. Radiographics 28 (4):1005-1017. https://doi.org/10.1148/rg.28407515
Mesurolle B, Sayag E, Meingan P, Lasser P, Duvillard P, Vanel D (1996) Retroperitoneal extramedullary hematopoiesis: sonographic, CT, and MR imaging appearance. AJR Am J Roentgenol 167 (5):1139-1140. https://doi.org/10.2214/ajr.167.5.8911166
Georgiades CS, Neyman EG, Francis IR, Sneider MB, Fishman EK (2002) Typical and atypical presentations of extramedullary hemopoiesis. AJR Am J Roentgenol 179 (5):1239-1243. https://doi.org/10.2214/ajr.179.5.1791239
Orphanidou-Vlachou E, Tziakouri-Shiakalli C, Georgiades CS (2014) Extramedullary hemopoiesis. Semin Ultrasound CT MR 35 (3):255-262. https://doi.org/10.1053/j.sult.2013.12.001
Zhou LP, Zhang B, Peng WJ, Yang WT, Guan YB, Zhou KR (2008) Imaging findings of Castleman disease of the abdomen and pelvis. Abdom Imaging 33 (4):482-488. https://doi.org/10.1007/s00261-007-9282-5
Cohn JE, Zhou J, Hu A (2018) Castleman disease. Ear Nose Throat J 97 (8):233-234. https://doi.org/10.1177/014556131809700819
Meador TL, McLarney JK (2000) CT features of Castleman disease of the abdomen and pelvis. AJR Am J Roentgenol 175 (1):115-118. https://doi.org/10.2214/ajr.175.1.1750115
Irsutti M, Paul JL, Selves J, Railhac JJ (1999) Castleman disease: CT and MR imaging features of a retroperitoneal location in association with paraneoplastic pemphigus. Eur Radiol 9 (6):1219-1221. https://doi.org/10.1007/s003300050821
Czeyda-Pommersheim F, Hwang M, Chen SS, Strollo D, Fuhrman C, Bhalla S (2015) Amyloidosis: Modern Cross-sectional Imaging. Radiographics 35 (5):1381-1392. https://doi.org/10.1148/rg.2015140179
Kawashima A, Alleman WG, Takahashi N, Kim B, King BF, Jr., LeRoy AJ (2011) Imaging evaluation of amyloidosis of the urinary tract and retroperitoneum. Radiographics 31 (6):1569-1582. https://doi.org/10.1148/rg.316115519
Acknowledgements
The authors thank Henry Douglas for his help with images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
CME activity This article has been selected as the CME activity for the current month. Please visit https://ce.mayo.edu/node/103122 and follow the instructions to complete this CME activity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1: Follicular Lymphoma in a 38-year-old female. Coronal reconstruction of a contrast enhanced abdomen CT cine clip show a large confluent nodal mass (arrows) in the retroperitoneum and the mesenteric root. Note the relatively homogenous (for the size of the nodal mass) contrast enhancement and contiguous but discrete enlarged lymph nodes in the mesentery. The retroperitoneal mass splays and narrows but doesn’t obstruct the right main renal artery (RMRA) and the left main renal artery (LMRA). Supplementary Material 1 (AVI 409318 kb)
Video 2 a,b: Paraganglioma in a 54-year-old male with retroperitoneal mass. A. Axial contrast enhanced CTcine clip shows a heterogeneous but predominantly markedly enhancing mass (M) in the retroperitoneum adjacent to the left kidney and adrenal gland. B. F-18 FDG Body PET-CT fusion cine clip obtained in axial plane demonstrates a very intense uptake in the locally invasive left retroperitoneal mass (SUVmax 26.3, image 145), consistent with malignancy. Supplementary Material 2 (AVI 174740 kb)
Video 3: Germ Cell tumor with retroperitoneal metastases in a 52-year-old male. Coronal T2W MRI cine clip of the abdomen and pelvis shows a large, confluent heterogeneous hyperdense to the skeletal muscle nodal mass (arrows) causing significant displacement of the left kidney (LK) and narrowing of the retroperitoneal vasculature most pronounced of the left renal vein. Note large well-circumscribed primary germ cell tumor in the scrotum. Multiple simple renal cysts are also present. Supplementary Material 3 (AVI 131472 kb)
Supplementary Material 4 (AVI 278170 kb)
Video 4 a, b: Presacral Epidermoid in a 29-year-old female with complex pelvic cyst. A. Sagittal T2 TSE cine clip shows a well-circumscribed lesion (M) with a T2 hypointense wall and T2 hyperintense internal contents in the presacral tissues. The contents are markedly T2 hyperintense due to gross fat. B. Axial T1W FS post-contrast image of the pelvis shows a cystic lesion (M) with a thin, enhancing wall and no internal enhancing components in the presacral tissues. Note that on the fat saturated image the cyst contents show complete signal loss due to gross fat content. Supplementary material 5 (AVI 197048 kb)
Supplementary Material 6 (AVI 371462 kb)
Video 5: Retroperitoneal fibrosis in a middle-aged woman. Contrast-enhanced T1 weighed fat suppressed axial image through the abdomen and pelvis shows enhancing soft tissue encircling the aorta (yellow arrows). Although less noticeable the ureters also were involved resulting in enhancing tissue thickening of the ureteral wall (green arrows) and resulting in ureteral obstruction for which bilateral nephroureteral stents (seen as signal void within the ureter lumen) were placed. The soft tissue tethers the right ovary (red arrow) which is anteriorly displaced. Supplementary material 7 (AVI 116773 kb)
Rights and permissions
About this article
Cite this article
Czeyda-Pommersheim, F., Menias, C., Boustani, A. et al. Diagnostic approach to primary retroperitoneal pathologies: what the radiologist needs to know. Abdom Radiol 46, 1062–1081 (2021). https://doi.org/10.1007/s00261-020-02752-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02752-8