Abstract
Purpose
To evaluate the utility of Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) in assessing treatment response in patients of intestinal tuberculosis (ITB).
Method and materials
MR Enterography (MRE) was done for patients with suspicion of ITB and 19 patients with pre- and post-treatment imaging were included in the analysis. MRE included T1W, T2W, post-contrast T1W, and DWI sequences. DWI was done using b values—0, 400 and 800 s/mm2, and ADC maps were generated. The trace DW images and ADC values were compared before and after therapy. Composite gold standard (clinical, colonoscopic criteria, and biopsy) was used to assess treatment response and to classify into no response, partial response, and complete response.
Results
Thirty-one bowel segments were evaluated at baseline and after treatment in 19 patients. Prior to therapy, restricted diffusion was seen in 29/31 (93.5%) segments. After treatment, patients with either complete or partial response (27/31 segments, 15 patients) showed significant rise in mean ADC values from 1.1 ± 0.37 × 10−3 to 2.1 ± 0.64 × 10−3 mm2/s (p value < 0.05), whereas no significant change was found in mean ADC values of non-responders (4/29 segments in 4 patients) which increased from 1.0 ± 0.1 × 10−3 mm2/s on baseline scan to 1.32 ± 0.2 × 10−3 mm2/s on post-treatment scan (p value = 0.318). An increase in ADC value was found to be a reliable and objective marker of improvement with response to therapy.
Conclusion
ADC values show good correlation with treatment response in ITB and can be used for objectively quantifying it.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is a multisystem chronic granulomatous disorder with both pulmonary and extra-pulmonary manifestations. Abdominal TB is one of the common extra pulmonary manifestations which may involve gastrointestinal tract, peritoneum, lymph nodes, and solid viscera. Intestinal TB (ITB) is one of the sites of involvement in abdominal TB. With the increasing burden of inflammatory bowel disease in India and other developing countries, the differential diagnosis of ulcero-constrictive intestinal disease has expanded from just ITB to include Crohn’s disease (CD) as well. Differentiating the two disorders has remained a challenge, and the dilemma is often resolved by a therapeutic anti-tubercular therapy (ATT) trial. Clinical and endoscopic response to ATT trial confirms the diagnosis of ITB [1,2,3]. Symptomatic improvement is no longer considered as a reliable measure of response, and mucosal healing needs to be documented. However, repeating the colonoscopy after ATT is often unacceptable to patients because of its invasive nature. The presence of intestinal strictures precludes the examination of the proximal intestine, and the small bowel disease that cannot be evaluated by ileocolonoscopy. Moreover, other endoscopic modalities like enteroscopy are often not available and are invasive, and capsule endoscopy cannot be done in presence of strictures. Therefore, cross-sectional imaging, which has the advantage of imaging the entire intestine non-invasively, can be used to document response to ATT. The imaging modalities used for the evaluation of ulcero-constrictive diseases of the bowel include barium examination, ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI). ITB requires a prolonged course of treatment and frequent monitoring, and its therapeutic endpoint is governed by complete response and mucosal healing on endoscopy and/or cross-sectional imaging.
MR Enterography (MRE) is a technique which is optimized for evaluation of bowel pathology and with the advent of novel imaging techniques like diffusion-weighted imaging (DWI); it is increasingly being used for assessment of disease activity and resolution of inflammation, and has been studied extensively in patients with CD [4, 5]. The literature on role of MRE in ITB is limited to a small study which evaluated the MRE findings of small bowel tuberculosis; however, it did not utilize DWI, and imaging was not repeated at follow-up [6]. The present study was carried out to assess the utility of DWI—qualitative as well as quantitative evaluation using apparent diffusion coefficient (ADC)—in distinguishing the inflamed from normal bowel segments in ITB and verifying if it can be used to assess response to treatment.
Subjects and methods
The data presented in this paper represent part of a larger study that was undertaken from January 2015 to December 2016, in which treatment-naïve patients with clinical suspicion of intestinal ulcero-constrictive lesions were prospectively recruited after taking informed consent. In this ethically approved study, all patients underwent MRE, ileocolonoscopy, biopsy, and histopathological examination. They were then divided into different categories based on a composite gold standard comprising clinical features, colonoscopy, and biopsy results, and response to ATT at follow-up. The final diagnoses were—ITB, CD, non-specific colitis, NSAID-induced enteropathy, and post-operative adhesions. Out of the 50 patients enrolled for the study, 28 were diagnosed with ITB, 18 with CD, and four patients had other alternate diagnoses. Patients with initial diagnosis other than ITB and without follow-up imaging were excluded from the study.
Finally, 19 patients were included in the analysis out of the total 50 patients, who had both pre- and post-treatment imaging as depicted in the Fig. 1. Of these, 12 patients were diagnosed as ITB upfront (as per Paustian’s criteria), and seven patients with the suspicion of ITB were started on empirical ATT. Among the seven patients, four were confirmed to have ITB, based upon clinical and endoscopic/radiological response to ATT (Logan’s modification of Paustian’s criteria). The remaining three patients showed no improvement in symptoms and based on the evolution of the disease, their diagnosis was revised and they were found to have CD.
Patients were assessed for clinical improvement, and the objective response to treatment was assessed on ileocolonoscopy and imaging. On completion of the treatment, a repeat MRE was done to assess the disease activity. In most cases, it was done at the end of 6 months; however, in cases with clinical and endoscopic features suggestive of persistent disease, therapy was prolonged. The duration of therapy and hence, the time interval between the baseline MRE and the follow-up, ranged between 6 and 9 months. Follow-up imaging helped in deciding the further course of management, and the treatment was modified based on the imaging, clinical, and endoscopic findings.
MR enterography
MRE was performed before start of therapy and after completion of therapy. Patients were advised to fast for at least 6 h prior to the examination, and a total of 1500 mL of diluted Mannitol (300 ml of 20% Mannitol diluted with 1200 ml of water) was given orally in divided aliquots over the course of 60 min, followed by 250 ml of plain water just before scanning to achieve adequate bowel distension. To reduce the artifacts due to peristaltic activity, 20 mg hyoscine butyl bromide (Inj. Buscopan®) was given intravenously before the acquisition of T1W contrast sequence. For contrast study, 0.1–0.2 ml/kg of Gadobenate dimeglumine was given intravenously followed by saline flush.
MRE was done on 3 Tesla scanner (Ingenia Philips, Netherlands) using 32 channel torso coil with the patient in supine position. The sequences performed are as depicted in Table 1:
Image interpretation
MRE images were interpreted by two experienced radiologists having at least 10 years’ experience in MR imaging who were blinded to the colonoscopic findings.
Conventional (T1W, T2W, and post-contrast T1W fat-suppressed) sequences were used to identify the mesenteric and bowel inflammation. On conventional sequences, imaging parameters like site of involvement (small or large intestine), length of involved segment (focal, segmental, diffuse), bowel wall thickness (3 mm or more), T2W signal intensity of the involved segment (hypointense, isointense or hyperintense), contrast-enhancement characteristic (homogeneous or stratified), surrounding mesenteric changes (vascular proliferation or fibrofatty proliferation), and mesenteric lymphadenopathy (homogeneous or necrotic) were assessed. The corresponding segments were then analyzed on trace DW images (as detailed below).
Based on the findings of ileocolonoscopy, MRE,and symptomatic relief following treatment, the patients were divided into two groups—responders (complete as well as partial) and non-responders. Responders included those who showed either partial or complete improvement after treatment. Non-responders showed no response or insignificant interval change even after 6–9 months of therapy.
DWI Analysis
Qualitative and quantitative analysis of DW images was done.
DW imaging was done using three b values (0, 400 and 800 s/mm2), and apparent diffusion coefficient (ADC) values were calculated. ADC values were obtained by drawing circular ROI on the b 800 image which was automatically transferred to the ADC map generated by vendor provided software. T2W and post-contrast images were used to ensure correct placement of ROI. Restriction of diffusion was seen as increased signal on the trace images (DW image) and decreased signal on the corresponding ADC map.
For qualitative analysis, small and large bowel were divided into different segments—duodenum, jejunum, ileum (proximal, mid and distal), terminal ileum, and ileocecal junction, cecum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum. The signal intensities of these segments were evaluated on trace images, and a qualitative 4-point scale devised by Oto et al. was used to grade them depending upon their signal intensities on the highest b value (800 s/mm2) image (Grade 0—normal, Grade 1—probably normal, Grade 2—probably abnormal, Grade 3—abnormal) [7].
In a similar manner, for the quantitative analysis, ADC values were calculated for both pre and post-treatment studies and compared in order to look for resolution of inflammatory activity. The thickened segments were identified on the T2W and post-contrast images, which showed hyperintensity on trace DW images (b value—800 s/mm2), and circular ROIs were placed on the thickened bowel wall. The ROI was placed such that it included only the inflamed bowel wall, while meticulously taking care to avoid the periphery and hence the contamination due to volume averaging. These ROIs were then copied on to the ADC maps generated by the software, and then ADC values were measured. In the case of normal bowel wall, ROIs were placed on the bowel wall. These were first drawn on T2W images and later copied to trace DWI and ADC images. The images were also zoomed on the work-station for the purpose of proper placement of ROI. The mean diameter of the ROI was 3 mm (range 2–4 mm, corresponding area ranged from 3.5 to 12.5 mm2). Resolution of inflammation is reflected by increased ADC value and vice versa.
For both qualitative and quantitative analyses, signal intensities of the normal bowel loops on b 800 s/mm2 image and ADC values were taken as internal reference.
Definitions
Diagnosis of intestinal TB
The diagnosis of ITB was made in an appropriate clinical setting with the demonstration of necrotizing granulomas on histopathology or demonstration of acid-fast bacilli (AFB) on histopathology or culture of intestinal tissue (Paustian’s criteria) [8]. In patients who did not fulfill the above definitions, therapeutic trial with ATT was given. Diagnosis of ITB was made if patient had clinical and endoscopic/radiological response to ATT (Logan’s modification). These defining criteria for diagnosis of ITB have been validated earlier in another study at our center [9].
Treatment and follow-up
Anti-tubercular therapy (ATT): Induction regimen of isoniazid 5 mg/kg, rifampicin 10 mg/kg, pyrazinamide 20–25 mg/kg, and ethambutol 15–20 mg/kg for 2 months followed by maintenance therapy of isoniazid and rifampicin for duration of 4–7 months was given. The total treatment duration was 6–9 months depending upon the response to ATT. Patients were routinely followed up at 2 monthly intervals and as and when required to assess symptom response and also to monitor drug toxicity by means of assessment of liver function tests.
Post-treatment evaluation
Complete response was defined as complete resolution of the inflammation on MRE as well as on ileocolonoscopy with symptomatic improvement clinically. Partial response was defined as reduction in the extent as well as severity of the inflammation both on MRE and ileocolonoscopy with either complete or partial improvement in the clinical symptoms.
Patients with no change in the disease activity on MRE as well as on ileocolonoscopy at the end of 6–9 months of treatment were regarded as non-responders. They were again thoroughly evaluated with the revision of initial diagnosis, and the treatment was modified in such cases.
Statistical analysis
Data were analyzed using the IBM SPSS software (version 24.0). The ADC values were calculated for the inflamed bowel segments on pre-treatment imaging, and these index segments were then evaluated on post-treatment imaging for comparison. On the basis of treatment response, patients were divided into three categories—complete, partial, and non-responders.
The mean ADC value was calculated for each category separately, and paired t test was used to identify change in mean ADC values on pre- and post-treatment imaging. A ‘p’ value < 0.05 was considered as statistically significant.
Results
Subjects
The age of the study population ranged between 13 and 64 years with a median age of 35 years (mean—35.5 years), and there were 11 women and 8 men. Most of the patients presented in the 3rd decade of life.
The major presenting complaints of the patients were abdominal pain (100%), altered bowel habits like diarrhea (57.8%) and constipation (26.3%), loss of weight (63.2%) and appetite (42.1%), subacute intestinal obstruction (31.5%), fever (26.3%), vomiting (26.3%), and other constitutional symptoms (Fig. 2).
Pre-treatment MRE
A total of 31 diseased segments were identified in 19 patients with different sites of involvement. Terminal ileum (45.2%) and ileocecal junction (45.2%) were the most commonly involved sites (Fig. 3) followed by cecum (25.8%), ascending colon (22.5%), transverse colon (19.3%), and distal ileum (19.3%). Splenic flexure, descending colon, and rectosigmoid involvement were not seen in any of the patients.
Most of the patients (15/19 patients) showed focal involvement with length of involved segment equal to or less than 5 cm (24/31). Few of them showed more than one site of bowel inflammation with varying lengths of involved segments at different sites. Such skip lesions were seen in 9/19 patients.
The inflamed segments were hyperintense on T2W images and showed intense contrast enhancement. The enhancement pattern was predominantly homogeneous, and only four segments showed stratified enhancement pattern.
Necrotic mesenteric lymph nodes were seen in 9 patients, and one of the patients had vertebral osteitis at multiple levels in the dorsolumbar spine.
DWI
Qualitative evaluation
On evaluation, 29 out of 31 diseased segments identified on conventional imaging were found to be hyperintense on DW images and showed dark signal on ADC map. Their numerical ADC values were then calculated. Two of the 31 diseased segments identified on conventional sequences did not show diffusion restriction (Fig. 4).
DWI—active inflammation in ITB—38-year-old man with ITB. MRE axial T2W image a showing mural thickening with hyperintense signal of ileocecal junction (arrow). Post-contrast T1W image b showing wall thickening with homogeneous enhancement (arrow) and enlarged draining lymph nodes. DW image at b 800 s/mm2, c shows hyperintense signal of the involved segment (arrow) and corresponding ADC map d showing dark signal (ADC 0.935 × 10−3 mm2/s) suggesting restricted diffusion (arrow)
At the baseline on qualitative analysis, 93.5% segments showed high signal intensity on b 800 s/mm2, of which 74.1% segments (n = 23) had Grade 3 and 19.35% segments (n = 6) had Grade 2 signal intensity. However, two diseased segments (n = 2,6.5%), one in the jejunum and other in the transverse colon, which were abnormal on conventional sequences including T2W and post-contrast T1W fat suppressed sequence, showed normal signal on b 800 s/mm2 image (Grade 1). Few segments (n = 10) which were normal on conventional MR sequences and endoscopy showed hyperintensity on high b value images (Grade 2). Most of these segments were identified in the jejunum (at least five) where the bowel loops were collapsed and not adequately distended with the intraluminal oral contrast agent. Similar segments were also seen in the large bowel particularly in the rectosigmoid region (three segments). Few of the ileal loops (two segments) also showed falsely high signal on trace DW images with no dark signal on corresponding ADC map and they appeared normal on conventional MR sequences.
Quantitative Evaluation
ADC values were calculated for the inflamed segments which were hyperintense on trace images (n = 29). The mean ADC value of actively inflamed bowel segments (n = 31 segments) was 1.122 ± 0.346 × 10−3 mm2/s. Mean ADC value of normal bowel loops was found to be much higher i.e. 2.61 ± 0.096 × 10−3 mm2/s and this difference was found to be statistically significant (p < 0.001) (Table 2).
Post-treatment MRE
Eight (13 segments) of the 19 patients showed complete resolution on MRE with resolution of diseased segments as well as necrotic and enlarged mesenteric lymph nodes. There was resolution of hyperintense signal on T2W images as well as of the abnormal contrast enhancement seen on the pre–treatment MRE images. Ileocolonoscopy showed complete mucosal healing and on clinical assessment patients reported remarkable improvement in symptoms with significant gain in weight and appetite. On DW images, there was no hyperintensity on b 800 s/mm2 images, and ADC map showed no dark signal. The pre-treatment mean ADC value of the inflamed bowel segments in complete responders was 1.015 ± 0.359 × 10−3 mm2/s, with significant rise in the ADC value on post-treatment imaging to 2.45 ± 0.59 × 10−3 mm2/s. The ∆ ADC in complete responders was 1.44 ± 0.65 × 10−3 mm2/s and the difference was found to be statistically significant (p < 0.005) (Fig. 5).
Complete responder—18-year-old lady with ITB showing complete response on follow-up imaging. Pre-treatment DW image at b 800 s/mm2a shows hyperintensity of ileocecal junction (arrow) with dark signal on corresponding ADC map b having ADC 0.9 × 10−3 mm2/s, suggesting restricted diffusion (arrow). Post-treatment axial DW image c shows complete resolution in the absence of hyperintensity on DW image (arrow) at b 800 s/mm2 and the absence of dark signal (arrow) on corresponding ADC map d having ADC value 1.68 × 10−3 mm2/s
Seven patients (with 14 diseased segments) showed partial resolution with decrease in the disease severity and number of inflamed segments on post–treatment imaging. Three (3/7 patients) of these patients showed complete mucosal healing on ileocolonoscopy and marked improvement in the symptoms. However, on conventional MRE, there was some residual activity with persistence of T2W hyperintense signal and mild mural thickening, along with abnormal homogeneous contrast enhancement on post-contrast T1W imaging. On DW imaging there was persistence of hyperintensity on trace images and dark signal on ADC map. Nevertheless, the quantitative evaluation revealed a significant rise in the ADC value as compared to the pre-treatment imaging examination. The treatment was stopped in these patients and they were kept on close follow-up.
Four patients (4/7 patients) showed partial improvement with evidence of residual disease both on MRE and ileocolonoscopy. These patients were given ATT for prolonged period of time (9 months). One of these patients developed fibrotic stricture at the ileocecal junction and another had a stricture at the transverse colon. For fibrotic stricture, serial endoscopic dilatation was done as it resulted in obstructive symptoms and abdominal pain (Fig. 6).
Partial responder—51-year-old man with ITB showed response on follow-up imaging. Pre-treatment DW image at b 800 s/mm2a shows hyperintensity in hepatic flexure with dark signal on corresponding ADC map b having ADC 0.8 × 10−3 mm2/s suggesting restricted diffusion (arrow). Post-treatment axial DW image c shows subtle hyperintensity of hepatic flexure and corresponding ADC map d shows significant rise in ADC value (ADC 1.44 × 10−3 mm2/s)
Patients with partial response showed decrease in the mural thickening, T2W hyperintensity of the involved segments as well as the abnormal contrast enhancement. They showed improvement on DW imaging with rise in ADC value.
The mean ADC value on pre-treatment imaging in partial responders was 1.216 ± 0.364 × 10−3 mm2/s and on post-treatment imaging was found to be 1.77 ± 0.50 × 10−3 mm2/s. The difference in the mean of these values (∆ADC mean) was 0.55 ± 0.54 × 10−3 mm2/s and it was found to be statistically significant (p < 0.05).
Patients who did not respond to treatment and showed only minor improvement in symptoms with no change either on ileocolonoscopy as well as MR imaging findings were thoroughly evaluated.
Four of the 19 patients (with four inflamed segments) included in the study were non-responders. In these patients, the diagnosis was revised at the end of the treatment. One of them showed increase in the extent of conglomerate necrotic mesenteric lymphadenopathy on follow-up imaging, hence modified ATT regime was started for the patient after testing for antibiotic sensitivity. There was inflammation of terminal ileum and ileocecal junction on pre-treatment imaging which was persistent even on follow-up imaging (Fig. 7).
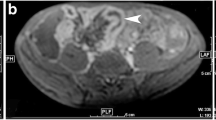
Non-responder—19-year-old lady with suspected ITB showed no response on follow-up imaging. Pre-treatment DW image at b 800 s/mm2a and corresponding ADC map b showed restricted diffusion in the distal ileum (ADC 1.2 × 10−3 mm2/s) (arrow). Post-treatment axial DW image c and ADC map d showed similar findings with persistent diffusion restriction (ADC 1.3 × 10−3 mm2/s. Final diagnosis: CD
However, three other patients showed long segment contiguous inflammation of the bowel with no evidence of necrotic lymph nodes or mesenteric lymphadenopathy. Since there were no skip lesions and there was involvement of terminal ileum and ileocecal junction in all these patients, they were thought to have ITB initially. But with no improvement in symptoms on ATT, their diagnosis was revised. These patients were started on steroids and aminosalicylates with the suspicion of inflammatory bowel disease, following which they showed dramatic improvement in symptoms. As CD is a close mimicker of ITB, and at times, due to overlap in the symptoms, imaging findings as well as non-specific colonoscopy and biopsy results, it becomes difficult to differentiate among these two conditions.
The mean ADC value on pre-treatment imaging in non-responders was 1.06 ± 0.14 × 10−3 mm2/s, and on post-treatment imaging, it was found to be 1.32 ± 0.21 × 10−3 mm2/s. The difference in the mean of these values (∆ADC mean) was 0.18 ± 0.30 × 10−3 mm2/s; although there was rise in the ADC value, it was not statistically significant (p = 0.318).
The responders (complete and partial) on the other hand showed significant resolution of bowel inflammation, on pre-treatment imaging their mean ADC value was 1.119 ± 0.37 × 10−3 mm2/s and on post-treatment imaging it was found to be 2.1 ± 0.64 × 10−3 mm2/s. The difference in the mean of these values was 0.98 ± 0.75 × 10−3 mm2/s and it was statistically significant (p < 0.05).
Patients showed multiple sites of bowel inflammation and each segment was dealt separately as not all showed consistent improvement or similar response to treatment. In patients (seven) who were partial responders and found to have residual disease activity, a few of the inflamed segments showed complete response (6/14 segments) to treatment, whereas a few others (8/14 segments) showed persistent disease activity. In complete responders, however, all segments (13 segments) showed response with no residual inflammation on follow-up.
The mean ADC value on pre-treatment imaging of inflamed segments (19/31) which later showed complete response to treatment was 1.11 ± 0.4 × 10−3 mm2/s, and their mean ADC value on post-treatment imaging was found to be 2.3 ± 0.54 × 10−3 mm2/s. The difference in the means of these values (∆ADC mean) was 1.27 ± 0.7 × 10−3 mm2/s, and it was found to be statistically significant (p < 0.05).
The mean ADC value on pre-treatment imaging of inflamed bowel segments (8/31) with partial response to treatment was 1.13 ± 0.26 × 10−3 mm2/s, and on post-treatment imaging, it was found to be 1.43 ± 0.24 × 10−3 mm2/s. The difference in the means of these values (∆ADCmean) was 0.30 ± 0.32 × 10−3 mm2/s, and it was found to be statistically significant (p = 0.03).
As there were no skip lesions or multiple sites of involvement in non-responders, the ADC parameters were same for patientwise as well as segmentwise distribution of disease as depicted in Table 3.
Discussion
This study highlights the importance of DWI in assessment of inflammation and disease severity in patients with ulcero-constrictive disease. DWI is a novel MR imaging technique based on the diffusivity of water molecules (Brownian motion) in the tissues. Tissue cellularity and presence of intact cell membranes determine the impedance of diffusion of water molecules. In normal tissues, the water molecules diffuse freely as a result of large extracellular space. However, in the presence of inflammation, there are increased tissue cellularity and cell density which result in restricted diffusion, manifesting as high signal on high b-value diffusion-weighted trace images and corresponding low signal on the ADC map and low ADC values. In recent times, DWI has been increasingly used in bowel imaging [10]. With the advent of parallel imaging and fast respiratory triggered sequences, it has become possible to reduce the motion- and breathing-related artifacts which earlier precluded its use for abdominal imaging.
This study demonstrates the relation between active inflammation and restricted diffusion, further reinforced objectively by lower ADC values in inflamed bowel segments. All the inflamed segments were analyzed separately, and treatment response was evaluated. The findings were confirmed by comparing them with the ileocolonoscopy and histopathology. To the best of our knowledge, there is no study in the literature where pre and post-treatment changes in intestinal tuberculosis were compared with emphasis on the DWI sequence. However, many studies in the western literature have assessed disease activity in Crohn’s disease using MRE with DWI and further compared them with the endoscopic findings. They found high degree of correlation between endoscopic findings and DWI results. For assessment of disease activity in CD, DWI has been accepted as one of the diagnostic techniques of choice, and multiple scoring systems have been devised [5, 11].
We used a four-point grading scale devised by Oto et al. in our study, for qualitative evaluation and assessment of inflammation of the bowel loops using diffusion restriction [7]. Out of 31 inflamed bowel segments, 29 segments (93.5%) were correctly identified by DWI on pre-treatment evaluation. The quantitative evaluation showed statistically significant difference in the mean ADC values of inflamed segments (1.122 ± 0.346 × 10−3 mm2/s) when compared to that of the normal segments (2.61 ± 0.096 × 10−3 mm2/s). These results were in concordance with the study performed by Oto et al. wherein they evaluated the role of DWI in CD patients by performing MRE along with DWI in eleven patients of CD. They used surgery and colonoscopy as the reference standard for evaluating disease extent and for confirming the diagnosis. The authors identified 53 segments in 11 patients, out of which 19 were inflamed and 34 were normal. They used a qualitative four-point grading score for identifying diffusion restriction, 18 of the 19 inflamed segments and 28 of the 34 normal segments were correctly identified using DWI. The mean ADC values of inflamed and normal segments were found to be 1.59 ± 0.45 × 10−3 mm2/s and 2.74 ± 0.68 × 10−3 mm2/s, respectively.
MRE along with DWI was performed at the completion of treatment to identify improvement in the index lesions. In our patients who showed complete or partial remission following treatment, there was significant rise in the ADC value with evidence of resolution of inflammation on conventional sequences as well as on ileocolonoscopy compared with pre-treatment MRE and ileocolonoscopy. However, among non-responders, the ADC value was found to be persistently abnormal with signs of bowel inflammation on post-treatment imaging. The difference in the mean ADC value among responders and non-responders was found to be statistically significant [ΔADCmean value: 0.78 (p < 0.05)].
Two similar studies by Huh et al. and Bhatnagar et al. have described similar results in CD patients following treatment. In the study performed by Huh et al., DWI was used to monitor bowel inflammation following medical therapy. They found a significant rise in the ADC value in patients who showed complete or partial remission. The change in the ADC value varied significantly between improved and unimproved lesions [12].
In the study by Bhatnagar et al. in CD patients, TNF-α antagonists were given and baseline and follow-up MRE with DCE and DWI were performed after a median interval of 51 weeks of therapy. They observed that following treatment with TNF-α antagonists, ADC increased significantly in responders but was not significantly different in non-responders [13].
We compared mean ADC value of the inflamed bowel segments with those of the adjacent morphologically normal bowel segments (confirmed on ileocolonoscopy) in our patients. Thirty such segments were identified in 19 of our patients which were normal on MRE including DWI as well as on ileocolonoscopy. The difference in the mean ADC value of the inflamed and normal bowel segments was found to be statistically significant (p < 0.05). Ninivaggi et al. in their study attempted to determine a discriminating ADC threshold value between pathological and normal bowel loops in 60 CD patients. They found a high mean ADC for normal appearing bowel loops (3.525 ± 0.07 × 10−3 mm2/s) compared to pathological ones (1.48 ± 0.058 × 10−3 mm2/s). They derived a threshold of 2.416 which had 100% sensitivity and 100% specificity for the discrimination between normal and pathological bowel loops [14]. However, these results are in contrast with the study performed by Pendse et al. who found no statistically significant differences in the mean ADC values of bowel segments with and without histological inflammation [15].
To further establish the role of DWI, Qi et al. described the diagnostic accuracies of MRE alone and MRE combined with DWI among CD patients. They showed that MRE combined with DWI had the highest sensitivity (93.5%), specificity (89.4%), and diagnostic accuracy (92%) for detection of lesions of CD compared to MRE or DWI alone, and b value 800 s/mm2 sequence showed the highest diagnostic sensitivity (74.1%) [16].
Our study showed the importance of DWI in the assessment of resolution of inflammatory disease activity where it was used as an adjunct to the conventional MRE sequences. It accurately identified 93.5% of the inflamed segments and proved to be of utmost importance in identifying post-treatment residual bowel inflammation just by qualitative assessment and reinforcing objectively by rise in ADC values. However, it has several limitations, most important being the small sample size. In addition, measurement of ADC value requires placement of ROI on the bowel wall, but due to the marked difference in the degree of bowel wall thickening among patients, it was not possible to maintain a uniform size of ROI for calculating ADC. In spite of meticulous care in placing the ROIs, there may have been inherent and unavoidable variability in sampling such a small area. To avoid this, the site for placing ROI was chosen after identifying the wall on T2W and post-contrast images, and the same person was assigned to place the ROIs in all studies. In patients with involvement of small bowel which could not be assessed either by upper GI endoscopy or colonoscopy, it was difficult to find a reference standard for confirming the diagnosis, as double balloon enteroscopy and capsule endoscopy are invasive and expensive. Also, their use was precluded in patients with tight strictures due to risk of capsule getting stuck at the stricture site. Hence, due to the risks involved with these procedures, in patients with small bowel disease not accessible by conventional endoscopy, symptomatic relief had to be taken as the reference standard for response to therapy.
Conclusion
DWI showed a high sensitivity (93.5%) for detecting inflamed bowel segments, and both qualitative and quantitative DWI data showed good correlation with disease activity. There was a significant increase in the ADC values post treatment among responders, whereas no change was noted in non-responders. Both qualitative and quantitative DWI data (ADC values) can be used for assessment of disease activity and for objectively assessing treatment response, and DWI is a useful adjunct to the conventional MRE for assessment of inflammatory activity and post-treatment changes.
References
Debi U, Ravisankar V, Prasad KK, Sinha SK, Sharma AK. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol. 2014;20(40):14831–40.
Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn’s disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008;14(5):741–6.
Munot K, Ananthakrishnan AN, Singla V, Benjamin J, Kedia S, Dhingra R, et al. Response to Trial of Antitubercular Therapy in Patients With Ulceroconstrictive Intestinal Disease and an Eventual Diagnosis of Crohn’s Disease. Gastroenterology. 2011;140(5):S-159.
Al-Hawary MM, Zimmermann EM, Hussain HK. MR imaging of the small bowel in Crohn disease. Magn Reson Imaging Clin N Am. 2014;22(1):13–22.
Li X-H, Sun C-H, Mao R, Huang S-Y, Zhang Z-W, Yang X-F, et al. Diffusion-weighted MRI Enables to Accurately Grade Inflammatory Activity in Patients of Ileocolonic Crohn’s Disease: Results from an Observational Study. Inflamm Bowel Dis. 2017;23(2):244–53.
Krishna S, Kalra N, Singh P, Kochhar R, Gupta R, Singh R, et al. Small-Bowel Tuberculosis: A Comparative Study of MR Enterography and Small-Bowel Follow-Through. AJR Am J Roentgenol. 2016;207(3):571–7.
Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of Diffusion-weighted MR Imaging for Detection of Bowel Inflammation in Patients with Crohn’s Disease. Acad Radiol. 2009;16(5):597–603.
Paustian F. Tuberculosis of the intestine. In: Bockus HL, ed. Bockus Gastroenterology. 5 ed. Philadelphia: Saunders; 1995. p. 3304.
Logan VS. Anorectal tuberculosis. Proc R Soc Med [Internet]. 1969;62(12):1227–30.
Park SH. DWI at MR Enterography for Evaluating Bowel Inflammation in Crohn Disease. American Journal of Roentgenology [Internet]. 2016;207(1):40–8.
Tielbeek JAW, Makanyanga JC, Bipat S, Pendsé DA, Nio CY, Vos FM, et al. Grading Crohn disease activity with MRI: interobserver variability of MRI features, MRI scoring of severity, and correlation with Crohn disease endoscopic index of severity. AJR Am J Roentgenol. 2013;201(6):1220–8.
Huh J, Kim KJ, Park SH, Park SH, Yang S-K, Ye BD, et al. Diffusion-Weighted MR Enterography to Monitor Bowel Inflammation after Medical Therapy in Crohn’s Disease: A Prospective Longitudinal Study. Korean J Radiol. 2017;18(1):162–72.
Bhatnagar G, Dikaios N, Prezzi D, Vega R, Halligan S, Taylor SA. Changes in dynamic contrast-enhanced pharmacokinetic and diffusion-weighted imaging parameters reflect response to anti-TNF therapy in Crohn’s disease. Br J Radiol. 2015;88(1055):20150547
Ninivaggi V, Missere M, Restaino G, Gangemi E, Di Matteo M, Pierro A, et al. MR-enterography with diffusion weighted imaging: ADC values in normal and pathological bowel loops, a possible threshold ADC value to differentiate active from inactive Crohn’s disease. Eur Rev Med Pharmacol Sci. 2016;20(21):4540–6.
Pendsé DA, Makanyanga JC, Plumb AA, Bhatnagar G, Atkinson D, Rodriguez-Justo M, et al. Diffusion-weighted imaging for evaluating inflammatory activity in Crohn’s disease: comparison with histopathology, conventional MRI activity scores, and faecal calprotectin. Abdom Radiol (NY). 2017;42(1):115–23.
Qi F, Jun S, Qi QY, Chen PJ, Chuan GX, Jiong Z, et al. Utility of the diffusion-weighted imaging for activity evaluation in Crohn’s disease patients underwent magnetic resonance enterography. BMC Gastroenterol. 2015;15:12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mathur, P., Sharma, R., Kandasamy, D. et al. Can ADC be used as a surrogate marker of response to therapy in intestinal tuberculosis?. Abdom Radiol 44, 3006–3018 (2019). https://doi.org/10.1007/s00261-019-02090-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02090-4