Abstract
The inclusion of diffusion-weighted imaging (DWI) into abdominal magnetic resonance (MR) protocols for the study of the large bowel is steadily rising. Reported potential benefits of DWI constitute the rationale for including these sequences in the study protocols: it may lead to an increased accuracy in the detection of colonic diseases and in the characterization of colonic wall abnormalities; it may help to increase MR accuracy regarding assessment of disease activity and treatment response in inflammatory conditions, most notably ulcerative colitis and Crohn’s disease; it could provide a noninvasive imaging evaluation that eventually may obviate the need for intravenous gadolinium administration, even—as suggested in some works—without the need for fasting or need for bowel cleansing and distension, therefore reducing patient discomfort and improving compliance. These theoretical benefits of DWI will be addressed in the present chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Magnetic resonance imaging (MRI) is being increasingly performed for the assessment of lesions of the large bowel, whereby mainly morphometric macroscopic tissue information is usually obtained. Yielding insights at a cellular level, diffusion-weighted imaging (DWI) provides images whose signal intensity is sensitized to the random motion of free water molecules. The mobility of water molecules within a given voxel is determined by the microscopic cellular structure, i.e., the presence of barriers, such as cell membranes and macromolecules. Thus, DWI offers a theoretical possibility for the assessment of colonic diseases, both inflammatory and neoplastic, on a more “functional” level. DW-MR images may be evaluated both qualitatively and quantitatively: the former results from a visual assessment of the DW-MR sequences, in which areas of restricted diffusion will appear hyperintense against a hypointense background on highest b-values obtained and hypointense on corresponding ADC map; quantitative evaluation of the water diffusion characteristics is performed by expressing them as an apparent diffusion coefficient (ADC) value. Both qualitative and quantitative information have been the subject of several works on colonic diseases.
A critical review of colonic polyp and cancer detection, characterization of colonic wall thickening, and assessment of inflammatory bowel disease of the colon will be performed in this chapter. A perspective on future applications and trends will also be discussed.
4.2 Technical Considerations
Imaging of the large bowel with DWI is challenging. In fact, relatively long acquisition times causing an increased sensitivity to bowel motion and the presence of T2 shine-through effect, which is frequently encountered in the bowel lumen, may hamper the diagnostic information conveyed by MR images [1].
A combination of high magnetic field MR scanners, decreased acquisition times, multichannel coils, and parallel imaging techniques have been helpful to reduce these technical limitations. New pulse sequences such as echo-planar imaging have contributed to a reduction in acquisition time, therefore also decreasing sensitivity to bowel and respiratory movements [1]. At present, no clear benefit from the use of antiperistaltic agents has been clearly shown, although theoretically they may reduce bowel motion artifacts.
The T2 shine-through effect may be reduced with the use of high b-values and short echo times.
For imaging of the bowel, both a low b-value ranging from 0 to 50 s/mm2 and at least one high b-value (800–1000 s/mm2) are useful. In our daily practice, usually three b-values (b = 50, b = 500, and b = 1000 s/mm2) are routinely acquired in a high-field magnet. This allows for the calculation of ADC values minimizing the effects of microperfusion allowing at the same time the acquisition of images with high contrast and signal-to-noise ratios.
Axial images should be preferably acquired, since they are less prone to motion artifacts than those acquired in other planes, namely in the coronal plane [1].
For image acquisition, different approaches may be used: e.g., a navigator-triggered technique, which helps reduce motion artifact and increases signal-to-noise ratio, the main drawback being the increased acquisition time. Sequences acquired with the patient breathing freely or in several breath holds are generally faster but suffer from motion artifacts and less detail [1].
The use of oral and rectal cleansing before DW-MRI is questionable nowadays. If it seems reasonable to think that colonic distention would improve detection and evaluation of lesions, it is also true that there is a growing body of evidence showing that colonic assessment without bowel preparation is feasible, yields satisfactory results, and improves patient compliance by reducing discomfort.
4.3 Detection of Polyps and Cancer
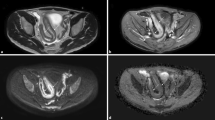
The rationale behind the use of DW-MRI for the detection of colonic polypoid lesions and cancer is that those lesions will exhibit restriction to diffusion and therefore will show high signal intensity at high b-value DW images. As such, the high lesion-to-background contrast that can be provided by DW-MRI will theoretically provide an advantage compared to conventional sequences (Fig. 4.1). Furthermore, DWI does not require the administration of any contrast agents and can be performed without any bowel preparation [2].
64-year-old male with a history of occult blood loss in stool. On the T2-weighted image (a), no abnormalities are found, whereas on the DWI sequence (b1000), a small focus of high signal intensity is apparent on the topography of the upper rectum (white arrow on b), which corresponded to a sessile polyp on colonoscopy. Both sequences were obtained without previous bowel preparation
A feasibility study published by Dutch authors on the detection of polyps included 26 patients and achieved a lesion-based sensitivity of 80.0% for clinically relevant lesions (polyps ≥6 mm and cancer) [2]. Nevertheless, the authors suggested that further technical developments are required in order to increase the diagnostic yield of DW-MRI in the detection of polypoid lesions of the colon.
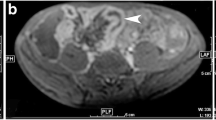
Some authors have also investigated the value of DW-MRI in the detection of colorectal cancer. Ichikawa et al. retrospectively assessed the diagnostic value of DWI for the detection of colorectal cancer in 33 patients with neoplasms and 15 controls and reported a sensitivity of 90.9% and specificity of 100% for the diagnosis of colonic adenocarcinoma [3]. Neoplastic lesions, due to its high cellularity, presented with high signal intensity on the high b-value images, by contrast to a dark background (Fig. 4.2). On the ADC maps, these lesions characteristically appeared with low signal intensity. Again, the detection of neoplastic lesions could be achieved on non-gadolinium-enhanced sequences, and previous bowel cleaning was not performed.
77-year-old female with an endoluminal lesion on colonoscopy. On the T2-weighted image, an irregular wall thickening is disclosed at the level of the distal sigmoid (white arrow on a) with some perilesional adenopathies (white arrowheads on a). On the DWI sequence (b1000), both the primary lesion (white arrow on b) and the adenopathies (white arrowheads on b) reveal predominant hyperintensity against a dark background
In polypoid cancers, DWI can clearly demarcate the areas within the polyp that show a high cellular content, helping to distinguish them from the low-cellular areas (Fig. 4.3).
62-year-old male with a polyp on colonoscopy. On the T2-weighted image, a large pedunculated polypoid cancer is seen at the level of the rectum (a). On the DWI sequence (b1000), the head of the polyp demonstrates high signal intensity, corresponding to the more cellular areas, in contrast to the stalk, which, because of the relatively low cellularity, appears dark on DWI (b)
4.4 Characterization of Wall Thickening
DW-MRI has been used as a tool to help characterize diffuse bowel wall thickening, namely to distinguish between malignancy and various benign conditions, including inflammatory, ischemic, or infectious bowel diseases.
Solak et al. retrospectively evaluated 26 patients with malignant disease and 15 patients with benign conditions of the colorectum by DW-MRI, visually assessing high b-value (b = 800 s/mm2) DWI images and ADC maps, and also quantifying ADC values, having defined endoscopic biopsy as the gold standard [4]. Results from this study demonstrated that the difference between the mean ADC values of benign and malignant conditions was statistically significant, with ADC values of benign lesions being significantly higher than those of malignant lesions. By applying a cutoff value of 1.21 × 10−3 mm2/s, ADC yielded a sensitivity of 100%, a specificity of 87.3%, and an accuracy of 89.3% in the discrimination of malignant colorectal pathology. With the combined visual assessment of the high b-value images and the measurement of ADC values, malignant and benign lesions could be differentiated with 100% sensitivity, 89.2% specificity, and 90.4% accuracy. Importantly also, although some benign lesions were interpreted as malignant, no malignant lesion was judged to be benign on the visual assessment [4].
Other authors directed their attention to the differentiation between a particular inflammatory condition (acute diverticulitis) and cancer. Both clinical conditions may show overlapping signs, symptoms, and imaging features—particularly on CT—and the coexistence of acute diverticulitis superimposed on a colon cancer may obscure the latter on imaging. Therefore, DW-MRI was tested as an alternative to CT in establishing the diagnosis of diverticulitis [5]. Öistämö et al. retrospectively examined patients presenting with either diverticulitis or sigmoid cancer with DW-MRI. This study reported a sensitivity and specificity for the diagnosis of colon cancer and diverticulitis of 100% when using DW-MRI, whereas the sensitivity and specificity for the diagnosis of colon cancer and diverticulitis were 67% and 93%, respectively, using CT [5]. However this study included only two groups of 15 patients, and its results should be confirmed and further validated in larger studies.
In these works, the differentiation between malignant and benign lesions of the colonic wall relied on the identification of hyperintense areas within the colonic wall on high b-value images and lower ADC values, in the former group of diseases.
4.5 Assessment of Inflammatory Bowel Disease
One major application of DW-MRI consists of the evaluation of inflammatory bowel diseases. In fact, the largest body of evidence on the role of DW-MRI in the colon comes from the assessment of ulcerative colitis and Crohn’s disease. Several works have been published in the field, regarding detection of colonic inflammation, assessment of disease activity, and evaluation of response to therapy.
4.5.1 Detection of Inflammatory Changes in the Colon
A feasibility study by Oto et al. was designed to determine the possibility of a role for DWI in the detection of bowel inflammation and to investigate the changes in ADC values of the inflamed bowel in patients with Crohn’s disease, with pathologic features as the gold standard [6]. Inflammation of the bowel wall causes restricted diffusion, and as such DWI yields both qualitative (increased signal intensity) and quantitative (decreased ADC values) information that can be helpful in the evaluation of bowel inflammation (Fig. 4.4). In addition to the increased number of inflammatory cells, dilated lymphatic channels, hypertrophied neuronal tissue, and the development of granulomas in the bowel wall can further narrow the extracellular space and therefore contribute to the restricted diffusion of water molecules.
31-year-old male with Crohn’s disease. On the T2-weighted image, there is thickening of the wall of the right colon (a) with correspondent hyperenhancement on the fat-suppressed, gadolinium-enhanced, T1-weighted image (b). On the DWI sequence (b1000), there is restriction to diffusion with hyperintensity of the colonic wall (c) and low signal intensity on the ADC map (d), compatible with inflammatory changes at that level
Diffusion-weighted magnetic resonance colonography (DW-MRC) without oral or rectal preparation proved to be a reliable tool for detecting colonic inflammation in several studies. The technique does not need fasting, is noninvasive, and does not require any bowel preparation. Oussalah et al. studied 96 patients with both ulcerative colitis and Crohn’s disease (68 had concomitant endoscopy) with DW-MRC without fasting or oral or rectal preparation [7]. On DW-MRC, six radiological signs were studied: (1) DWI hyperintensity, (2) rapid gadolinium enhancement after intravenous contrast medium administration, (3) differentiation between the mucosa-submucosa complex and the muscularis propria, (4) bowel wall thickening, (5) parietal edema, and (6) the presence of ulceration(s). In the ulcerative colitis group, the presence of a DWI hyperintensity demonstrated a sensitivity and a specificity of 90.79% and 80%, respectively, for the detection of endoscopic inflammation, with an area under the ROC curve of 0.854. DWI hyperintensity was statistically more effective for the detection of endoscopic colonic inflammation in ulcerative colitis than in Crohn’s disease. Comparatively, in the ulcerative colitis group, rapid gadolinium enhancement correlated with endoscopic inflammation in the colon with a sensitivity and specificity of 72.37% and 96.67%, respectively, with an area under the ROC curve of 0.845. Rapid gadolinium enhancement was significantly more effective for the detection of endoscopic inflammation in ulcerative colitis than in Crohn’s disease. Of note, there was no statistically significant difference in accuracy between DWI hyperintensity and rapid gadolinium enhancement areas under the ROC curves in ulcerative colitis and Crohn’s disease. In ulcerative colitis, ROC analyses for the four remaining parameters of the MR score for the detection of endoscopic inflammation demonstrated a good sensitivity (88.16%) and specificity (83.33%) for the “differentiation between the mucosa-submucosa complex and the muscularis propria.” For the three other items, the sensitivity was low, ranging from 38.16% to 67.11%, with excellent specificities ranging from 93.33% to 96.67%. In Crohn’s disease, ROC analyses for the same four parameters revealed low sensitivities ranging from 36.11% to 62.5% and good to excellent specificities ranging from 75% to 100%. Among these four parameters, the “differentiation between the mucosa-submucosa complex and the muscularis propria” and “ulcerations” exhibited better accuracy for the detection of endoscopic inflammation in ulcerative colitis than in Crohn’s disease. The accuracy was similar for the other two items in both ulcerative colitis and Crohn’s disease. Logistic regression analysis showed that DWI hyperintensity was predictive of the presence of endoscopic inflammation in both the ulcerative colitis and the Crohn’s disease groups (odds ratio = 13.26 and 2.67, respectively) [7].
Similarly, Sirin et al. used DW-MRC to assess whether intravenous contrast was needed to depict inflammatory lesions in the bowel when DWI was also available, in a pediatric population [8]. In this retrospective study, patients received bowel preparation, and optical colonoscopy was the gold standard for the 37 individuals studied. Mean sensitivity and specificity for two readers for the depiction of inflammatory lesions were, respectively, 78.4% and 100% using gadolinium-enhanced T1-weighted MRC, 95.2% and 100% using DWI, and 93.5% and 100% combining both imaging techniques compared with colonoscopy (including results of the histopathological samples). In six patients, inflammatory lesions were only detected by DWI; in another six patients, DWI detected additional lesions. The preferred b-value with the best detectability of the lesions was b = 1000 s/mm2 in 28 of the 30 patients (93.3%) (8).
A recent study also assessed the role of DW-MRI without bowel preparation in the detection of ulcerative colitis in 20 patients with optical colonoscopy as the gold standard [9]. The authors assessed the following imaging signs: (1) DWI hyperintensity (b = 800 s/mm2), (2) rapid gadolinium enhancement after intravenous contrast medium administration (20–25 s after gadolinium infusion), (3) differentiation between the mucosa-submucosa complex and the muscularis, (4) bowel wall thickening (exceeding 5 mm), (5) parietal edema, (6) the presence of ulceration(s), and (7) comb sign of engorged vasa recta that perpendicularly penetrated the bowel wall. The results showed that DWI provided qualitative and quantitative information when this technique was combined with conventional magnetic resonance imaging without bowel preparation; the combined technique demonstrated a good diagnostic performance to detect colonic inflammation in ulcerative colitis. DWI hyperintensity at b = 800 s/mm2 detected endoscopic colonic inflammation with a sensitivity of 93.0% and a specificity of 79.3% with an area under the ROC curve of 0.867. With rapid gadolinium enhancement, endoscopic colonic inflammation was detected with a sensitivity of 73.2%, a specificity of 93.1%, and an area under the ROC curve of 0.853. The accuracy between DWI hyperintensity and rapid gadolinium enhancement was not significantly different. Differentiation between the mucosa-submucosa complex and the muscles revealed a good sensitivity (80.3%) and specificity (86.2%). The four other signs demonstrated low sensitivities (range, 43.7–66.2%) and excellent specificities (range, 89.7–93.1%) [9].
From the analysis of the published works, it seems reasonable to recognize that DW-MRC, which combines morphological MRI and DWI, even without oral or rectal preparation might be used in clinical practice to evaluate colonic inflammation, particularly in ulcerative colitis. DWI has the potential to replace gadolinium-enhanced sequences in order to detect inflammatory changes in the colon, therefore reducing the likelihood of adverse effects from the use of gadolinium-based contrast media and, at the same time, reducing examination costs.
4.5.2 Assessment of Disease Activity
With the aim of assessing disease activity in patients with ulcerative colitis, Kılıçkesmez et al. prospectively studied 28 patients in different stages of the disease by means of DW-MRI without preparation, measuring ADC of the bowel wall in sigmoid colon and rectum and comparing the findings with endoscopy [10]. Results disclosed no statistically significant difference in the ADC of the sigmoid colon in patients with active, subacute, and remissive ulcerative colitis. On the contrary, ADC values of the rectum were statistically different between patients in the active (1.08 ± 0.14 × 10−3 mm2/s) and subacute phases (1.13 ± 0.23 × 10−3 mm2/s) of disease and those in remission (1.29 ± 0.17 × 10−3 mm2/s). Therefore, an increased activity of the disease was correlated with lower ADC values [10].
Similarly, Kiryu et al. investigated the application of free-breathing DW-MRI to assess active Crohn’s disease [11]. The findings of a conventional barium study or surgery were regarded as the gold standard. The ADC was significantly lower in the disease-active segments than in the disease-inactive segments in the large bowel (1.52 ± 0.43 × 10−3 mm2/s versus 2.31 ± 0.59 × 10−3 mm2/s, respectively). The sensitivity, specificity, and accuracy were 85.7%, 75.7%, and 77.3%, respectively, in the large bowel. The accuracy was 82.6% in the ascending colon, 85.0% in the transverse colon, 80.8% in the descending colon, 72.4% in the sigmoid colon, and 70.0% in the rectum [11].
Diffusion-weighted magnetic resonance enterocolonography (DW-MREC) with no bowel cleansing and no rectal enema was performed by Buisson et al. to prospectively evaluate patients with Crohn’s disease, specifically for the indirect detection of ulcerations in this setting [12]. Forty-four patients were studied and results were compared to the gold standard (ileocolonoscopy): a total of 158 colorectal segments were assessed. The authors showed that not only the segmental ADC measured on the bowel wall in these segments was correlated with endoscopic scores but also that MRI accuracy to detect endoscopic ulcerations, using a ADC < 1.88 for colon/rectum, ranged from 63.2% (cecum/right colon) to 84.6% (left/sigmoid colon). This work also disclosed a relationship between ulcer size and ADC: the segmental ADC values decreased when the ulceration size increased [12].
Sato et al. also aimed to compare the findings of DW-MREC with endoscopically identified lesions according to inflammatory grades and assess the diagnostic accuracy of DW-MREC for sensitivity and grading severity in Crohn’s disease [13]. A total of 27 patients were evaluated. A positive lesion was defined as having at least one of the following: wall thickness, edema, high intensity on DWI images, and relative contrast enhancement on MREC. The sensitivities were 100% for ulcer, 84.6% for erosion, and 52.9% for redness, suggesting an ability to detect milder lesions such as erosion or redness in MREC. For DWI in specific, the sensitivities for endoscopic ulcer, erosion, and redness were 80.9%, 69.2%, and 33.3%, respectively. The specificities for endoscopically identified lesions were high (92.1%). When the lesion was defined as having two or three positive MREC findings, sensitivity increased: sensitivities for either wall thickness or DWI high intensity, either edema or DWI high intensity, and either wall thickness or edema or DWI high intensity were 80.9%, 80.9%, and 80.9% for ulcer, respectively; 76.9%, 69.2%, and 76.9% for erosion, respectively; and 41.2%, 35.3%, and 41.2% for redness, respectively. Specificities were 92%, 92%, and 92%, respectively [13].
Therefore, DWI could be an adjunct to differentiate between active inflammation and quiescent disease (Fig. 4.5).
Two different patients (a, b, 19-year-old male; c, d, 16-year-old female) with Crohn’s disease showing areas of wall thickening at the level of the right colon on the T2-weighted images (a, c). On the DWI sequence (b1000), there is restriction of diffusion with hyperintensity of the colonic wall in the first patient (b) indicating active disease, whereas in the second patient, no areas of restricted diffusion are seen, corresponding to quiescent disease
4.5.3 Evaluation of Response to Therapy
A recently published paper aimed to assess DW-MREC parameters as predictors of remission after anti-TNF induction therapy in Crohn’s disease [14]. Forty consecutive patients were enrolled in this prospective study, being evaluated by DW-MREC with no rectal distension and no bowel cleansing. Patients were evaluated before treatment and at week 12. The authors showed that a mean ADC cutoff of 1.96 × 10−3 mm2/s was predictive of remission at week 12 (area under the ROC curve = 0.703) with sensitivity, specificity, positive predictive value, and negative predictive value of 70.0%, 65.0%, 66.7%, and 68.4%, respectively. In a multivariate analysis, mean ADC < 1.96 × 10−3 mm2/s (odds ratio = 4.87), reflecting high inflammatory activity, was predictive of remission at week 12. These results suggest that DW-MREC may help to select patients with objective digestive inflammation who could benefit from anti-TNF therapy and could be helpful to predict remission after anti-TNF induction therapy [14]. However, results from this pilot study need to be confirmed in an independent larger cohort.
Similarly, Sakuraba et al. evaluated 13 individuals 1 year after infliximab induction therapy by DW-MRI scans which were assessed as predictors of maintained response, or remission, through 3 years of treatment in patients with CD [15]. Examinations were performed 1 and 3 years after the starting point of the infliximab therapy. DW-MRI predicted the presence of synergistic mucosal changes on colonoscopy with a sensitivity of 80.52% and specificity of 66.67%. DW-MRI at 1 year was able to predict the presence of endoscopic inflammation with a sensitivity of 66.67%, a specificity of 80.52%, and an area under the ROC curve of 0.7359. Also, DW-MRI at 3 years suggested endoscopic inflammation with a sensitivity of 94.12%, a specificity of 73.91%, and an area under the ROC curve of 0.8402 [15].
Evaluation of therapy response by DW-MRI is naturally regarded with increasing interest by both clinicians and radiologists working on the field of inflammatory bowel disease, since the patient’s discomfort and risk of injury are minimized because of the noninvasiveness of the method. Furthermore, lesions that are not accessible by endoscopy because of stenosis or adhesion can be evaluated, as well as the extraintestinal tissues. In the future, biomarkers of response to treatment based on DW-MRI might be helpful for optimizing the indications for endoscopy and further treatment of these patients.
4.6 Future Applications and Perspectives
Many expectations are being raised by the application of DW-MRI in the field of colorectal oncology. DW-MRI may theoretically be used to assess and monitor therapy response of colorectal cancer.
An animal study by Schneider et al. performed the early monitoring of antiangiogenic therapy in an experimental tumor model [16]. Using quantitative DW-MRI, the authors found that therapy of human colon carcinoma xenografts with the multi-tyrosine kinase inhibitor regorafenib significantly increased water diffusivity in tumorous tissue after 6 days of treatment. Regorafenib significantly reduced tumor growth compared to the control group. Using either tumor ADC changes or tumor growth to distinguish between therapy and control group resulted in a diagnostic accuracy of about 78% and 83%, respectively, which was improved by the approach to combine both parameters using Fisher’s linear discriminant analysis to about 96%, thus highlighting the potential of multiparameter MRI as an imaging biomarker for noninvasive monitoring of early tumor therapy and allowing in this way a more patient-tailored therapeutic approach [16].
Another experimental study aimed to assess the potential value of combined MR elastography and DW-MRI in the detection of microstructural changes of murine colon tumors during growth and antivascular treatment for two models of implantation (ectopic and orthotopic) [17]. DW-MRI was sensitive to tumor cell alterations, including cellularity and micronecrosis; ADC decreased significantly for the ectopic model between early and angiogenic stages, whereas no significant ADC change was observed for the orthotopic model between these stages. MR elastography allowed monitoring of changes in vascularization. The authors concluded that MR elastography and DW-MRI have the potential of being complementary for noninvasive surveillance of tumor evolution [17].
In addition to conventional ADC measurements in the monoexponential range, the development of high-performance gradient coils enables DWI measurements with stronger diffusion weighting using higher b-values (e.g., 1500 s/mm2) and increased diffusion contrast. Under these conditions the signal attenuation is often non-monoexponential. This is a consequence of restricted diffusion as the mean-squared displacements of diffusing protons are no longer Gaussian distributed. Quantitative non-Gaussian diffusion models have been developed to fit diffusion signals with high b-values. Several of these non-Gaussian diffusion models have been implemented in cancer imaging and appear to show new information or higher sensitivity compared with conventional ADC measurements [18].
In fact, Xu et al. tested both conventional ADC and non-Gaussian model measurements and analyses in order to assess the early therapeutic response of human colon cancer to barasertib [19]. The results suggest that the non-Gaussian DWI model-derived parameters were capable of detecting earlier tumor changes to treatment in comparison with conventional ADC. Non-Gaussian DWI may potentially provide an opportunity to better evaluate tumor status earlier than ADC and tumor volume changes that are currently widely used in clinical cancer research, therefore yielding an opportunity to assist clinicians to better enable necessary therapeutic adjustments in a timely manner to enhance treatment efficacy and avoid unnecessary treatment delays, toxicity, and expenses [19].
The role of DWI-MR as a biomarker of response in colon cancer should be, at present, regarded as an adjunct to clinical tools (e.g., endoscopy and biopsy). The results published so far are obviously still premature for clinical decision-making, but their promise warrants further validation by large and prospective patient studies.
References
Dohan A, Taylor S, Hoeffel C, et al. Diffusion-weighted MRI in Crohn’s disease: current status and recommendations. J Magn Reson Imaging. 2016;44(6):1381–96.
Leufkens AM, Kwee TC, van den Bosch MA, Mali WP, Takahara T, Siersema PD. Diffusion-weighted MRI for the detection of colorectal polyps: feasibility study. Magn Reson Imaging. 2013;31(1):28–35.
Ichikawa T, Erturk SM, Motosugi U, et al. High-B-value diffusion-weighted MRI in colorectal cancer. AJR Am J Roentgenol. 2006;187:181–4.
Solak A, Genç B, Solak I, et al. The value of diffusion-weighted magnetic resonance imaging in the differential diagnosis in diffuse bowel wall thickening. Turk J Gastroenterol. 2013;24(2):154–60.
Öistämö E, Hjern F, Blomqvist L, Von Heijne A, Abraham-Nordling M. Cancer and diverticulitis of the sigmoid colon. Differentiation with computed tomography versus magnetic resonance imaging: preliminary experiences. Acta Radiol. 2013;54(3):237–41.
Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol. 2009;16(5):597–603.
Oussalah A, Laurent V, Bruot O, et al. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut. 2010;59(8):1056–65.
Sirin S, Kathemann S, Schweiger B, et al. Magnetic resonance colonography including diffusion-weighted imaging in children and adolescents with inflammatory bowel disease: do we really need intravenous contrast? Invest Radiol. 2015;50(1):32–9.
Yu LL, Yang HS, Zhang BT, et al. Diffusion-weighted magnetic resonance imaging without bowel preparation for detection of ulcerative colitis. World J Gastroenterol. 2015;21(33):9785–92.
Kilickesmez O, Atilla S, Soylu A, et al. Diffusion-weighted imaging of the rectosigmoid colon: preliminary findings. J Comput Assist Tomogr. 2009;33(6):863–6.
Kiryu S, Dodanuki K, Takao H, et al. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging. 2009;29(4):880–6.
Buisson A, Hordonneau C, Goutte M, Boyer L, Pereira B, Bommelaer G. Diffusion-weighted magnetic resonance imaging is effective to detect ileocolonic ulcerations in Crohn’s disease. Aliment Pharmacol Ther. 2015;42(4):452–60.
Sato H, Tamura C, Narimatsu K, et al. Magnetic resonance enterocolonography in detecting erosion and redness in intestinal mucosa of patients with Crohn’s disease. J Gastroenterol Hepatol. 2015;30(4):667–73.
Buisson A, Hordonneau C, Goutte M, et al. Diffusion-weighted magnetic resonance enterocolonography in predicting remission after anti-TNF induction therapy in Crohn’s disease. Dig Liver Dis. 2016;48(3):260–6.
Sakuraba H, Ishiguro Y, Hasui K, et al. Prediction of maintained mucosal healing in patients with Crohn’s disease under treatment with infliximab using diffusion-weighted magnetic resonance imaging. Digestion. 2014;89(1):49–54.
Schneider MJ, Cyran CC, Nikolaou K, Hirner H, Reiser MF, Dietrich O. Monitoring early response to anti-angiogenic therapy: diffusion-weighted magnetic resonance imaging and volume measurements in colon carcinoma xenografts. PLoS One. 2014;9(9):e106970.
Jugé L, Doan BT, Seguin J, et al. Colon tumor growth and antivascular treatment in mice: complementary assessment with MR elastography and diffusion-weighted MR imaging. Radiology. 2012;264(2):436–44.
Maier SE, Sun Y, Mulkern RV. Diffusion imaging of brain tumors. NMR Biomed. 2010;23:849–64.
Xu J, Li K, Adam Smith R, et al. A comparative assessment of preclinical chemotherapeutic response of tumors using quantitative non-Gaussian diffusion MRI. Magn Reson Imaging. 2017;37:195–202.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Semedo, L.C. (2019). Large Bowel. In: Gourtsoyianni, S., Papanikolaou, N. (eds) Diffusion Weighted Imaging of the Gastrointestinal Tract. Springer, Cham. https://doi.org/10.1007/978-3-319-92819-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-92819-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92818-0
Online ISBN: 978-3-319-92819-7
eBook Packages: MedicineMedicine (R0)