Abstract
Purpose
To evaluate the utility of real-time contrast-enhanced ultrasound (CEUS)-guided coaxial needle biopsies for focal liver lesions (FLL) that were inconspicuous or could not be accurately identified the active site on B-mode ultrasound (US).
Materials and methods
This prospective study included 76 patients who had CEUS-guided coaxial needle biopsies for FLL between December 2015 and June 2017. We recorded characteristics of target lesions. We evaluated conspicuity of target lesions and accuracy of identifying the active site of target lesions on B-mode US and CEUS using a 5-point scale. Patients were divided into three groups, and analyzed according to body mass index (BMI). Based on the final diagnosis, the diagnostic performance was evaluated.
Results
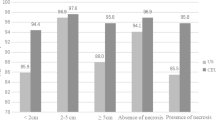
The mean size and depth of target lesions were 41.5 ± 28.5 and 47.9 ± 18.9 mm on CEUS, respectively. In arterial phase, the enhanced pattern of target lesions varied. The conspicuity of target lesions and accuracy of identifying the active site of target lesions was significantly improved on CEUS compared to B-mode US (p < 0.05). The three BMI groups had significant differences in conspicuity of target lesions after using CEUS (p < 0.05). The high BMI group had a greater change in conspicuity of lesions compared to the normal BMI group or the low BMI group (p < 0.05). The sensitivity, specificity, and accuracy of this technique for the diagnosis of FLL were 92.8%, 100%, and 93.4%, respectively.
Conclusion
Real-time CEUS-guided coaxial needle biopsy can be very useful for FLL that are inconspicuous or cannot be accurately identified the active site on B-mode US.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Ultrasound (US) is generally the preferred imaging modality for guidance of biopsy of focal liver lesions (FLL) due to several advantages, such as safety, low cost, absence of radiation hazard, and convenience [1,2,3]. However, when the target lesion had poor sonographic conspicuity or the target lesion was large with consistent necrosis, it could not be confidently localized on B-mode US. Thus, advanced techniques are required to detect target lesions difficult to confidently localize on B-mode US. According to some studies [4,5,6,7,8,9], contrast-enhanced ultrasound (CEUS) has been used to augment US-guided interventional procedures, especially in those lesions that are inconspicuous on B-mode US. CEUS is performed using the intravenous injection of an ultrasound contrast agent (UCA) composed of a microbubble stabilized with a coating of a biocompatible surfactant or polymer-like phospholipids or proteins. This contrast is not excreted by the kidneys and thus can be used in patients with impaired renal function in whom CT and MR contrast studies are contraindicated. Moreover, CEUS has a substantially higher temporal resolution than CT and MR, allowing a real-time study of contrast-enhancement behavior of FLL. This is especially true for second-generation UCAs, which have a prolonged lifespan for the microbubbles in the bloodstream and allow a continuous, real-time scanning in all vascular phases [10]. Percutaneous liver biopsy can be done either with a conventional non-coaxial technique or with a coaxial technique. The majority of percutaneous liver biopsies in the literature have been performed using a noncoaxial technique. However, a few articles have evaluated the usefulness of real-time CEUS-guided coaxial needle biopsy as a biopsy method for FLL.
The purpose of the current study was to evaluate the utility of real-time CEUS-guided coaxial needle biopsies for FLL that were inconspicuous or could not be accurately identified as the active site on B-mode US.
Materials and methods
Patients
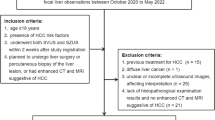
This prospective study was approved by the ethics committee of our institution. Informed consent was obtained from all patients prior to enrollment. Between December 2015 and June 2017, there were 419 consecutive patients who underwent US guided coaxial needle biopsies of FLL in the Department of Ultrasonography in our institution. Three hundred forty-one patients were excluded because biopsies were performed with conventional US guidance without use CEUS guidance. There were 78 patients who underwent CEUS-guided coaxial needle biopsies of FLL. Of these, 2 patients were excluded because the US system was unable to store biopsy images. Therefore, our study eventually included 76 patients (Fig. 1).
The inclusion criteria were as follows: (1) A target lesion was inconspicuous such that two radiologists could not confidently localize it on B-mode US; or (2) The target lesion was large and was partly necrotic such that two radiologists could not confidently determine the active portion of the target lesion on B-mode US. Exclusion criteria were as follows: (1) A target lesion that could be confidently localized and the active site accurately identified on B-mode US; or (2) A target lesion was located in a sonographically blind area; or (3) Bleeding tendency of patients or patients with severe systemic disease; or (4) Patients with contraindications for the use of the contrast agent.
CEUS
B-mode US and CEUS were performed with the Supersonic Aixplorer system (SuperSonic Imagine, France), which was equipped with a convex probe with a bandwidth of 1-6 MHz. The principles of CEUS were as follows. Pulse- or phase-inversion US sums the signals returned from two 180 degree ultrasound pulses. Linear scattering from tissue results in a signal void while nonlinear signals from microbubbles stand out. A second-generation US contrast agent SonoVue (sulfur hexafluoride microbubbles, Bracco, Milan, Italy) was used in this study. SonoVue was approved for CEUS of the liver in China. UCAs were usually injected as a 2.4 mL bolus (equivalent to a 0.003 mL/kg for 70 kg body weight) using a peripheral intravenous 20-gauge cannula (usually positioned in the left arm), followed by a flush of 10 mL of sterile saline [11]. In the study, we generally injected the contrast agent twice. The first dose of 2.4 mL was administered intravenously to assess the target lesion. After the assessment, flash mode was used to destroy the bubbles, and then the second dose of 1–2.4 mL was used for CEUS-guided biopsy.
Biopsy procedure
All procedures were performed by one of two radiologists with over 5 years of clinical experience performing CEUS diagnosis and biopsies of liver. The biopsy procedure was displayed in the split-screen mode on a single monitor: the left side of the CEUS image was used to observe the lesion, while the right B-mode was used to observe the puncture needle. In the case where the target lesion was inconspicuous on B-mode US, the biopsy was performed in the parenchymal or delayed phase when the lesions washed out, and the tissue to lesion contrast ratio was maximal. In the case where the active portion of the target lesion could not be determined on B-mode US, the coaxial needle was inserted into the enhanced areas in the arterial phase. The patients underwent biopsy with a coaxial Tru-Cut needle (18-gauge Bard Magnum Needle and Magnum Biopsy Instrument, 17-gauge Bard TruGuide Disposable Coaxial Needle; C. R. Bard, Tempe, USA). After sterilizing the skin and administering local anesthesia, a coaxial needle was advanced just to the edge of the target lesion, and samples were taken by needle biopsy. When enough samples were obtained, the co-axial needle was removed. All operations were performed using a free-hand technique. The biopsy process time was defined as the time from the initial injection of US contrast agents to the biopsy termination. Complications associated with biopsy or the use of contrast media within 24 h after the procedure were recorded.
Outcome analysis
The two operators who performed the biopsy, assessed conspicuity and confidence score of target lesions using a 5-point scale. When there was a lack of unanimity of initial scoring, an agreement was reached by the two doctors to arrive at a final score after discussion. One of the scales addressed the conspicuity of target lesions and another assessed the active portion of target lesions. The conspicuity on the basis of the visibility of target lesions compared with adjacent liver parenchyma used a 5-point scale (1, definitely invisible; 2, probably invisible; 3, barely visible; 4, fairly visible; and 5, clearly and distinctly visible) [12]. The confidence score of the active portion of target lesions also used a 5-point scale (1, definitely uncertain; 2, probably uncertain; 3, barely certain; 4, fairly certain; 5, clearly and distinctly certain). The final diagnosis was determined based on histopathological, clinical, and radiographic follow-up results. The diagnostic performance of this technique for FLL was evaluated according to the final confirmed diagnosis.
Statistical analysis
Descriptive statistics were presented as the mean ± standard deviation (SD) and median according to normality for the continuous variable and with frequency (percentage) for the categorical variable. To compare inter-observer variability, the Cohen kappa statistic were used. To compare the conspicuity and confidence scores of target lesions on B-mode US and CEUS, Wilcoxon signed rank tests were used. Subjects were divided into three groups according to body mass index (BMI). To compare differences between the three BMI groups, covariance analysis was used. To identify differences between each two groups, Bonferroni correction were used. All statistical analyses were performed using the SPSS software package (SPSS Statistics, version 22.0; SPSS Inc., Chicago, IL, USA). A p value of < 0.05 was considered statistically significant.
Result
Patient characteristics
The demographic characteristics of the 76 patients are summarized in Table 1. There were 48 (63.2%) men and 28 (36.8%) women. The ages ranged from 28 to 77 years, the median age was 60 years, and the mean age was 58.6 ± 8.9 years (mean ± SD). There were 9 (11.8%) patients with low BMI, 39 (51.3%) patients with normal BMI, and 28 (36.9%) patients with high BMI. There were 23 (30.3%) patients with hepatitis B, 3 (3.9%) with hepatitis C, and 50 (65.8%) with no history of hepatitis. Twenty-three (30.2%) patients had a liver CT and a liver MRI examination in our hospital within 2 weeks prior to biopsy, 42 (55.3%) patients had a liver CT or a liver MRI in our hospital, and 11 (14.5%) patients had CT or MRI examinations at other hospitals. There were 55 (72.4%) patients with 1 lesion, 4 (5.2%) patients with 2 lesions, and 17 (22.4%) patients with 3 or more lesions. Among them, 17 (22.4%) patients had elevated AFP levels, 41 (53.9%) had normal APF levels, and 18 (23.7%) had not had AFP testing.
Lesion characteristics
The lesion characteristics are summarized in Table 2. Twenty-eight lesions were located in the left liver, and 48 lesions were located in the right liver. The size of target lesion ranged from 8 to 126 mm, with a median size of 34.5 mm and a mean diameter of 41.5 ± 28.5 mm. The depth of the target lesion ranged from 15 to 96 mm, with a median depth of 45 mm and a mean depth of 47.9 ± 18.9 mm. The puncture distance ranged from 40 to 130 mm, the median puncture distance was 71.5 mm, and the mean puncture distance was 74.6 ± 20.0 mm. The number of needle passes ranged from 1 to 4, with a median number of 2.5, and the mean number of needle punctures was 2.6 ± 0.8. The procedure time ranged from 5 to 29 min, with a median puncture time of 8 min and the mean puncture time of 9.3 ± 4.2 min. In the arterial phase, there were 42, 18, and 9 lesions that were hyper-vascular, iso-vascular, and hypo-vascular, respectively. Most lesions showed washout in portal phase or late phase (67/76). Two lesions showed hyper-enhancement in arterial phase, iso-enhancement in portal and late phases. In addition, 7 lesions showed iso-enhancement in arterial, portal, and late phases. The 7 cases were diagnosed finally as benign lesions.
The conspicuity score of target lesions on B-mode US and CEUS
Kappa statistics showed that agreement of two observers was good in conspicuity score of target lesions either on B-mode US or CEUS (k = 0.65; k = 0.66). Moreover, on imaging with B-mode US, the mean conspicuity score of target lesions was 2.04 ± 0.94. On imaging with CEUS, the mean conspicuity score of target lesions was 4.58 ± 0.57. Most of target lesions showed better in target lesion conspicuity on CEUS imaging (Fig. 2). Meanwhile, Wilcoxon signed rank tests showed that the conspicuity score of target lesions was significantly better on CEUS than B-mode US (p < 0.05; Table 3). Moreover, covariance analysis showed that the three BMI groups had significantly different conspicuity of target lesions after the use of CEUS (p < 0.05; Table 4). Specifically, Bonferroni correction showed that the high BMI group had a greater change in the conspicuity of target lesions than the normal BMI group or the low BMI group (p < 0.05; Table 5).
Hepatic metastasis from gastric cancer in a 61-year-old man. (A) Axial contrast-enhanced CT showing a low-density nodule in segment 7 of the liver (arrow). (B) B-mode US showing no obvious focal lesion in the corresponding area of the CT image. (C) Split-screen mode showing no focal lesion in the region (right) where a 8-mm target lesion (left, arrow) is clearly showed on the corresponding region. (D) Biopsy was performed under the real-time guidance of CEUS. The biopsy needle passes through the center of the target lesion (left, arrow)
The confidence score of target lesions active portion on B-mode US and CEUS
Kappa statistics showed that agreement of two observers was good based on the confidence scores of the active portion of target lesions either on B-mode US or CEUS (k = 0.69; k = 0.67). Moreover, on imaging with B-mode US, the mean confidence score of target lesions was 1.64 ± 0.68. On imaging with CEUS, the mean confidence score of target lesions was 4.50 ± 0.55. Most of target lesions showed better identification of the active portion of target lesions on CEUS imaging (Fig. 3). Meanwhile, Wilcoxon signed rank tests showed that the confidence scores of the active portion of target lesions was significantly improved on CEUS compared to B-mode US (p < 0.05; Table 3). However, covariance analysis showed that the three BMI groups had no significant difference in confidence scores of the active portion of target lesions after the use of CEUS (p > 0.05; Table 4). In addition, the choice of biopsy location within the tumor was changed following administration of contrast agent in 19 cases.
Hepatic metastasis from cholangiocarcinoma in a 49-year-old man. (A) Axial contrast-enhanced CT showing a low-density nodule in segment 6 of the liver (arrow). (B) T2-weighted MRI showing a high signal nodule in the corresponding area of the CT image (arrow). (C) B-mode US showing a poorly defined focal lesion in the corresponding area of the CT image. (D) Split-screen mode showing a poorly defined focal lesion in the region (right) where the target lesion (left, arrow) is clearly located on the corresponding region. (E) Biopsy was performed under the real-time guidance of CEUS. The biopsy needle passes through the active site of the target lesion (left, arrow)
Final diagnosis and diagnostic performance
Final diagnosis in the 76 patients are listed in Table 6. The final diagnosis was as follows: malignant neoplasm (n = 69) and benign lesion (n = 7). Sixty-nine malignant neoplasms included primary hepatocellular carcinoma, primary cholangiocarcinoma, hepatocytes and cholangiocarcinoma, differentiated cancer, leiomyosarcoma, and metastatic cancer in 16, 8, 1, 7, 1, and 36 patients, respectively. Five cases were diagnosed initially as benign lesions, but according to clinical and radiological follow-up (at least 10 months), they were finally diagnosed as malignant tumors. In the current study, the sensitivity, specificity, accuracy, false positive rate, and false negative rate of CEUS-guided coaxial biopsy for the diagnosis of liver tumors were 92.8%, 100%, 93.4%, 0%, and 7.2%, respectively. Biopsy was performed on all 76 patients without major puncture-related complications requiring additional treatment or hospitalization.
Discussion
The correct diagnosis of FLL obtained by biopsy is critical in patients because misdiagnosis of a malignant lesion can potentially deprive patients of the opportunity for curative treatment. On the other hand, misdiagnosis of a benign lesion can lead to unnecessary invasive surgery and anxiety. Therefore, in clinical work, the choice of the appropriate biopsy technique is particularly important. Our current study found that most of target lesions showed better conspicuity and better identification of the active portion of the target lesion on CEUS imaging. Moreover, patients in the various BMI groups had different changes in the conspicuity of the lesion after using CEUS. The high BMI group had a greater change in the conspicuity of the lesion compared to the normal BMI group or the low BMI group. Moreover, the real-time CEUS-guided coaxial biopsy of FLL also has better diagnostic performance compared with the previous literature.
In recent years, there have been many studies that reported on the importance of biopsy under CEUS guidance. Goto et al. and Mishima et al. [13, 14] reported that CEUS using Sonazoid improved the detection rate of hepatocellular carcinoma and liver metastases. Francica et al. [15] reported that CEUS could be useful in assessing invisible or necrotic liver lesions. Kang et al. [16]. reported that real-time CEUS-Fusion imaging improved the lesion visibility and the viable portion assessment. Similarly, in the current study, the results showed that CEUS-guided biopsy increased the conspicuity and the accuracy of the assessment of the active site of FLL, especially, in patients with high BMI. Moreover, Sparchez et al. [1, 17] reported that CEUS-guided percutaneous biopsy could increase the accuracy of biopsy. Yoon et al. [18] reported the sensitivity in the diagnosis of malignancy was 88%. Sparchez et al. [4] reported the overall sensitivity of US guided biopsy in the tumor diagnosis was 90%. Park et al. [19] reported the technical success rate in the less-experienced radiologists was 90.9%. Kang et al. [20] reported the technical success rate of biopsy was 87.6%. Partovi et al. [21] reported a technical success rate in their study of 88.5%. Compared with the above mentioned results, the diagnostic efficacy in the current study is better. The sensitivity, specificity and accuracy of this technique in the current study were 92.8%, 100%, and 93.4%, respectively. Only 5 of 76 cases of biopsy were misdiagnosed as benign lesions after at least 10 months of follow-up.
In the current study, the operation was performed by using coaxial technique, in split-screen mode, and free-hand technique. As previously reported [22,23,24,25], co-axial technique has many advantages, such as easy positioning, shortened biopsy time, reduced bleeding, easy multi-needle sampling, and reduced chances of needle track planting. In the current study, there were no major puncture-related complications that required additional treatment or hospitalization. Yoon et al. [18] reported a mean and median time for their study of 11.7 and 8.7 min, respectively. In the current study, the mean and median times of CEUS-guided coaxial needle biopsy were 9.3 and 8 min, respectively. Thus, as mentioned earlier, this coaxial technique can save time. Secondly, the split-screen mode can simultaneously utilize the advantages of CEUS and B-mode US. The CEUS mode clearly delineates the tumor borders, but fails to show needles and tissue clearly. However, on the other side of monitor, the B-mode US display of the puncture needle shows good fundamental imaging, but the tumor display is not ideal due to absence of microbubble signal outline. Using a free-hand puncture technique, the operator can adjust the puncture angle in real-time without the limitation of the probe puncture bracket, and the cost can also be decreased. However, this technology requires the operator’s right and left hands to cooperate well.
This study has several limitations. First, the number of cases was limited. Therefore, the patients analyzed may not represent the overall population requiring CEUS imaging guidance. Second, there is inevitable subjectivity in evaluating the conspicuity and the confidence score of FLL due to lack of quantitative parameters. To reduce subjectivity, we used two independent radiologists to identify and assess target lesions. Third, tumor biopsy is performed for histologic diagnosis, and preferably also for mutational analysis. However, the current study did not include a mutational analysis. This would be especially pertinent for cases in which the CEUS led the operators to biopsy an area of more active contrast-enhancement. In the future, we plan to conduct a study which includes mutational analysis.
In conclusion, our results show that a coaxial biopsy technique under real-time CEUS guidance could enhance the conspicuity of target lesions and help identify the active portion of the target lesions. Therefore, it could be useful for FLL that are inconspicuous or cannot be accurately identified as an active site on B-mode US.
References
Sparchez Z, Radu P, Zaharia T, et al. (2010) Contrast enhanced ultrasound guidance: a new tool to improve accuracy in percutaneous biopsies. Med Ultrason 12(2):133–138
Caliskan KC, Cakmakci E, Celebi I, Basak M (2012) The importance of experience in percutaneous liver biopsies guided with ultrasonography: a lesion-focused approach. Acad Radiol 19(2):256–259
Kim JW, Shin SS (2017) Ultrasound-guided percutaneous core needle biopsy of abdominal viscera: tips to ensure safe and effective biopsy. Korean J Radiol 18(2):309–322
Sparchez Z, Radu P, Zaharia T, et al. (2011) Usefulness of contrast enhanced ultrasound guidance in percutaneous biopsies of liver tumors. J Gastrointest Liver Dis 20(2):191–196
Jung EM, Friedrich C, Hoffstetter P, et al. (2012) Volume navigation with contrast enhanced ultrasound and image fusion for percutaneous interventions: first results. PLoS ONE 7(3):e33956
Lee MW (2014) Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography 33(4):227–239
Min JH, Lim HK, Lim S, et al. (2014) Radiofrequency ablation of very-early-stage hepatocellular carcinoma inconspicuous on fusion imaging with B-mode US: value of fusion imaging with contrast-enhanced US. Clin Mol Hepatol 20(1):61–70
Minami T, Minami Y, Chishina H, et al. (2014) Combination guidance of contrast-enhanced US and fusion imaging in radiofrequency ablation for hepatocellular carcinoma with poor conspicuity on contrast-enhanced US/fusion imaging. Oncology 87(Suppl 1):55–62
Park HS, Kim YJ, Yu MH, et al. (2015) Real-time contrast-enhanced sonographically guided biopsy or radiofrequency ablation of focal liver lesions using perflurobutane microbubbles (sonazoid): value of Kupffer-phase imaging. J Ultrasound Med 34(3):411–421
Chung YE, Kim KW (2015) Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography 34(1):3–18
Piscaglia F, Nolsøe C, Dietrich CA, et al. (2012) The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 33(1):33–59
Lee MW, Kim YJ, Park SW, et al. (2010) Sequential changes in echogenicity and conspicuity of small hepatocellular carcinoma on gray scale sonography after transcatheter arterial chemoembolization. J Ultrasound Med 29(9):1305–1312
Goto E, Masuzaki R, Tateishi R, et al. (2012) Value of post-vascular phase (Kupffer imaging) by contrast-enhanced ultrasonography using Sonazoid in the detection of hepatocellular carcinoma. J Gastroenterol 47(4):477–485
Mishima M, Toh U, Iwakuma N, et al. (2016) Evaluation of contrast Sonazoid-enhanced ultrasonography for the detection of hepatic metastases in breast cancer. Breast Cancer 23(2):231–241
Francica G, Meloni MF, de Sio I, et al. (2017) Biopsy of liver target lesions under contrast-enhanced ultrasound guidance: a multi-center study. Ultraschall Med. https://doi.org/10.1055/s-0043-122496
Kang HJ, Kim JH, Lee SM, et al. (2018) Additional value of contrast-enhanced ultrasonography for fusion-guided, percutaneous biopsies of focal liver lesions: prospective feasibility study. Abdom Radiol (NY). https://doi.org/10.1007/s00261-018-1608-y
Sparchez Z, Radu P, Kacso G, et al. (2015) Prospective comparison between real time contrast enhanced and conventional ultrasound guidance in percutaneous biopsies of liver tumors. Med Ultrason 17(4):456–463
Yoon SH, Lee KH, Kim SY, et al. (2010) Real-time contrast-enhanced ultrasound-guided biopsy of focal hepatic lesions not localised on B-mode ultrasound. Eur Radiol 20(8):2047–2056
Park HJ, Lee MW, Lee MH, et al. (2013) Fusion imaging-guided percutaneous biopsy of focal hepatic lesions with poor conspicuity on conventional sonography. J Ultrasound Med 32(9):1557–1564
Kang TW, Lee MW, Song KD, et al. (2017) Added value of contrast-enhanced ultrasound on biopsies of focal hepatic lesions invisible on fusion imaging guidance. Korean J Radiol 18(1):152–161
Partovi S, Lu Z, Kessner R, et al. (2017) Contrast enhanced ultrasound guided biopsies of liver lesions not visualized on standard B-mode ultrasound-preliminary experience. J Gastrointest Oncol 8(6):1056–1064
Maturen KE, Nghiem HV, Marrero JA, et al. (2006) Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. Am J Roentgenol 187(5):1184–1187
Schulze R, Seebacher G, Enderes B, et al. (2015) Complications in CT-guided, semi-automatic coaxial core biopsy of potentially malignant pulmonary lesions. Rofo 187(8):697–702
Jandaghi AB, Habibzadeh H, Falahatkar S, et al. (2016) Transperineal prostate core needle biopsy: a comparison of coaxial versus noncoaxial method in a randomised trial. Cardiovasc Intervent Radiol 39(12):1736–1742
Jandaghi AB, Lebady M, Zamani AA, et al. (2017) A randomised clinical trial to compare coaxial and noncoaxial techniques in percutaneous core needle biopsy of renal parenchyma. Cardiovasc Intervent Radiol 40(1):106–111
Acknowledgments
We would like to thank all participants for their support in this study. No grant support needs to be reported.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work did not receive funding.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration, and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants prior to enrollment in the study.
Rights and permissions
About this article
Cite this article
Cao, X., Liu, Z., Zhou, X. et al. Usefulness of real-time contrast-enhanced ultrasound guided coaxial needle biopsy for focal liver lesions. Abdom Radiol 44, 310–317 (2019). https://doi.org/10.1007/s00261-018-1713-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1713-y