Abstract
Purpose
Interpretation of water-soluble contrast enema following laparoscopic low anterior resection can be very challenging for both radiologists and colorectal surgeons. Discriminating the radiological appearances secondary to anastomotic configuration from those caused by actual anastomotic dehiscence is a common problem and may be made worse with the advent of laparoscopic surgery. The aim of this study is to identify potential novel appearances of the water-soluble contrast enema (WSCE) images of rectal anastomosis following laparoscopic low anterior resection to radiologists and surgeons.
Methods
We enrolled 45 patients who underwent laparoscopic low anterior resection with proximal de-functioning loop ileostomy within a specialized colorectal unit. The water-soluble contrast enema reports were reviewed. Two blinded colorectal radiologists independently reviewed the images of patients suspected of anastomotic leak. All of these patients also underwent a flexible sigmoidoscopy to confirm or exclude anastomotic leak before reversal of loop ileostomy. Inter-observer concordance was calculated.
Results
Seven out of eighteen patients (38.9%) were found to have true anastomotic leaks on flexible sigmoidoscopy (15% overall leak rate). In the remaining eleven patients the image appearances were attributed to the appearance of the anastomotic ‘dog-ear effect’, created by the anastomotic configuration due to multiple firing of the intra-corporeal laparoscopic stapling device. Radiologist inter-observer concordance was 83%. Sensitivity was 100%, specificity 71%, positive-predictive value (38.9%) and negative-predictive value (100%).
Conclusions
The novel appearances of laparoscopic-stapled rectal anastomoses in WSCE can be mistaken for anastomotic leak. To avoid delay in reversal of ileostomy, a flexible sigmoidoscopy can be used to confirm or exclude a leak.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Leakage of low rectal anastomosis after open or laparoscopic surgery occurs in about 10%–15% of patients following surgery for cancer [1]. Anastomotic leakage is associated with increased local recurrence and diminished survival after colorectal cancer surgery [2, 3]. The vast majority of surgeons choose to protect low rectal anastomoses with a defunctioning loop ileostomy. The diversion of faeces in itself does not reduce anastomotic leak rates but significantly mitigates the consequences, which include mortality of 10%–15% [4, 5].
Provided there are no contraindications, the ileostomy would be closed 8–12 weeks post-operatively. It is common practice to perform a water-soluble contrast enema (WSCE) to assess the integrity of low rectal anastomoses prior to closure of a defunctioning ileostomy. The interpretation of WSCE or CT images with the advent of intracorporeal laparoscopic stapling techniques, sometimes involving multiple staple lines, can be challenging; in particular discriminating radiological appearances secondary to anastomotic configuration abnormalities from those that are caused by actual anastomotic dehiscence. With interval repeat examinations, the ‘radiological leak’ may resolve or persist, posing a huge challenge to the surgeon faced with a patient keen on stoma reversal. This suspected leak in WSCE is exaggerated by the configuration of the laparoscopic intracorporeal low rectal anastomosis (multiple firing) as opposed to open low anterior resection (single firing).
Unfortunately the use of more than one linear firing can exaggerate a wedged anastomosis (dog-ear). The appearance of this anastomotic dog-ear can be mistaken for anastomotic leak. Radiologists may not be aware of this enhanced appearance following laparoscopic low anterior resection (LapLAR). In open LAR almost always one stapler is required to transect the distal rectum, so this misleading appearance is often not seen. This potential WSCE mis-interpretation has not been reported to date and we believe recognition of this will help to reduce the rates of false positive reports and improve patients’ experience by timely reversal of the defunctioning loop ileostomy.
Methods
Setting
A retrospective review of a prospectively maintained database in a high-volume specialized colorectal unit. This identified 45 consecutive patients between 2007 and 2013 who underwent laparoscopic low anterior resection (LapLAR), where the colorectal anastomosis was below peritoneal reflection. All patients had a proximal defunctioning loop ileostomy created at the time of surgery.
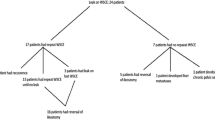
Study protocol (Fig. 1)
Following surgery, a WSCE was performed between 8–12 weeks to assess the anastomosis. Patients who had normal WSCE reported by Consultant Radiologists proceeded to reversal surgery. Where the WSCE report identified a suspected leak, as per departmental policy, these cases underwent flexible sigmoidoscopy to correlate with the radiological findings. Two Consultant Radiologists with a specialist interest in colorectal gastrointestinal radiology and are members of the colorectal cancer multidisciplinary team then independently reviewed the original blinded WSCE images of these patients with suspected leaks. The radiologists independently reported their conclusions, and their reports were then compared for inter-observer concordance and then with flexible sigmoidoscopy findings to confirm or exclude anastomotic leak before ileostomy closure. Criteria used at WSCE for the diagnosis of an anastomotic leak was the presence of contrast in a location not conforming to the expected bowel lumen (Fig. 2).
To differentiate an anastomotic ‘dog-ear’ from true anastomotic leak following laparoscopic low anterior resection, the following criteria were used at flexible sigmoidoscopy: presence of a defect visualised at flexible sigmoidoscopy of a calibre that would not permit the flexible sigmoidoscope; presence of a similar adjacent defect; absence of significant surrounding tissue induration; easy passage of the flexible sigmoidoscope into the proximal true colonic lumen; and no history of associated symptoms of ongoing discharge or perianal pain before ileostomy closure.
Procedure: anastomosis (Fig. 3)
Following mobilisation of the left colon and laparoscopic total mesorectal excision (TME) the distal rectum is divided intracorporeally using 1–3 firings of the Ethicon Echelon™Flex ® or Covidien EndoGIA ® nonvascular roticulating stapler (45/60 mm roticulated). A straight end-to-end anastomosis is then constructed using a circular stapling device (Ethicon CDH 29 mm).
Appearances of Anastomosis by Stapling Technique. A Shows open end-to-end anastomosis with single firing with circular stapler. B Shows open end-to-end anastomosis with single firing of linear stapler followed by single firing of circular stapler. C Shows laparoscopic end-to-end anastomosis with dog-ear created with double firing of linear stapler followed by single firing of circular stapler
Procedure: water-soluble contrast enema
Prior to reversal of the loop ileostomy all patients underwent WSCE which was performed with the patient in left lateral decubitus position. A size 18–22 Channel Foley Catheter was inserted per anus into the rectum distal to the stapled anastomosis without inflating the balloon. 300 mls Gastrograffin diluted with 300 mls water was instilled using a controlled instillation from a hanging bag via the Foley catheter. No air insufflation, no added pressure was used. The contrast was instilled under fluoroscopic vision acquiring hard images of the anastomosis.
Results
Forty-five consecutive cases were available for analysis; all procedures were carried out for rectal cancer. Median age was 66 years (age range: 45–82 years); gender balance was 78% male/22% female; and preoperatively 18 patients had short course radiotherapy (40%), 5 patients had long course chemoradiotherapy (11%), and 22 patient had no preoperative oncological intervention (49%). All resections were R0 (complete excision) with a median distance from anal verge of 8 cm on MRI (distance range: 2.4–18.5). Fifteen patients received postoperative chemotherapy (33%).
Eighteen patient (40%) had WSCE reported as suspected leak. Seven of the eighteen patients (38.9%) were confirmed as having a true leak after flexible sigmoidoscopy, giving an overall anastomotic leak rate of 15.6% following LapLAR (7/45). The suspected reported leak in the remaining eleven patients was attributed at flexible sigmoidoscopy to the appearance of the anastomotic ‘dog-ear’. This anomaly was created by the anastomotic double-stapled configuration which may be exaggerated in laparoscopic surgery due to angled multiple-firing of the intra-corporeal laparoscopic stapling device (Figs. 4, 5, 6, 7 and 8).
Appearances of WSCE images (A) and (B); and Low Rectal Anastomosis on Flexible Sigmoidoscopy (C). Figure 4 A and B showing the normal appearance of WSCE with no leak with rectal catheter in place, Figure 4 C is the endoscopic appearance of normal low rectal anastomosis. Figure 5 A, 6 A, and 7 A WSCE demonstrating a questionable anastomotic leak. Figure 5 B, 6 B, and 7 B further views from same patients with WSCE demonstrating the dog-ear radiological appearance with a smooth outline. Figure 5 C, 6 C, and 7 C is the endoscopic appearance of the dog-ear/normal intact anastomosis with no leak
A Laparoscopic view of the distal rectal transection using EndoGIA 45 stappler. The white open arrow is the staple line from the first EndoGIA firing, The EndoGIA stapler doing the second firing. B Laparoscopic view showing distal rectal staple line after transaction of the distal rectum. The white circle showing the site of the colorectal anastomosis before using the circular stapler. The white solid arrows is the areas where the potential dog-ear is created and mistaken for a leak
The sensitivity of WSCE was 100% for true anastomotic leak (for example, Fig. 2), but only 71% specific. Given that 38.9% of WSCE with suspected leaks actually had true anastomotic leaks, the positive predictive value is rather low, however the negative predictive value of 100% is reassuringly high, indicating that no true leaks were missed.
Between the independent blinded Consultant Radiologists who reviewed the WSCE images with suspected anastomotic leaks, there was inter-observer concordance in 83% (15/18) of cases (Fig. 9).
Discussion
Division of the distal rectum using laparoscopic intra-corporeal stapling devices can be technically demanding due to the confinement of the pelvis, its width (particularly in male patients), and limited roticulation (rotation of an articulated instrument) of the devices [6]. The rectal stump often ends up wedged due to multiple firings of the stapler. It is this novel configuration that is thought to be responsible for the exaggerated ‘dog-eared’ appearance of the anastomosis compared to open surgery (Fig. 3) and is often misconstrued as an anastomotic leak.
In this series, flexible sigmoidoscopy was carried out routinely for those with suspected leaks on WSCE to compare to the radiological findings. The anastomosis was found to be intact in most cases (61.1%) but showed a characteristic rectal transection stapling effect at opposite sides of the circular staple line at the site of the anastomotic “dog-ear”. Figures 4, 5, 6, and 7 illustrate the findings of the contrast enemas alongside the endoscopic appearance of the anastomosis. Figure 8A and B illustrate the line of transection using an intracorporeal stapler with a maximum angulation of 45°. This would usually require two firings of the stapler resulting in the wedging that is illustrated.
It is now widely accepted that though ‘protecting’ a low rectal anastomosis with an ileostomy does not reduce leak rates, the morbidity and mortality are reduced. Though ileostomy closure is not in itself a technically demanding operation, it is fraught with complications and up to 25% of patients develop complications [7]. Water-soluble contrast enema has been shown to be superior to computerised tomography (CT) in the diagnosis of anastomotic leakage especially for low rectal anastomosis [8]. In this study, the high sensitivity and relatively low specificity are similar to those found by Karsten et al. [9], however the high negative predictive value and high sensitivity give support to the fact that leaks are effectively detected by this WSCE technique. However, the technique has limitations including subjectivity of image interpretation and variability in technique between radiologists. Our study however shows relatively high inter-observer reliability of 83%. To differentiate a true anastomotic leak from a ‘dog-ear’ at WSCE following laparoscopic low anterior resection it is essential for the reporting radiologist to have a detailed understanding of the anatomy of the anastomotic configuration created at the original surgery (open vs. laparoscopic as well as end-end vs. end-side) prior to embarking on the water soluble contrast enema examination (Fig. 3). Contrast in a location that does not conform to the expected course of the described bowel lumen can then be considered suspicious of a true anastomotic leak (Fig. 2). Alternatively, a ‘dog-ear’ may be suspected if the WSCE image is in keeping with a laparoscopic anastomosis (Fig. 3C).
Many surgeons question the need for contrast enemas prior to reversal of ileostomy. A recent systematic review and meta-analysis by Habib et al. [10] concluded that WSCE is effective in excluding clinically significant anastomotic leak, however, false positive results can be observed in asymptomatic patients. From this systematic review it was unclear whether these false positives were in patients who underwent laparoscopic low rectal anastomosis or open anastomosis. Our study may provide an alternative hypothesis for the cause of the false positives in laparoscopic rectal anastomosis due to the formation of the exaggerated anastomotic ‘dog-ear’.
There is evidence that radiological leak rates are very low (0–6%) in uncomplicated patients [11]. There is also evidence that a proportion of patients with low rectal anastomosis do not have subsequent closure of their ileostomies possibly due to suspected radiological leak [12]. It would therefore seem reasonable to offer further investigation in the form of flexible sigmoidoscopy in this group of patients to prevent delay in reversal of ileostomy in asymptomatic patients created by a suspected radiological leak on WSCE.
Conclusion
Contrast enemas remain a useful tool in the diagnosis of anastomotic leakage following low rectal surgery. Their interpretation in laparoscopic surgery may prove more challenging because of the novel appearances of laparoscopic-stapled rectal anastomoses which can be easily mistaken for anastomotic dehiscence. To avoid delay in reversal of ileostomy, clarity regarding anastomotic configuration is necessary when reporting WSCEs and a flexible sigmoidoscopy can be recommended when in doubt to confirm or exclude the presence of an anastomotic leak. As laparoscopic low anterior resection with intracorporeal anastomosis is gaining preference in the management of rectal cancer, so has the confidence of radiologists in reporting water-soluble contrast enemas. Correlation with the clinical picture, flexible sigmoidoscopy and communication between radiologist and surgeon is of paramount importance.
References
Taflampas P, Christodoulakis M, Tsiftsis DD (2009) Anastomotic leakage after low anterior resection for rectal cancer: facts, obscurity, and fiction. Surg Today 39(3):183–188
Walker KG, Bell SW, Rickard MJ, et al. (2004) Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg 240:255–259
McArdle CS, McMillan DC, Hole DJ (2005) Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg 92:1150–1154
Den Dulk M, Marijnen CAM, Collette L, et al. (2009) Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg 96(9):1066–1075
Bokey EL, Chapuis PH, Fung C, et al. (1995) Postoperative morbidity and mortality following resection of the colon and rectum for cancer. Dis Colon Rectum 38:480–487
Brannigan AE, De Buck S, Suetens P, Penninckx F, D’Hoore A (2006) Intracorporeal rectal stapling following laparoscopic total mesorectal excision: overcoming a challenge. Surg Endosc 20(6):952–955
Williams LA, Sagar PM, Finan PJ, Burke D (2008) The outcome of loop ileostomy closure: a prospective study. Colorectal Dis 10(5):460–464
Nicksa GA, Dring RV, Johnson KH, et al. (2007) Anastomotic leaks: what is the best diagnostic imaging study? Dis Colon Rectum 50(2):197–203
Karsten BJ, King JB, Kumar RR (2009) Role of water-soluble enema before takedown of diverting ileostomy for low pelvic anastomosis. Am Surg 75(10):941–944
Habib K, Gupta A, White D, Mazari FA, Wilson TR (2015) Utility of contrast enema to assess anastomotic integrity and the natural history of radiological leaks after low rectal surgery: systematic review and meta-analysis. Int J Colorectal Dis 30(8):1007–1014
Khair G, Alhamarneh O, Avery J, et al. (2007) Routine use of gastrograffin enema prior to the reversal of a loop ileostomy. Dig Surg 24(5):338–341
Lewis OA, McCallum IJ, Dixon S, Katory M (2015) Long-term -ostomy as a quality marker: comparison of outcomes from a 6 year series of laparoscopic surgery in MRI defined low rectal cancer. Int J Sur 23:108–114
Acknowledgments
Dr. Christopher Dennison, Consultant Radiologist, Queen Elizabeth Hosital Gateshead for the application of the water-soluble contrast enema technique.
Authors contribution
Mark Katory MD FRCS: Conception and design, acquisition of data, analysis and interpretation of data; and final approval. Ross McLean MRCS: Interpretation of data; and final approval. Khalid Osman PGCMEd FRCS: Conception and design, drafting the article and final approval. Mukhtar Ahmad, MMedSci, FRCS: Conception and design, acquisition of data, drafting the article and final approval. Tracey Hughes FRCR: Acquisition of data and revising it critically and final approval. Mike Newby FRCR: Acquisition of data and revising it critically and final approval. Christopher Dennison FRCR: Acquisition of data and revising it critically and final approval. Paul O’Loughlin FRCS: Analysis and interpretation of data; revising it critically and final approval.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no funding. There are no financial interests for any author in this manuscript.
Conflict of interest
There are no disclosures for any of the authors involved in this study.
Ethical approval
The requirement for review by the Medical Ethics Committee or informed consent was waived because of the retrospective nature of this study with preexisting anonymized data.
Rights and permissions
About this article
Cite this article
Katory, M., McLean, R., Osman, K. et al. The novel appearance of low rectal anastomosis on contrast enema following laparoscopic anterior resection: discriminating anastomotic leaks from “dog-ears” on water-soluble contrast enema and flexible sigmoidoscopy. Abdom Radiol 42, 435–441 (2017). https://doi.org/10.1007/s00261-016-0885-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0885-6