Abstract
Background
To compare magnetic resonance enterography (MRE) and computed tomography enterography (CTE) in detecting inflammatory bowel disease activity (IBD) in patients with Crohn’s disease (CD).
Methods
A total of 29 patients (M 20; F 9; mean age 43.8 ± 15.9) with known CD underwent MRE. MRE was performed at 1.5 T using phased-array sense body coil, after oral administration of 1.5–2 L of PEG solution as oral contrast agent. MRE protocol included T1-weighted, sSShT2, sBTFE and gadolinium-enhanced THRIVE sequences acquired on coronal and axial planes. CTE was performed using a 16 multidetector-row computed-tomography before and after intravenous administration of 120 mL of iodinated contrast. MRE images and CTE scans were reviewed by a radiologist for bowel thickness and enhancement, mesenteric lymph nodes, vascular engorgement, fibrofatty proliferation, fistulas and abscesses. The disease activity was also defined by CDAI > 150.
Results
MRE has demonstrated a good sensitivity in detection of CD activity, particularly in depiction of mural thickening, mural enhancement, and vascular engorgement. The level of agreement between the two technique was excellent in evaluating wall thickening with mucosal hyperenhancement (κ = 1), comb (κ = 0.90) and halo signs (κ = 0.86). In detecting fibrofatty proliferation and mesenteric lymph nodes, CTE was superior to MRE (accuracy: P < 0.05), while MRE was superior in visualization of fistulas.
Conclusion
MRE is an accurate method in monitoring the activity of CD as compared to CTE and may be considered an alternative to CTE in assessing degree of CD and evaluating therapeutic effectiveness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Crohn’s disease (CD) is an autoimmune disorder of unknown origin that mainly affects young population with a chronic and relapsing course [1]. It can affect any part of the gastrointestinal tract, often involving multiple discontinuous sites [2]. Assessment of inflammation activity is important in order to determine the optimal medical or surgical therapy, while patient monitoring is essential in order to evaluate the efficacy of treatment. Identification of location of fibrotic restricted lumen tracts and evaluation of prestenotic dilation may help surgeons to plan intervention [1].
Assessment of activity is usually made using a combination of clinical symptoms, physical findings, laboratory investigations, endoscopy, and imaging tests. A variety of imaging tools is also available in current clinical practice. Such imaging techniques should ideally be able to demonstrate the extent, evaluate the severity, reveal the complications and identify the subtypes of the disease [3]. Enteroclysis is widely recognized as the most reliable method for imaging evaluation of small intestinal CD; however, its main limitation still remains its inability to demonstrate associated extraenteric pathology [3, 4].
The role of cross-sectional imaging in the diagnosis of Crohn disease has expanded with recent technologic advances in computed tomography (CT) and magnetic resonance (MR) imaging that allow rapid acquisition of high-resolution images [2].
During CT or MR imaging, the intestines should be distended with a fairly large amount of intraluminal contrast material for better visualization of the anatomy and of morphologic changes caused by the disease. Contrast material is injected intravenously to demonstrate the presence of lesions and to help assess their inflammatory activity [2].
CT enterography differs from routine abdomino-pelvic CT in that it makes use of thin sections and large volumes of enteric contrast material to better display the small bowel lumen and wall. The use of neutral enteric contrast agents such as water, combined with the use of intravenously administered contrast material, permits excellent assessment of hypervascular lesions and hyperenhancing segments [5]. In recent years, MRI has emerged as a diagnostic alternative applied to CD due to new pulse-sequence developments, along with the lack of ionizing radiation, which makes it more favorable to the usually young patient population. Different studies have confirmed the capability of MRI to detect pathologic bowel segments with high sensitivity and specificity. This technique plays an important role in diagnosing complications such as fistulas and abscesses [6, 7]. Nevertheless, few studies have investigated MRI’s capability to assess treatment response after a relapse of CD [8]. Recent advances in MR imaging, including the routine use of breath-hold imaging, fat suppression, and the addition of intravenous and oral contrast materials, have improved image quality and the depiction extrahepatic disease and GI tract diseases [6, 9–12]. Various contrast agents have been proposed for small bowel MRI applications [13, 14]. The most important characteristics of an intraluminal contrast agent suitable for intestinal application may be summarized as follows: uniform and homogeneous lumen opacification, a high contrast resolution between the lumen and bowel wall, a lack of significant adverse effects and low cost [3].
The purpose of our study was to compare the agreement of magnetic resonance enterography (MRE) and CT enterography (CTE) in detecting and quantifying inflammatory bowel disease activity (IBD) in patients with CD.
Materials and methods
Study design
The study protocol was approved by our institutional review board, and informed consent was obtained from each participant.
We undertook a prospective study of 29 clinically symptomatic patients (20 M, 9 F, range 14–70 years old) with previously proven CD who were suspected of having relapse of CD and were referred for enterographic study imaging by gastroenterologists. The symptoms ranged widely from mild, nonspecific abdominal discomfort and dyspepsia to frequent bloody diarrhea and weight loss. The study population reflected a cross section of patients who are undergoing regular follow-up in the gastroenterology outpatient clinic.
At enrolment, clinical activity was evaluated using Crohn’s Disease Activity Index (CDAI) (active disease CDAI > 150) and laboratory tests, such as CRP.
The inclusion criteria were (a) a known CD biopsy proven with clinical suspicion of relapse, (b) CDAI > 150, and (c) at least one elevated acute phase reactant (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP] active > 5 mg/dL). The exclusion criteria were (a) contraindications for MRI (electrically, magnetically, or mechanically activated devices; central nervous system hemostasia clips, or the inability to administer a gadolinium contrast agent because of known allergic problems), (b) pregnancy, (c) renal insufficiency, and (d) documented adverse reaction to iodinated contrast material.
A solution of PEG was given to the patients to distend the bowel loops. Using PEG electrolyte solution, we typically administer a total of 1500–2000 mL within 60 min prior to scanning, with the first 500 mL ingested over the first 15 min and two 500-mL aliquots consumed 25 and 15 min prior to scanning, respectively, to obtain a sufficient luminal distension and to guarantee an accurate detection of the lesions.
MRI technique
The MR enterography was performed by using a 1.5 T magnet (Philips, Achieva MR system), with a phased array body coil and the exam was acquired with patients in prone position.
To reduce bowel peristalsis and related motion artefacts, 20 mg of N-butyl-scopolamine (Buscopan; Boehringer, Ingelheim, Germany) were i.v. administered immediately before imaging.
After acquiring standard three-plane scout images, the precontrast protocol included the following sequences: transverse and coronal single shot fast SE MR images with fat saturation (TR infinite; TE 100 ms; matrix 4 mm thickness with no gap; FOV 400; matrix 210 × 250) were acquired during breath hold; coronal and axial BTFE sequences (TR 447 ms; TE 100 ms; matrix 256 × 180; 6 mm thickness with no intersection gap; FOV: 395); coronal and axial pre-contrast 3D T1 THRIVE (T1 High Resolution Isotropic Volume Examination) gradient echo sequences with fat saturation (1 mm thickness; TR 4 ms; TE 1.9 ms; matrix 192 × 144; FOV: 400) repeated also after intravenous administration of gadolinium DTPA.
The arterial phase MR imaging was initiated immediately after the visual detection of contrast material at the descending aorta by using a real-time bolus displayed method; the venous phase was performed with a fixed image delay of 70 s, followed by delayed transerve imaging at 2 min and subsequent coronal imaging at 3 min after contrast agent injection.
The table time for an MR enterographic examination was less than 30 min for all of the cases.
CT technique
The time between the completion of MR-enterography and the initiation of CT-enterography was less than 10 min.
Contrast-enhanced CT was performed by using 270 mA, 120 kVp, 0.5-s tube rotation time, and 120 mL of intravenous contrast material (Xenetix 350; Guerbet, Aulnay, France) containing 350 mg of iodine per milliliter. The contrast agent was injected at a rate of 3.5 mL/s, with scanning initiated after a 70 s delay.
A 2-mm section thickness was used, and images were reconstructed every 1 mm. CT scans are obtained from the dome of the liver to the level of the perineum to cover the entire course of the intestine. Imaging with the patient in the prone position was obtained to disperse the small bowel loops. Coronal reformatted images are generated at the workstation from the axial images.
Image analysis
MR and CT images were interpreted by a radiologist who reached a consensus without being aware of the clinical scoring. MRI features that are reported to reflect or measure disease activity included mucosal hyperemia, intramural edema, wall thickening and enhancement, and mesenteric involvement, in terms of either vascular engorgement (the comb sign) or the presence of inflammatory mesenteric lymph nodes. A number of studies suggest that the activity of CD can be assessed using such MRI and CT parameters.
The following cross-sectional imaging findings were analyzed at both CT and MR imaging: (a) small bowel wall thickening (i.e., mural thickness greater than 3 mm); (b) mural stratification, known as “halo sign” (i.e., visualization of two or three layers of the bowel wall); (c) mural hyperenhancement (i.e., segmental regions of increased mural enhancement compared with enhancement in the adjacent bowel); (d) fibrofatty proliferation (soft-tissue stranding in the perienteric mesenteric fat, along the mesenteric border of bowel segments affected by Crohn disease); (e) comb sign (is created by engorged vasa recta, vessels that penetrate the bowel wall perpendicular to the bowel lumen) that has been described previously as well-known signs of Crohn disease correlating with active mucosal and mural inflammation; (f) lymph nodes enlargement; and (g) presence of fistulas and abscesses.
According to previous literature [15], after reviewing the images, the radiologist reached agreement as to the presence or absence of active disease in each patient. A patient was deemed to have active disease if one or more inflamed bowel loops were identified that showed abnormal wall thickening (>3 mm) and increased bowel wall enhancement, with or without perienteric changes.
Statistical analysis
Cohen’s kappa was used to summarize the agreement between MRE and CTE. The 95% confidence intervals (CI) for the Cohen’s kappa index were also reported. The McNemar test was considered in order to compare the marginal distributions of the proportions of findings obtained with the two techniques. The test was two-sided test, with a significant level of 0.05.
Results
MRE and CTE were well tolerated by all the patients without clinically significant side effects occurring in either adults and/or pediatric population. Only in three cases we observed a mild diarrhea, and the motion artefacts were more severe for MR enterography than for CT enterography.
As shown in Table 1, of the 29 patients who underwent a combined transaxial study, CD was classified as active both by MRE and CTE in 19 patients (65.5%) and inactive in the remaining 10 patients. MRE completely agreed with CTE in the classification of disease activity (κ = 1). Of the seven visual markers considered, the most frequent in the detection of active CD was mural hyperenhancement combined with mural thickening. The level of agreement between the two techniques was excellent in evaluating wall thickening with mucosal hyperenhancement (κ = 1), comb (κ = 0.90) and halo signs (κ = 0.86).
The number of depicted fistulas was higher with MRE compared to CTE (P = 0.083). In particular, MRE revealed five fistulas while CTE only two (κ = 0.52). An important sign better depicted in CTE study than MRE was mesenteric lymphadenopathy. CTE showed the presence of lymph node enlargement in 15 patients, despite the 12 of MRE (P = 0.083). A significant difference was observed in the detection of fibrofatty proliferation (P = 0.045).
Of overall seven sectional imaging findings analyzed for all of the patients in 13 cases, as shown in Table 2, we obtained discordant findings between the two different techniques employed.
Discussion
The assessment of CD activity is a clinical issue of paramount importance as it may categorize which patients will benefit from prompt and optimal treatment. It is currently accomplished by clinical and/or laboratory parameters, although no single test has been established as the gold standard. The most widely used method in everyday clinical practice or in therapeutic trials is CDAI [3]. The CDAI, used as a clinical reference for disease activity [16], provides an overall assessment of the clinical status, irrespectively from the site and activity of disease, even though it is often influenced by subjective factors [1].
Radiologic evaluation, however, remains particularly important, especially when involvement is confined to the mesenteric small intestine between the ligament of Treitz and the ileocecal valve, because this part of the GI tract is not generally available endoscopically. Enteroclysis has been considered the technique of choice for the evaluation of Crohn disease of the small intestine. Adequate distention of the entire small bowel is a key requirement because collapsed bowel loops may hide or simulate small bowel disease. However, conventional enteroclysis has two major disadvantages: the limited information concerning extramural extension of Crohn disease and its complications and the radiation dose administered to patients, mostly at young age [17].
In a recent study, Amitai et al. [18], in a comparative study between MSCT and bowel follow-through in the evaluation of patients with CD, have shown how MSCT could demonstrate the precise location of a dominant stricture, its length, and its adjacent complications, having the advantage of showing the colon and potential extra-enteric complications. It was concluded that the bowel follow-through has no added value in CD patients and can be omitted in patients who have been already studied by MSCT, being the modality of choice in imaging all patients with CD at all stages of the disease.
CT and MR presented an increasing role in defining intra-, trans- and extra-parietal lesions; however, CT induces exposure to ionizing radiation, which is of extreme importance when considering that examinations are often performed during follow-up and that patients are predominantly young. On the contrary, MRI has been advocated as a non-invasive tool in the diagnostic work-up of CD [1].
CT and MR imaging with intraluminal and intravenous contrast material are limited in the depiction of subtle mucosal lesions but provide excellent visualization of most intestinal lesions and demonstrate their mural and extramural extent. Bowel obstruction, strictures, abscesses or phlegmon, fistulas, and sinus tracts are common complications of advanced disease [2]. In the clinical setting, CT is currently the imaging modality of choice at most institutions; however, MR imaging is expected to soon play a similar role. CT or MR imaging should be included in a comprehensive evaluation of patients with Crohn disease, along with conventional imaging and clinical and laboratory tests [2].
CT enterography is a relatively new imaging technique that combines the improved spatial and temporal resolution of MDCT with large amounts of ingested neutral contrast material. Unlike routine CT, which uses high-density oral contrast material, CT enterography uses a low-density or neutral oral contrast material, allowing the detection of the abnormal mucosal enhancement that is common in CD [19].
MRI is able to detect significant variations in bowel wall thickness and contrast enhancement, reflecting favorable clinical response to medical treatment of CD’s relapse. In addition to its lack of ionizing radiation, this may allow MRI to be the imaging technique of choice for the follow-up of patients with active CD [8]. Optimum small bowel imaging should include fast and ultrafast pulse sequences. The spatial resolution of these sequences should be high enough to permit the demonstration of small lesions, such as ulcers or mucosal nodularity.
Small bowel wall contrast uptake is considered to be the most important indicator of disease activity [16, 20, 21] and can be appreciated on T1-weighted three-dimensional THRIVE images. Wall thickening, significant enhancement of the mucosa and relatively hypointense submucosal edema have been reported as common findings on post-gadolinium THRIVE (Fig. 1) images in active CD [3]. As feces can show bright signal intensity on T1-weighted sequences, it is important to perform a pre-contrast T1-weighted sequence in order to be able to determine whether high signal intensity was already present before intravenous contrast administration, indicating the presence of stool [22]. Luminal narrowing and associated pre-stenotic small bowel dilatation are easily recognized with all sequences. MRE has been reported to be in full agreement with conventional enteroclysis in detecting, localizing and estimating the length of all involved small bowel segments and in assessing thickening of bowel wall, luminal narrowing or high-grade stenosis [3].
Active CD in terminal ileum in a 35-year-old man. A axial venous phase gadolinium-enhanced T1 fat suppressed gradient echo images (THRIVE) shows mural hyperenhancement, mural stratification (halo sign) and wall thickening in terminal ileum (arrow). B axial CT enterographic image in portal venous phase at the same plane shows same findings of active terminal ileitis.
These small bowel attenuation patterns are important for radiologists to recognize and to take into consideration when evaluating patients for active Crohn disease, because segmental mural hyperenhancement at CT and magnetic resonance (MR) imaging is indicative of active inflammation [8, 23, 24].
Our findings confirm the previous ones suggesting that CT and MR enterography have a similar accuracy in the identification of active CD of small bowel, but conflicting results have been reported for the detection of fistulas and detection of pathologic lymph nodes, in particular, in our findings, the MR enterography is more accurate than CT enterography in detection of enteric fistulas and sinus tracts (Fig. 2), while CT enterography is more sensitive in depiction of mesenteric pathologic lymph nodes (Fig. 3), in relation to the greater motion artefacts of MRE than CTE.
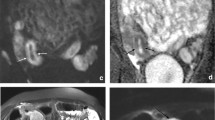
Perianal fistula in a 40-year-old woman. A axial CT enterographic image in portal venous phase does not show a significant finding except a focal thickening (arrow) or right anal wall. B T2 weighted fat-saturation axial image at the same plane shows, clearly, an hyperintense fistulous tract of anum of lateral wall (arrow).
Active Crohn lesions at the distal ileum in a 28-year-old woman. A axial venous phase gadolinium-enhanced T1 fat suppressed gradient echo image shows bowel wall thickening at the distal ileum (arrowheads). Increased mesenteric vascularity with engorgement of vasa recta (“comb sign”) is noted around the involved segment (arrow) and presence of multiple small and large-sized mesenteric lymph nodes (curved arrow). B Same findings are observed in the coronal reformatted 3-mm-thick images at CT enterographic study in portal venous phase.
Both CT and MR imaging demonstrate fibrofatty proliferation (Fig. 3), which has slightly increased CT attenuation and slightly decreased MR imaging signal intensity compared with normal fat separating the bowel loops. Fistulas (Fig. 4) are well demonstrated at fat-saturated T2-weighted MR imaging and can be distinguished reliably, which aids in management planning.
Our findings confirm those of Koelbel et al., that is the extramural complications are well shown on CT, although the lower contrast resolution of CT makes this modality less suitable for the detection of fistulas and abscesses than MRI in patients with CD [5].
In only one small study, by Wold et al., CT enteroclysis and CT enterography were compared, but both the degree of luminal distension and the diagnostic accuracy did not show significant differences.
Similar results have been reported by Hara et al. [19] in a preliminary study where CTE was used in the monitoring of CD progression or regression, to attempt the distinction of symptoms of CD from functional symptoms and to assess the complications of disease.
In our study, MR imaging proved to be useful in assessing the activity of CD, and in accordance with the report of Koh et al. [15] bowel thickening of more than 3 mm, increase in bowel wall enhancement, and increased mesenteric vascularity may be useful discriminatory signs of active disease. In addition, a layered enhancement pattern after the IV administration of gadolinium appears to be highly specific for active disease (Fig. 1).
The data in our series of patients confirm the previous study of Low et al. [6] where the MR technique was preferred to CTE in distinguishing liquefactive fluid collections, which is an important point in the assessment of surgery or interventional treatment.
Advantages of CT enterography for the evaluation of CD include its non-invasive nature, fast examination time (<1 min), and multiplanar projections. The multiplanar projections of CT enterography improve anatomic presentation of the bowel and can help clarify the presence of extramural complications.
However, the patient’s exposure to ionizing radiation limits its excessive use, according to patient age and the relapsing nature of the disease.
MR enterography has several advantages, including the lack of ionizing radiation and the ability to give gadolinium contrast in patients with iodinated contrast allergies. The main limitations of MR enterography are its high cost and its lesser availability. CT enterography is more widely available, cheaper, and faster (20 s scanning time vs. 30 min with MR enterography).
Thus, MR enterography is likely considered the best test for young patients because of the radiation dose saving, while in older patients at decreased risk from radiation exposure, the benefit of MR enterography is debatable.
Our results show that MRI clearly allows the distinction of pathologic from normal bowel wall in CD, as it detects significant variations in bowel wall thickness with clinical improvement and is able to reflect pathologic inflammatory changes of the bowel wall based on variations in the CE. Consequently, this technique is reliably applicable to the follow-up of patients with CD. More extensive series should be investigated that involve defined disease patterns (fibrosing, inflammatory, fistulizing) to more precisely define the role of MRI in patients with CD.
High soft-tissue contrast, static and dynamic imaging capabilities, and the absence of ionizing radiation exposure represent advantages of MR imaging over CT.
The main problem in this study, as in previous studies on CD, is that an ideal reference standard method to define disease activity, during the follow-up of patients, does not exist, but it is usually assessed by a combination of clinical symptoms, physical findings, laboratory investigations, and imaging tests. Abdominal symptoms may be non-specific and can result from active inflammation or from fibrotic scarring and stricture formation. Therefore, the CDAI, used as a clinical reference for disease activity, provides an overall assessment of the clinical status, irrespectively from the site and activity of disease, even though it is often influenced by subjective factors [25].
The limitations of our study include the relatively small number of patients, who were limited to symptomatic individuals.
Advantages of CT over MR imaging include greater availability, shorter examination times, flexibility in choosing imaging thickness and planes after data acquisition with multi-detector row CT, and higher spatial resolution.
In conclusion, in this preliminary study, CT and MR enterography appear to be equally accurate in the identification of active inflammation in the small intestine and are capable of depiction of more complications in patients with CD.
Because the MR enterography has a diagnostic effectiveness comparable to that of CT enterography, we suggest this technique as an alternative that can be used in evaluation of patients with CD, being radiation-free alternative, especially for young patients who may require repeated examinations.
References
Del Vescovo R, Sansoni I, Caviglia R, et al. Dynamic contrast enhanced magnetic resonance imaging of the terminal ileum: differentiation of activity of Crohn’s disease. Abdom Imaging. 2008 Jul-Aug;33(4):417-24.
Furukawa A, Saotome T, Yamasaki M, et al. Cross-sectional imaging in Crohn disease. Radiographics. 2004 May-Jun;24(3):689-702.
N.C. Gourtsoyiannis, MD, N. Papanikolaou, MSc, A.Karantanas, MD, Magnetic resonance imaging evaluation of small intestinal Crohn’s disease Best Practice & Research Clinical Gastroenterology Vol: 20 Issue: 1, February, 2006 pp: 137-156.
Barloon T.J., Lu C.C., Honda H., Berbaum K.S., Does a normal small-bowel enteroclysis exclude small-bowel disease? A long-term follow-up of consecutive normal studies, Abdom Imaging, Volume: 19, (1994), pp. 113–115.
Paulsen SR, Huprich JE, Fletcher JG, et al. CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases..Radiographics. 2006 May-Jun;26(3):641-57.
Low RN, Francis IR, Politoske D, Bennett M. Crohn’s disease evaluation: comparison of contrast-enhanced MR imaging and single-phase helical CT scanning. J Magn Reson Imaging 2000;11:127–135.
Rieber A, Aschoff A, Nussle K, et al. MRI in the diagnosis of small bowel disease: use of positive and negative oral contrast media in combination with enteroclysis. Eur Radiol 2000;10:1377–1382.
Sempere GA, Martinez Sanjuan V, Medina Chulia E et al. MRI evaluation of inflammatory activity in Crohn’s disease. AJR Am J Roentgenol. 2005 Jun;184(6):1829-35.
Low RN, Francis IR. MR imaging of the gastrointestinal tract with IV gadolinium and diluted barium oral contrast media compared with unenhanced MR imaging and CT. AJR AJR Am J Roentgenol 1997;169:1051–1059.
Low RN, Barone RM, Lacey C, et al. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology 1997;204:513–520.
Lee JKT, Marcos HB, Semelka RC. MR imaging of the small bowel using the HASTE sequence. AJR Am J Roentgenol 1998;170:1457–1463.
Regan F, Beall DP, Bohlman ME, et al. Fast MR imaging and the detection of small-bowel obstruction. AJR 1998;170:1465–1469.
Giovagnoni A., Fabbri A., Maccioni F. Oral contrast agents in MRI of the gastrointestinal tract, Abdom Imaging, Volume: 27, (2002): 367-375.
Laghi A., Paolantonio P., Iafrate F., et al. Oral contrast agent for MRI of the bowel, Topics MRI, Volume: 13, (2002): 389-396.
Koh DM, Miao Y, Chinn RJ, et al. MR imaging evaluation of the activity of Crohn’s disease.. AJR Am J Roentgenol. 2001 Dec;177(6):1325-32.
Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index: National Cooperative Crohn’s Disease Study. Gastroenterology 70(3):439–444.
Masselli G, Brizi GM, Parrella A, et al. Crohn disease: magnetic resonance enteroclysis. Abdom Imaging. 2004 May-Jun;29(3):326-34.
Amitai MM, Arazi-Kleinman T, Hertz M, et al. Multislice CT compared to small bowel follow-through in the evaluation of patients with Crohn disease. Clin Imaging. 2008 Sep-Oct;32(5):355-61.
Hara AK, Alam S, Heigh RI, et al. Using CT enterography to monitor Crohn’s disease activity: a preliminary study. AJR Am J Roentgenol. 2008 Jun;190(6):1512-6.
Maccioni F., Viscido A., Broglia L., et al. Evaluation of Crohn’s disease activity with magnetic resonance imaging, Abdom Imaging, Volume: 25, (2000), pp. 219—228.
Schunk K., Kern A., Oberholzer K., et al. Hydro-MRI in Crohn’s disease. Appraisal of disease activity, Invest Radiol, Volume: 35, (2000), pp. 431–437.
Horsthuis K, Stokkers PC, Stoker J. Detection of inflammatory bowel disease: diagnostic performance of cross-sectional imaging modalities Abdom Imaging. 2008 Jul-Aug;33(4):407-16.
Bodily KD, Fletcher JG, Solem CA, et al. Crohn disease: mural attenuation and thickness at contrast-enhanced CT enterography correlation with endoscopic and histologic findings of inflammation. Radiology 2006;238:505–516.
Booya F, Fletcher JG, Huprich JE, et al. Active Crohn disease: CT findings and interobserver agreement for enteric phase CT enterography. Radiology. 2006 Dec;241(3):787-95.
Florie J, Wasser MN, Arts-Cieslik K, et al. Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn’s disease. AJR Am J Roentgenol. 2006 May;186(5):1384-92.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ippolito, D., Invernizzi, F., Galimberti, S. et al. MR enterography with polyethylene glycol as oral contrast medium in the follow-up of patients with Crohn disease: comparison with CT enterography. Abdom Imaging 35, 563–570 (2010). https://doi.org/10.1007/s00261-009-9557-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-009-9557-0