Abstract
The purpose of this study was to determine whether MRE performed with diffusion-weighted imaging (DWI) sequences is comparable to contrast-enhanced MRE in the detection of active small-bowel inflammation in pediatric patients with Crohn’s disease (CD). We included in our study 68 patients with diagnosis of CD between April 2015 and June 2018 that underwent MRE examination. Examination protocol includes coronal and axial FISP, T2-w half-Fourier RARE and DWI sequences, a baseline coronal T1-w fat-saturated ultrafast (GRE) sequence followed by contrast 3D T1-w GRE. All images were assessed by two radiologists who graded each of bowel segments for the presence of inflammation on a four-point confidence scale on the basis of wall thickening and wall signal on DWI and ADC maps and comparing their results with post-contrast images. When considering all bowel segments, we found 41 true positive and 25 true negative on DWI. One false positive case corresponded to the absence of inflammatory histopathology changes at the level of the terminal ileum in a 15-year-old male, and one false negative case was in a 10-year-old female with only jejunal lesion. The corresponding sensitivity, specificity, PPV, NPV and accuracy were 97.6% (95% CI 67.7–99.7), 96.1% (95% CI 66.7–98.5), 97.6% (95% CI 70.8–98.4), 96.1% (95% CI 64.2–90.6) and 97% (95% CI 84.2–97.5), respectively. Analyzing the gadolinium-enhanced set, 35 true positive and 25 true negative results were found. One false positive case was found, and it was the same as with DWI. The corresponding sensitivity, specificity, PPV, NPV and accuracy were 83.3% (95% CI 65.9–86.7), 96.1% (95% CI 68.7–88.9), 97.2% (95% CI 84.3–98.7), 78.1% (95% CI 27.9–72.1) and 88.2% (95% CI 41.2–85.6), respectively. Sensitivity for the detection of active IBD lesion was significantly better with DWI than with CE-T1-w imaging (p = 0.002), whereas the specificity was similar (p = 0.743). Our study has shown that DWI sequences have a high accuracy in detecting the bowel segment affected by CD. These results emphasize the utility to include the DWI/ADC in standard MR enterography protocols and suggest that DWI could replace T1-weighted post-contrast sequences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a chronic inflammatory and remitting disorder of the gastrointestinal tract; in 25% of cases, it is diagnosed in childhood with an increasing incidence in children. One recent study showed that the incidence of pediatric CD has doubled in the USA over the last 12 years [1].
Magnetic resonance enterography (MRE) is an increasingly important pediatric imaging modality that is most often used to evaluate inflammatory bowel disease (IBD), sparing children and adolescents from potential risks of ionizing radiation exposure [2,3,4,5,6]. Conventional MRE requires intravenous administration of gadolinium-based contrast agents (CA); the most current concern about CA in children has been the accumulation of Gd in the basal ganglia, which is of uncertain clinical significance but should be considered in patients with IBD, who undergo multiple imaging examinations over time to evaluate symptom recurrence [7, 8].
A non-contrast MRE does not need glucagon injection and would improve patient comfort and compliance, reducing scanning time and in some cases avoiding the need for pediatric sedation; moreover, it would reduce sanitary costs (in the USA, contrast adds up to $1000 to the MRI price for out-of-pocket paying patients) [9].
In this regard, diffusion-weighted imaging (DWI) has been increasingly used for abdominal and pelvic examinations due to its ability to measure molecular water diffusion into the extracellular space and to detect microscopic changes of inflammatory processes. Apparent diffusion coefficient (ADC) maps calculated from DWI images provide a quantitative measure of the diffusion characteristics of tissue. This reflects all forms of intravoxel incoherent motion and is independent from the underlying scan parameters [2, 5]. Its values decrease with increased tissue cellularity or cell density and may help in the quantitative analysis of disease activity [10,11,12].
DWI appears well adapted for children thanks to rapid acquisition time, a free breathing mode that reduced motion artifacts, high tissue contrast and finally because it obviates the need for contrast enhancement. Currently, DWI is considered an additional sequence in the MRE and only a few studies have been done in order to evaluate its potential and diagnostic accuracy alone and in comparison with standard MRI sequences [13, 14].
The aim of this study was to determine whether MRE performed with DWI sequences, without administration of intravenous Gd-CA, is comparable to contrast-enhanced MRE (CE-MRE) in the detection of small-bowel inflammation in pediatric patients with CD.
Materials and methods
Patients and methods
This prospective study was approved by the institutional review board of our University. Parent or guardian informed consent and subject assent were obtained at the time of study enrollment.
Sixty-eight consecutive patients (38 females and 30 males; mean age 10.3 years; range, 6–16 years) treated at the IBD pediatric unit of our University Hospital were consecutively and prospectively included between April 2015 and June 2018.
Inclusion criterion was the diagnosis of CD according to Lennard–Jones criteria [6]. The patients enrolled in the study either had symptomatic disease or underwent imaging follow-up to monitor CD activity under treatment.
All the patients underwent complete clinical assessment, and blood samples were obtained for cell blood counts, C-reactive protein (CRP) measurements and evaluation of renal function (MDRD).
Clinical disease activity was assessed using the pediatric CD activity index (PCDAI) [7], and all patients underwent MRI within 72 h from clinical assessment.
Patients with claustrophobia or other common MRI contraindications, such as allergy to gadolinium or severe renal failure (MDRD < 30 mL/min), were excluded.
MRI examination was performed using a 1.5 T MR imaging system (Avanto, Siemens Medical Solution, Malvern, PA, the USA) with a 16-channel phased-array abdominal coil.
Children fasted for 4 h and were asked to drink 500–1000 of mL polyethylene glycol (PEG) solution 45 min before the examination. Images were acquired with patients in the prone position. Coronal and axial FISP images were obtained first, and if distension was adequate, T2-w images were obtained in the coronal and axial planes with a single-shot half-Fourier RARE sequences (i.e., HASTE half-Fourier acquisition single-shot turbo spin echo).
Coronal DWI and axial DWI were obtained by using a single-shot spin-echo-type echo-planar imaging (EPI) sequence with fat suppression and parallel technique (reduction factor 2). Imaging parameters for DWI were as follows: TR/TE 2500 ms/80 ms; matrix 128 × 128; field of view 280 × 400 mm; slice thickness/gap 6 mm/1 mm; 6 averages; bandwidth 1930 Hz/pixel. The time required to acquire the DWI set was 3 min. b Values of 50, 400 and 800 s/mm2, as used in other abdominal applications [13], were employed (Table 1). Fat suppression was obtained with a selective frequency to reduce chemical-shift artifacts. Isotropic ADC maps were generated with a commercially available software workstation system (Leonardo; Siemens, Erlangen, Germany) using all b values and taking an average value of the three directions of diffusion sensitization.
All images were transferred to the workstation (Leonardo; Siemens, Erlangen, Germany) for quantitative analysis.
A dose of 0.5 mg glucagon (glucagen 1 mg/mL) was intravenously administered 1 min before a 3D T1-weighted spoiled gradient echo (GRE) sequence.
A baseline coronal T1-w fat-saturated ultrafast (GRE) sequence was applied first, followed by, after administration of 0.2 mmol/kg of Gadolinium Chelate Gadoterate Meglumine (Gd-DOTA, Dotarem, Guerbet) with an injection rate of 3 mL/s, 3D T1-w GRE sequences in the coronal plane during both the arterial and the portal phase. Finally, axial T1-w GRE acquisition covering the entire abdomen was acquired.
Image analysis
Images were assessed by two radiologists (with 12 and 15 years of body MR experience, respectively) who were blinded to the clinical and endoscopic examination and surgical results using a picture archiving and communication system (Carestream, 5.3 sp1.1; Kodak, Rochester, NY).
Three small-bowel segments were evaluated separately: (1) jejunum, (2) ileum proximal to the terminal ileum and (3) terminal ileum defined as the most distal 20 cm of the small bowel.
For this study, jejunal loops were distinguished from ileal loops both by their respective locations (left abdomen for jejunum, right abdomen for ileum) and by the appearance of their folds (more closely packed, jejunum; sparse, ileum).
Each segment was graded for the presence of inflammation on a four-point confidence scale on the basis of wall thickening and wall signal on DWI and ADC maps as follows: 0 = definitely absent (imperceptible wall, both in signal and in thickness), 1 = probably absent (normal thickness, signal intensity and thickness are similar to the surrounding bowel segments), 2 = probably present (normal wall thickness, but signal intensity is increased on DWI and decreased on ADC map), and 3 = definitely present (thick bowel wall, and signal intensity is increased on DWI and decreased on ADC map). The bowel wall was considered to be thickened when it was > 3 mm. On DWI, grading scores of 0 and 1 were regarded as indicating normal bowel wall and scores of 2 and 3 bowel wall inflammation.
On T2-w images, we analyzed the following signs: wall thickness (setting a grading score of 1: 3 mm, 2: 4–6 mm and 3: > 6 mm), submucosal edema, aphthous ulcers, lymphadenopathy.
On T1-w post-contrast images, we analyzed the following signs: comb sign (defined as mural stratification with engorged vasa recta that penetrate the bowel wall perpendicular to the bowel lumen), mesenteric hyperemia and stratified contrast enhancement of the mucosa.
To minimize recall bias, two reading sessions were performed 4 weeks apart from each other and MR images (T2, contrast T1 and diffusion-weighted images) were randomly analyzed during the different sessions.
Patient information was removed from all images. During each imaging analysis, the readers were asked to determine the presence and location of small-bowel inflammation.
Moreover, after 3 weeks, during a third interpretation, the two readers (1) resolved discrepancies in consensus and determined the diagnoses that were compared with the reference standard diagnoses; and (2) evaluated image quality of T1-weighted images post-contrast and DWI images by using a point scale: a score of 0 indicated non-diagnostic images; 1, diagnostic images with numerous artifacts; 2, diagnostic images with a few artifacts; and 3, diagnostic images without artifacts; (3) objective measurements of ADCs signal intensity were calculated using the standard tools of the workstation. Regions of interest (ROIs) on the ADC maps were drawn by an MR imaging physicist (S.S., 10 years of experience in abdominal MR imaging) and the two radiologists in consensus by using visual correlation of the ADC maps with the anatomic information derived from the corresponding T2-weighted images. Three values for each zone were recorded, and the final ADC value was the average.
The largest possible polygonal-shaped regions of interest (ROIs), ranging from 8 to 18 mm2, were placed at the level of the small-bowel wall.
Reference standards
The standard of reference for the presence of active lesion of IBD was histopathological findings (obtained by biopsy during endoscopic examination in 63 cases and by surgical specimens in 5 cases: 1 lesion of the jejunum and 4 lesions of the ileum).
The MR findings were correlated with the corresponding histological references for each segment by a coordinator not involved in the studies.
Histopathology activity score
The intestinal biopsies were stained with hematoxylin–eosin and retrospectively reviewed by an experienced pathologist > 10 years of experience, who was unaware of clinical information or MRI findings. The histopathologist applied an endoscopic biopsy acute inflammatory score (eAIS: Table 2) based on the typical morphological features of Crohn’s disease described in guidelines published by the European Crohn’s and Colitis Organization.
At least three samples of biopsy were collected for each patient, and the highest score for each was used for that patient, in accordance with the standard procedure in our institution.
Statistical analysis
Quantitative variables are given as means and standard deviation (s.d.) or as medians in the case of an abnormal distribution. Proportions are expressed as percentages and 95% confident intervals (CIs).
Differences in quantitative measures were tested by Student’s test or Kruskal–Wallis’ test if the conditions of Student’s test were not met. Qualitative variables were compared by exact Fisher’s test or Chi-squared test.
Using the histopathological standard of reference, ADC values were compared between patients with no histological inflammation (eAIS = 0) and those with histopathological inflammation (AIS score > 1) using the Mann–Whitney U test.
eAIS scores were then compared across qualitative DWI grades using Kruskal–Wallis test with post hoc correction.
The sensitivity and specificity of DWI grades 2 and 3 for active disease (AIS > 1) were calculated. A threshold was determined by calculating receiver operating characteristic (ROC) curves.
All correlations were studied using Spearman’s nonparametric correlation coefficients (r and p value, respectively). Inter-observer agreement between paired evaluations of MR by two radiologists (VP and AJ) was performed with kappa statistics for comparisons of qualitative variables and Pearson correlation coefficient or Lin concordance correlation coefficient for quantitative data.
A p value of 0.05 was considered significant. Calculations were done with STATA v10 (STATA Corp., College Station, TX, USA).
The strength of agreement was considered poor for κ values less than 0.20, fair for κ values of 0.21–0.40, moderate for κ values of 0.41–0.60, good for κ values of 0.61–0.80 and very good for κ values of 0.81–1.00.
Results
The mean time between MRE and endoscopy with biopsy was 5.5 days (range 1–21 days).
Based on the histopathology, findings were classified 48/68 (70%) patients in acute inflammatory phase and 20/68 (30%) patients in no-acute inflammatory phase, respectively.
Baseline patient characteristics are shown in Table 3.
The mean image quality score of DWI and contrast T1-weighed sequence was 2.9 ± 0.3 and 2.0 ± 0.5, respectively (p value = 0.074).
When considering all bowel segments, 41 true positive and 25 true negative results were found on DWI.
One false positive case corresponded to the absence of inflammatory histopathology changes at the level of the terminal ileum in a 15-year-old male, where endoscopic examination showed the presence of thickening folds.
One false negative case was in a 10-year-old female with only jejunal lesion.
The corresponding sensitivity, specificity, PPV, NPV and accuracy were 97.6% (95% CI 67.7–99.7), 96.1% (95% CI 66.7–98.5), 97.6% (95% CI 70.8–98.4), 96.1% (95% CI 64.2–90.6) and 97% (95% CI 84.2–97.5), respectively.
Analyzing the gadolinium-enhanced set, 35 true positive and 25 true negative results were found (Fig. 1). One false positive case was found, and it was the same as with DWI.
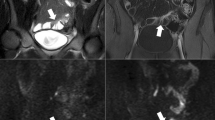
Diagnosis of ileal CD in a 14-year-old female with active Crohn’s disease. a Coronal steady-state true FISP image showed thickening of the wall of the terminal ileum (arrows) with evidence of increased layered enhancement on b coronal post-contrast T1-weighted image. Hyperintensity of the wall of the terminal ileum was observed on c coronal DWI at b = 800 mm2/s with low signal on d ADC (ADC value 1130 × 10–6 mm2/s (arrows). e Axial T2-weighted single-shot fast spin-echo image with fat saturation shows thickness of the wall of the terminal ileum (short arrow) with a hyperintense fluid collection (arrow). f Transverse single-shot echo-planar DW image at b = 800 mm2/s shows the hyperintense signal of the fluid collection, indicative of an abscess. g On T1-weighted image after contrast administration, the abscess shows thick, rim enhancement (arrows). Interventional drainage confirmed the diagnosis of an abscess
The number of false negative findings at contrast MRI (n = 7) was higher than that at DWI (n = 1) at the level of the jejunum (5 vs 1) rather than at the ileum (2 vs 0) (Figs. 2 and 3).
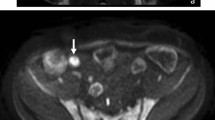
Diagnosis of ileal CD in a 8-year-old female with Crohn’s disease. a Coronal post-contrast T1-weighted image depicted no clear differences in terms of transmural enhancement at the level of the ileal loops. Hyperintensity of the wall of the terminal ileum compared to adjacent intestinal loops was observed on b coronal DWI at b = 800 mm2/s with low signal on c ADC (ADC value 1200 × 10–6 mm2/s (arrows). Based on the restriction of the diffusion CD in the active phase was diagnosed that was confirmed by endoscopy
Diagnosis of jejunal CD in a 13-year-old male with Crohn’s disease. a Coronal T2-weighted HASTE image showed mild thickening of jejunal small-bowel loop (arrows) with no evidence of increased enhancement on b coronal post-contrast T1-weighted image. Hyperintensity of the wall of the jejunal small-bowel loop was observed on c coronal DWI at b = 800 mm2/s with low signal on d ADC (ADC value 1170 × 10–6 mm2/s (arrows). Based on the restriction of the diffusion CD in the active phase was diagnosed that was confirmed by endoscopy
The corresponding sensitivity, specificity, PPV, NPV and accuracy were 83.3% (95% CI 65.9–86.7), 96.1% (95% CI 68.7–88.9), 97.2% (95% CI 84.3–98.7), 78.1% (95% CI 27.9–72.1) and 88.2% (95% CI 41.2–85.6), respectively.
Mean bowel wall ADC measurements were as follows: 1150 ± 200 × 10–6 mm2/s in patients with active disease (n = 48) and 1400 ± 250 × 10–6 mm2/s in patients with no active disease (n = 20), respectively. The comparison between two groups showed a statistically significant difference between the two samples, showing that the distribution of lower ADC values in patients with active disease is not random (p = 0.0367).
Sensitivity for the detection of active IBD lesion was significantly better with DWI than with CE T1-w imaging (p = 0.002), whereas the specificity was similar (p = 0.743).
Excellent inter-observer agreement regarding lesion detection was achieved (k = 0.970, 95% CI: 0.929, 1.000 for diffusion sequence and k = 0.937, 95% CI: 0.877, 0.998 for contrast MRI).
Discussion
Our results found significantly better accuracy and sensitivity of T2 in combination with DWI rather than in combination with CE sequences in detecting small-bowel acute inflammation in pediatric patients with CD.
Our results are in accordance with a retrospective study that has shown that DWI has a sensitivity of 94% for the detection of small- bowel and colonic lesions in the case of CD [20]. Another study has shown in 44 adults that DWI MRE is not inferior to contrast material-enhanced MRE for the evaluation of inflammation in CD, except for the diagnosis of penetration [11].
Most of the studies have demonstrated the accuracy of the MR enterography in radiological diagnosis of CD in pediatric patients using standard T2 and post-contrast T1 sequences [15, 16].
Recently, some studies [17, 18] have reported that the presence of inflammation in the active phase arises with a limited diffusivity or with an increased signal in DWI sequences and a corresponding reduction of the signal in the ADC maps [19].
Performance of high-quality MR enterography in children, compared with adults, poses additional challenges, such as limiting body movements during imaging and tailoring sequences to match pediatric patients’ ability to hold their breath, especially during intravenous administration of contrast medium [20].
DWI has still to be properly investigated in small-bowel imaging in pediatric population for its advantages such as rapid image acquisition, high tissue contrast and lack of intravenous contrast material [21,22,23,24,25,26]. Particularly, this last characteristic could avoid the venous access, the risk of adverse reactions to the contrast medium and minimize patient discomfort particularly important in the pediatric population that can preclude their cooperation at this examination.
There are several advantages in using DWI instead of CE T1-w images: a shorter examination duration (if post-gadolinium MR imaging is not performed) and no need for catheter placement and for glucagon injection.
In our study, images obtained by DWI displayed better image quality than CE T1-w images. This quality difference may be due to a longer time of examination with T1-w imaging and to movements artifacts. Furthermore, gadolinium MR imaging is performed in apnea, while DWI MR imaging can be performed in free breathing, which is better tolerated by pediatric patients. Still, there are some disadvantages of using DWI because it has a poorer spatial resolution that requires a joint analysis with T2 sequences; for this reason, the MR protocol requires the performance of T2-w imaging together with DWI.
In our study, DWI showed a better diagnostic performance than post-contrast sequences; an explanation could be that DWI was performed in free breathing avoiding the uncomfortable apnea required for CE MR and is less prone to motion artifacts.
Most of the lesions missed at CE-T1-w images were at the level of jejunal loops; an explanation could be that CE-T1-w images are less accurate in detecting lesions in the presence of sub-optimal distension, as it is frequently seen at the level of jejunum, whereas the accuracy of the diffusion sequence seems to depend less on the grade of distension [27, 28].
Some authors have suggested increasing bowel wall restricted diffusion in CD as a radiological biomarker for increasing disease activity without the need for intravenous contrast material [29,30,31,32].
The limitations of our study were several: the small sample of patients; however, our number is similar to that in previous studies [32, 33]; another limitation of the study was that we arbitrarily selected a b value of 800 s/mm2. In the future, further studies investigating DWI findings using different b values may determine their role in the detection of bowel inflammation.
Finally, comparison of imaging results with histological activity as gold standard is debatable since inflammation in Crohn’s disease is discontinuous, leading to sampling error for histological assessment. Transmural inflammation in Crohn’s disease may also mean that histological mucosal healing does not represent quiescent disease, since deeper inflammation, although invariably in the form of lymphoid hyperplasia, and therefore reflecting chronic inflammation rather than acute inflammation, may persist [34, 35].
In conclusion, our study has shown that DWI sequences with the corresponding ADC maps have a high accuracy in detecting the bowel segment affected by CD and that, associated with the standard T2-weighted images, allows to increase the diagnostic accuracy.
This evidence suggests the utility to include the DWI/ADC in the standard protocols of MR enterography. DWI could replace T1-weighted post-contrast sequences for detecting small-bowel acute inflammation in patients with CD, avoiding injection of contrast medium and reducing the examination time making the examination better tolerated by pediatric patients.
References
Malaty HM, Fan X, Opekun AR et al (2010) Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatric Gastroenterol Nutr 50(1):27–31
Dillman JR, Smith EA, Sanchez RJ et al (2015) Pediatric small bowel crohn disease: correlation of US and MR enterography. Radiographics 35(3):835–848
Masselli G, Casciani E, Polettini E et al (2013) Magnetic resonance imaging of small bowel neoplasms. Cancer Imaging 21(13):92–99
Li XH, Sun CH, Mao R et al (2015) Assessment of activity of crohn disease by diffusion-weighted magnetic resonance imaging. Medicine (Baltimore) 94(43):1819
Masselli G, Di Tola M, Casciani E et al (2016) Diagnosis of small-bowel diseases: prospective comparison of multi-detector row CT enterography with MR enterography. Radiology 279(2):420–431
La Seta F, Buccellato A, Tesè L et al (2006) Multidetector-row CT enteroclysis: indications and clinical applications. Radiol Med 111(2):141–158
Chavhan GB, Alsabban Z, Babyn PS (2014) Diffusion-weighted imaging in pediatric body MR imaging: principles, technique, and emerging applications. Radiographics 34(3):E73–E88
Neubauer H, Pabst T, Dick A et al (2013) Small-bowel MRI in children and young adults with Crohn disease: retrospective head-to-head comparison of contrast-enhanced and diffusion weighted MRI. Pediatr Radiol 43:103–114
Lee SM, Kim WS, Choi YH (2015) Pediatric magnetic resonance enterography: focused on Crohn’s disease. Pediatr Gastroenterol Hepatol Nutr 18(3):149–159
Ognibene NM, Basile M, Di Maurizio M et al (2016) Features and perspectives of MR enterography for pediatric Crohn disease assessment. Radiol Med 121(5):362–377
Seo N, Park SH, Kim KJ et al (2016) MR enterography for the evaluation of small-bowel inflammation in Crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology 278(3):762–772
Masselli G, Gualdi G (2013) CT and MR enterography in evaluating small bowel diseases: When to use which modality? Abdom Imaging 38(2):249–259
Wilkins T, Jarvis K, Patel J (2011) Diagnosis and management of Crohn’s disease. Am Fam Physician 84(12):1365–1375
Torkzad MR, Masselli G, Halligan SH et al (2015) Indications and selection of MR enterography vs. MR enteroclysis with emphasis on patients who need small bowel MRI and general anaesthesia: results of a survey. Insights Imaging 6(3):339–346
Masselli G, Gualdi G (2012) MR imaging of the small bowel. Radiology 264(2):333–348
Schooler GR, Hull NC, Mavis A, Lee EY (2019) MR imaging evaluation of inflammatory bowel disease in children where are we now in 2019. Magn Reson Imaging Clin N Am 27(2):291–300
Li Y, Hauenstein K (2015) New imaging techniques in the diagnosis of inflammatory bowel diseases. Visz Med 31(4):227–234
Masselli G, Polettini E, Laghi F et al (2014) Non-inflammatory conditions of the small bowel. Magn Reson Imaging Clin N Am 22(1):51–65
Scardapane A, Ambrosi A, Salinaro E et al (2015) Assessment of disease activity in small bowel Crohn’s disease: comparison between endoscopy and magnetic resonance enterography using MRIA and modified MRIA score. Gastroenterol Res Pract 2015:159641
Oto A, Zhu F, Kulkarni K et al (2009) Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol 16:597–603
Oussalah A, Laurent V, Bruot O et al (2010) Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut 59:1056–1065
Kiryu S, Dodanuki K, Takao H et al (2009) Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging 29(4):880–886
Moore WA, Khatri G, Madhuranthakam AJ et al (2014) Added value of diffusion weighted acquisitions in MRI of the abdomen and pelvis. Am J Roentgenol 202:995–1006
Towbin AJ, Sullivan J, Denson Lee A et al (2013) CT and MR enterography in children and adolescents with inflammatory bowel disease. Radiographics 33(7):1843–1860
Ream JM, Dillman JR, Adler J et al (2013) MRI diffusion-weighted imaging (DWI) in pediatric small bowel Crohn disease: correlation with MRI findings of active bowel wall inflammation. Pediatr Radiol 43:1077–1085
Dubron C, Avni F, Boutry N et al (2016) Prospective evaluation of free-breathing diffusion-weighted imaging for the detection of inflammatory bowel disease with MR enterography in childhood population. Br J Radiol 89:20150840
Cappabianca S, Granata V, Di Grezia G et al (2011) The role of nasoenteric intubation in the MR study of patients with Crohn’s disease: our experience and literature review. Radiol Med 116:389–406
Masselli G, Brizi MG, Menchini L et al (2005) Magnetic resonance enteroclysis imaging of Crohn’s. Radiol Med 110(3):221–233
Church PC, Greer MC, Cytter-Kuint R et al (2017) Magnetic resonance enterography has good inter-rater agreement and diagnostic accuracy for detecting inflammation in pediatric Crohn disease. Pediatr Radiol 47(5):565–575
Qi F, Jun S, Qi QY et al (2015) Utility of the diffusion weighted imaging for activity evaluation in Crohn’s disease patients underwent magnetic resonance enterography. BMC Gastroenterol 5(15):12
Kiryu S, Watanabe M, Kabasawa H et al (2006) Evaluation of super paramagnetic iron oxide-enhanced diffusion-weighted PROPELLER T2-fast spin echo magnetic resonance imaging: preliminary experience. J Comput Assist Tomogr 30:197–200
Dohan A, Taylor S, Hoeffel C et al (2016) Diffusion-weighted MRI in Crohn’s disease: current status and recommendations. J Magn Reson Imaging 44(6):1381–1396
Gee MS, Nimkin K, Hsu M et al (2011) Prospective evaluation of MR enterography as the primary imaging modality for pediatric Crohn disease assessment. Am J Roentgenol 197:224–231
Di Mizio R, Maconi G, Romano S et al (2004) Small bowel Crohn disease: sonographic features. Abdom Imaging 29(1):23–35
Masselli G, Picarelli A, Di Tola M, Libanori V, Donato G, Polettini E, Piermattei A, Palumbo P, Pittalis A, Saponara A, Gualdi G (2010) Celiac disease: evaluation with dynamic contrast-enhanced MR imaging. Radiology 256(3):783–790
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical standards
The present study was conducted in accordance with the institutional ethical committee of Sapienza University of Rome.
Informed consent
Parent or guardian informed consent was obtained at the time of study enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masselli, G., De Vincentiis, C., Aloi, M. et al. Detection of Crohn’s disease with diffusion images versus contrast-enhanced images in pediatric using MR enterography with histopathological correlation. Radiol med 124, 1306–1314 (2019). https://doi.org/10.1007/s11547-019-01067-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-019-01067-z