Abstract
Purpose
The successful labelling of bisphosphonates (BP) with 68Ga using macrocyclic chelators such as the based triazacyclononane (NO2AP) is a step forward in the in-house availability of a novel bone-seeking PET radiopharmaceutical with dual advantage of PET/CT imaging and generator production. In this study, we compared the novel generator-based skeletal radiotracer 68Ga-1,4,7-triazacyclonone-1,4-diacetic acid (68Ga-NO2AP-BP) with sodium fluoride (18F-NaF) for the detection of skeletal metastases in breast cancer patients. In addition, dosimetric analysis of 68Ga-NO2AP-BP was performed in a subset of patients.
Methods
This was a prospective study of histopathologically proven cases of breast cancer patients who were referred for bone scintigraphy and underwent positron emission tomography/computed tomography (PET/CT) with 18F-NaF and 68Ga-NO2AP-BP within a week in random order. The scans of each patient were compared both qualitatively for image quality and quantitatively for number of lesions and SUVmax of lesions. Dosimetric analysis was performed in five patients. Their PET/CT scans were acquired at multiple time points and urine and blood samples were collected. Dosimetric calculations were performed using OLINDA/EXM 1.1 software. Statistical analysis was done using Stata 13 (StataCorp) software package. An agreement analysis regarding number of lesions detected with the two skeletal radiotracers was carried out.
Results
The image quality of 68Ga-NO2AP-BP PET/CT scans were comparable to that of 18F-NaF. There was no statistically significant difference in the SUVmax of lesions, normal bone and lesion to background ratio between the two skeletal radiotracers. There was good agreement in the number of lesions detected by both skeletal radiotracers. The mean whole body effective dose for 68Ga-NO2AP-BP was 0.00583 mSv/MBq and the effective dose equivalent was 0.0086 mSv/MBq.
Conclusion
The excellent lesion detection agreement between 68Ga-NO2AP-BP and 18F-NaF favours the former as an alternative for skeletal scintigraphy in centres without an on-site cyclotron. The favourable dosimetric results and its potential to be used as a theranostic agent makes it an important generator-based skeletal radiotracer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Skeletal scintigraphy using 18F-sodium fluoride (18F-NaF) has proven superior to the conventional 99mTc-phosphonate bone scan, in particular because of the use of positron emission tomography/computed tomography (PET/CT) technology, which offers a higher sensitivity and spatial resolution along with routine CT acquisition, thus improving specificity of lesions detected [1, 2]. While 18F-NaF is cyclotron produced, PET radionuclide generator systems like 68Ge/68Ga generator serve as instant sources of short-lived radionuclides in institutions not equipped with an onsite cyclotron. The successful labelling of bisphosphonates (BP) with 68Ga using macrocyclic chelators such as DOTA is a step forward in the in-house availability of a novel bone-seeking PET radiopharmaceutical with dual advantage of PET/CT imaging and generator production. Pre-clinical studies have further reported alpha amino propionic acid-based 1,4,7 triazacyclononane) [3, 4], in particular 68Ga-NO2AP-BP, to have excellent tumour to background ratio and to facilitate imaging of small bone metastases [5]. As 18F-NaF is the agent with the highest sensitivity and specificity for evaluating skeletal metastases in breast cancer [6–9], in this prospective study, the primary objective was to compare head-to-head 68Ga-NO2AP-BP and 18F-NaF PET/CT for the detection of skeletal metastases in breast carcinoma patients. A secondary objective was to perform dosimetric analysis of 68Ga-NO2AP-BP as a new skeletal radiotracer.
Materials and methods
This was a prospective single centre study to compare the novel skeletal radiotracer 68Ga-NO2AP-BP with the reference standard 18F-NaF for skeletal PET/CT imaging. Figure 1 illustrates the structures of NO2AP-BP and (4-{bis-(phosphonomethyl)carbamoyl]methyl}-7,10-bis)carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl)acetic acid (BPAMD). Breast cancer was chosen for the study as both osteoblastic and osteolytic lesions can occur and we can compare the localisation of the two skeletal PET tracers in both types of lesions. The study was approved by the Institute Ethics Committee (IESC/T-236/15.06.2013), and written informed consent was taken from each patient before inclusion.
Patient characteristics
Inclusion and exclusion criteria
Pathologically proven breast carcinoma patients who underwent 99mTc-methylene diphosphonate (99mTc-MDP) skeletal scintigraphy were recruited for the study. Patients with one or more sites of abnormal tracer accumulation on the 99mTc-MDP bone scan which were suspicious for metastases were mainly focused. Pregnant and lactating female patients were excluded. Patients who refused to give informed consent were also excluded from the study.
As reported in the literature, 5–6 % of breast cancer patients [10] progress to skeletal metastases, and taking 10 % clinical error, a sample size of 22 patients was required to conduct the study. However, in the current study we have successfully analysed 28 patients.
Methods
Sample size and place of study
The study included 28 patients and was conducted between 2013 and 2015 in the Department of Nuclear Medicine, AIIMS, New Delhi.
Synthesis of 68Ga-NO2AP-BP
68Ga-NO2AP-BP was synthesised as described in detail by Holub et al. [5]. 68Ga3+ was obtained from the 68Ge/68Ga generator and complexed with the bisphosphonate as detailed below. 68Ga was eluted as cation from a 1850 MBq (50 mCi) 68Ge/68Ga generator (Isotope Technology Garching GmbH, Germany) with 5–7 ml of 0.05 M HCl (elution yield is ∼80 %). Following the standard protocol from Mainz [11], this eluate was then passed through a cation exchange resin strata (X-C phenomenex) online, which removed impurities and pre-concentrated the generator eluate. 68Ga was adsorbed onto the cartridge and the whole eluate solution passed into the waste. N2 solution (400 μl mixture of 97.6 % acetone and 0.05 N HCl) was used to release concentrated and purified 68Ga from the strata X-C. 68Ga was delivered into the reaction vial that contained the precursor NO2AP (20 μg dissolved in 1.5 mL 0.25 M sodium acetate). The reaction vessel was heated for 15 min at 95 °C. After heating, the labelled product was allowed to cool down. For purification purposes, the product was diluted with 5 ml of sterile water and passed through a STRATA X-C cartridge where the free Gallium was trapped on the resin and the pure labelled 68Ga-NO2AP-BP was present in the solution. The product was diluted with saline and was made sterile by passing through a 0.22 μm millipore filter. All synthesis steps were carried out in an automated module (Modular lab, Eckert and Ziegler, Germany). The total synthesis time was 18 min. For each run, a routine quality control test was performed with the help of radio-TLC, pH paper and dose calibrator. Labelling efficiency achieved in each synthesis was > 98 %.
Synthesis of 18F-NaF
The detailed procedure for synthesis and purification 18F-NaF has been described by our centre [12]. In brief, 18O water was irradiated by protons to produce 18F ions in an 11 MeV RDS 111 Medical Cyclotron (Siemens). 18F was transferred from the target to trap and release the column of the Explora FDG-4 module through the V-vial. Residual 18F in the V-vial was transferred to the second Explora module and 18F fluoride ion was trapped in the trap and release column. The trap and release column removes the silver content coming from the silver target. Trapped 18F fluoride ion was eluted with eluting agent (mixture of K222, K2CO3, water and acetonitrile) and transferred to a sterile empty vial in the hot cell through reaction vessels after filtering through a Millipore Milex 0.22 μm GS vented filter. Cation exchange resin was used for removal of K222. Dilution was done with normal saline (0.9 % NS) to form 18F-NaF. Mean total purification time was 8 min. Quality control was done as per United State Pharmacopia (USP) guidelines.
Study protocol
Patients who fulfilled inclusion criterion underwent 18F-NaF and 68Ga-NO2AP-BP PET/CT in random fashion were scheduled within one week of each other. It was ensured that good hydration was maintained on both scintigraphy days. The dose of either tracer used (18F-NaF or 68Ga-NO2AP-BP) was kept between 111 and 185 MBq (3–5 mCi) and was injected intravenously. After approximately 30 min, the patients were instructed to empty their bladder and thereafter were positioned in supine position with hands by the side on the PET/CT scanner, biograph mCT (Siemens). An initial scan (20 mA,120 kVp) of the whole body was followed by the low dose CT (140 mA, 120 kVp) from vertex to toe, and then the 3D emission scan was acquired at 2 min per bed position for the same landmarks. For dosimetry purpose of 68Ga-NO2AP-BP, we also enrolled five breast cancer patients with suspected skeletal metastases. The detailed pharmacokinetics and dosimetric parameters were undertaken in these five patients.
Analysis and Interpretation
Each study was reconstructed using iterative reconstruction (two iterations, 21 subsets). A multimodality work port (MMWP – Siemens) was used for viewing each study and included the maximum intensity projection image, PET, CT and fused PET/CT images of each tracer. All 68Ga-NO2AP-BP and 18F-NaF images were independently analysed by two nuclear medicine physicians. These physicians were blinded to the results of the 99mTc-MDP bone scan. The total number of lesions in each patient for 68Ga-NO2AP-BP and 18F-NaF was scored for physician 1 and similarly for physician 2. Each image was also scored visually for quality on the basis of a three-point scale: 1-good quality; 2-moderate quality; 3- poor quality. All foci of abnormal tracer uptake were counted and analysed with respect to their location, SUVmax and characteristic of the lesion on CT; and then classified as benign or metastatic. For calculation of SUVmax, circular regions of interest were drawn around areas with focally increased uptake on transaxial slices.
For dosimetric analysis, all the serial PET/CT scans were conducted in the biograph mCT PET/CT scanner (SIEMENS, Germany). The scan protocol consisted of a scout acquisition, followed by a low dose CT and multiple PET acquisitions at 5, 20, 30, 70, 120 and 240 min post-intravenous injection of an average activity of 159.1 MBq (4.3 mCi) of 68Ga-NO2AP-BP. To maintain uniformity, the time of acquisition of PET images applied per bed was 1 min at all the time points for all patients. Whole blood samples at 5, 15, 30, 60, 80 and 120 min on scanner and urine samples at 50, 160, 180, 220 and 300 min from the time of injection were collected. Whole blood was counted in a scintillation well counter to know the exact amount of activity.
Organs such as liver, right and left kidneys and skeleton were included in the image analysis. Quantification (Bq/ml) for each organ was done using ROI-based method in each slice with automatic thresholding. Whole body counts were taken at respective time points for all the patients. The number of disintegrations (residence times) of the skeleton was based on the measurements determined in ROI around the left humerus. To obtain the values for the whole skeleton, the values were scaled based on the fraction weight that the humerus represents with respect to the whole skeleton. These values were obtained from ICRP 70: 187.4 g for the humerus of one side and 4000 g for the female whole skeleton [13]. Dosimetric calculations were performed using OLINDA/EXM 1.1 software (Organ Level Internal Dose Assessment/Exponential Modelling computer software, Vanderbilt University). The percentage injected activity (%IA) was calculated, in each time point image. Percentage of injected activity was defined as the ratio between the activities in each organ and the injected activity. Values of %IA were entered in the OLINDA software and time activity curves were derived for each organ. The number of disintegrations of each organ was derived from the time activity curves. The blood and urine sample at each time point were counted and decay corrected. The %IA of both blood and urine samples were also entered in the OLINDA software to derive the number of disintegrations (residence time). The numbers of disintegrations of all the organs were entered in the kinetic input model and the absorbed doses of each organ and whole body effective doses were calculated.
Statistical Methods
Statistical analysis was done using Stata 13 (StataCorp) software package. The agreement analysis regarding number of lesions detected on 18F-NaF and 68Ga-NO2AP-BP was carried out, for which the mean difference between observations with 95 % confidence interval (CI), correlation coefficient, regression coefficient through origin (with 95 % CI) and intraclass correlation coefficient were calculated. A p value of < 0.05 was considered significant.
Results
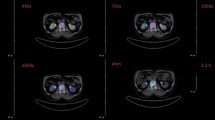
For the entire study, 28 patients were recruited. In five patients, in addition to lesion detection, dosimetric analysis was performed. For comparison between 68Ga-NO2AP-BP and 18F-NaF, a total of 28 patients were finally included in the study and had 68Ga-NO2AP-BP and 18F-NaF PET/CT performed. Figure 2 shows a comparison of 18F-NaF and 68Ga-NO2AP-BP PET MIP images. There were 27 females and one male patient with pathologically proven breast cancer. The age ranged from 26 years to 67 years (43.8 ± 12.3 years). Referral pattern for bone scintigraphy was as follows: ten patients prior to surgery, one patient after lumpectomy, two patients post-neoadjuvant chemotherapy and 15 patients for post-treatment surveillance. None of the patients received radiotherapy. Histopathologically, 14 patients had infiltrating ductal carcinoma, 13 patients had invasive ductal carcinoma and one patient had invasive lobular carcinoma.
Figure shows a comparison of 18F-NaF (a) and 68Ga-NO2AP-BP (b) PET/CT MIP images of a histopathologically proven case of breast carcinoma. Both the scans show increased radiotracer accumulation in skull, multiple cervico-dorsal lumbar vertebrae, multiple bilateral ribs, bilateral pelvic bones, sacrum and bilateral femora
Image quality was graded as good for 26 68Ga-NO2AP-BP and 27 18F-NaF studies. There were a total of 199 lesions on consensus reading of both scans, of which 178 lesions were characterised as metastatic and 21 as degenerative/traumatic (Table 1). One patient had a metastatic superscan that was well appreciated on both studies and the patient was excluded from lesion-based analysis (Fig. 3). SUVmax for metastatic lesions on 68Ga-NO2AP-BP and 18F-NaF ranged from 5.2 to 112 (mean = 27.2) to 6.2 to 46.7 (mean = 26.7), respectively (Table 2). Lesion to normal bone ratio ranged from 2.1 to 7.4 (mean = 3.1) for 68Ga-NO2AP-BP and from 2.1 to 6.7 (mean = 3.4) for 18F-NaF (Table 2). Normal bone SUVmax ranged from 4.2 to 39 for 68Ga-NO2AP-BP and 1.3 to 17.7 for 18F-NaF. There was no significant difference found in any of these parameters evaluated on the two studies (p > 0.05). Degenerative lesions like osteophytes, facet arthritis and rib fractures showing tracer localisation were noted in five patients on both scans and were correctly interpreted by both physicians using CT correlation. CT demonstrated a total of 187 lesions, among which purely lytic lesions were seen in five patients, predominantly sclerotic lesions in two patients; the remaining lesions were mixed in nature. Both tracers localised in all purely lytic, predominantly sclerotic and lytic/sclerotic lesions. During the study, it was seen that both tracers localised to brain metastases in three patients, which was confirmed on subsequent magnetic resonance imaging [14] and appropriate therapy was instituted. The total number of lesions detected on 99mTc-MDP in the 27 patients was 150, with 12 degenerative lesions mimicking metastases in one patient.
The number of lesions on 68Ga-NO2AP-BP were correlated between physician 1 and physician 2, and the correlation coefficient was significantly high (r =0.99) (Fig. 4a). Similarly, the number of lesions on 18F-NaF were also correlated between physician 1 and physician 2, and the correlation coefficient was also significantly high (r =0.99) (Fig. 4b). The numbers of lesions detected on 68Ga-NO2AP-BP and 18F-NaF were correlated between both the physicians. The mean difference for number of lesions detected on 68Ga-NO2AP-BP and 18F-NaF was not significant for both the readers, with a high correlation coefficient of 0.99 (Table 3) (Fig. 4c & d). Interobserver regression coefficient was highly significant. Intraclass correlation for both readers was excellent (Table 3).
a shows the correlation for number of lesions on 68Ga-NO2AP-BP PET/CT between physician 1 and physician 2, and b shows the correlation for number of lesions on 18F-NaF PET CT between physician 1 and physician 2. c shows the correlation of number of lesions between 18F-NaF PET CT and 68Ga-NO2AP-BP PET CT for physician 1 and d shows the correlation of number of lesions between 18F-NaF PET/CT and 68Ga-NO2AP-BP PET /CT for physician 2
No adverse reactions to tracer injection was noted on 68Ga-NO2AP-BP studies. The dosimetric analysis showed that the mean doses to kidneys, red marrow, urinary bladder and skeleton were 0.00763 mSv/MBq, 0.0203 mSv/MBq, 0.000113 mSv/MBq and 0.058 mSv/MBq, respectively. The mean whole body effective dose and effective dose equivalent were calculated to be 0.00583 mSv/MBq and 0.0086 mSv/MBq, respectively.
Discussion
The present study revealed excellent correlation in the detection efficiency of metastatic lesions on 68Ga-NO2AP-BP PET/CT when compared to 18F-NaF PET/CT with good localisation in both lytic and sclerotic lesions. All metastatic and degenerative lesions were classified correctly on 68Ga-NO2AP-BP PET/CT. Simultaneous CT acquisition enabled accurate identification of degenerative and traumatic lesions. Although the patient number was small, interobserver correlation, agreement and intraclass correlation was excellent for both studies. Extraskeletal tracer localisation in brain metastases was noted on both studies and reported earlier by us [14]. All three patients were asymptomatic for the same, and subsequent MRI demonstrated cerebral metastases. The possible explanation for extraosseous accumulation of 68Ga-NO2AP-BP includes presence of iron deposits (presence of hemorrhage on MRI), hyperemia or altered capillary permeability of the metastatic lesion. Extraosseous intracranial accumulation of conventional bone scintigraphy agent 99mTc-MDP in metastatic neoplasms has been reported [15]. 18F-NaF has also been reported to accumulate in treated brain metastases [16]. This possibility should be kept in mind while interpreting skeletal PET/CT, as this would facilitate early appropriate management to be instituted.
In vivo experiments have shown that 68Ga-NO2AP-BP has a high binding (93.8 ± 4.4 %) to hydroxyapatite and a fast renal clearance [5]. In addition, 68Ga-NO2AP-BP is taken up by osteoclasts, reflecting the farnesyl diphosphate synthase enzyme dynamics in the HMG-CoA reductase pathway, which may be responsible for a higher detection of lytic lesions [17]. Interestingly, in this study, all purely lytic lesions were detected equally on both 18F-NaF and 68Ga-NO2AP-BP. We could achieve good image quality with acquisition starting at 30 min post-injection at 2 min per bed, 3-D emission scans, for both 68Ga-NO2AP-BP and 18F-NaF. Similarly, the dose of approximately 111 MBq (3 mCi) resulted in good image quality on both scans.
The effective dose for 25 mCi of 99mTc-MDP is 5.3 mSv (0.0057 mSv/MBq) [18], and for 5 mCi of 18F-NaF, it is 4.44 mSv (0.024 mSv/MBq) [19]. Thus, for the injected dose of 18F-NaF (185 MBq), the effective dose is in the same range as that of 99mTc-MDP. The effective dose for 68Ga-NO2AP-BP was calculated to be 0.00583 mSv/MBq; hence, the effective dose for 159.1 MBq (4.3 mCi) of 68Ga-NO2AP-BP is 0.928 mSv. Thus, from all the three skeletal radiotracers, the effective dose estimated for 68Ga-NO2AP-BP was lower by a factor of five than for the two other diagnostic radiopharmaceuticals. The radiation dose to each patient can further be minimised with good hydration and frequent bladder emptying, which we ensured.
The total number of lesions detected on 68Ga-NO2AP-BP and 18F-NaF was more than that on the 99mTc-MDP scan planar and SPECT/CT, again demonstrating the superiority of PET/CT technology over SPECT/CT technology. Degenerative lesions in one patient mimicked metastases on planar imaging because they appeared to be involving the vertebral body, but were clearly identified as degenerative on both 68Ga-NO2AP-BP and 18F-NaF PET/CT. Though SPECT/CT would have also clarified the issue, it was not done in all cases due to technical reasons. The number of lesions detected on 68Ga-NO2AP-BP and 18F-NaF PET/CT (p = 0.16) was more than those detected on CT, which reiterates the age-old teaching that radiological lesions in the skeleton are discernible only after at least 30–50 % loss of bone mass.
The DOTA bisphosphonate, BPAMD [4], has also been labelled with 68Ga, resulting in a novel bone seeking radiotracer 68Ga-BPAMD. It has been shown to have faster clearance with high bone to soft tissue ratios [11]. Micro-PET animal experiments have revealed high accumulation of 68Ga-BPAMD in pronounced areas of bone remodelling such as bone metastases [3]. An initial report of its use to image bone metastases in prostate cancer has shown excellent image quality [17]. With the present study we confirm that a triaza-structure of the 68Ga-chelate is also perfectly usable for new 68Ga-macrocyclic bisphosphonate tracers.
The theeoretical prediction that the image quality achieved with 68Ga mono-conjugated bisphosphonate cannot compete with that of 18F-NaF, as 18F possesses a lower positron energy than 68Ga, resulting in lower positron tissue penetration (0.54 mm and 2.12 mm for 18F and 68Ga respectively) and thus lower image blurring, appeared to be not true. As the difference in resolution for a clinical PET scanner is small (3.05 mm for 18F and 3.57 mm for 68Ga), a successful application of 68Ga bone-imaging agents in patients is not precluded [20]. The results of this study agree with these observations and prove the utility of 68Ga-NO2AP-BP for skeletal imaging with equally good results in terms of spatial resolution as for 18F-NaF.
This study validates the utility of 68Ga-NO2AP-BP when compared to 18F-NaF, which is approved by the United States Food and Drug Administration (FDA) and has been the standard agent for skeletal PET/CT till date. 18F-NaF has been shown to be more accurate for detecting skeletal metastases in both breast cancer and prostate cancer patients when compared to 99mTc-MDP [9]. The 68Ge/68Ga generator produced 68Ga-NO2AP-BP can be an excellent alternative to 18F-NaF. The major advantages perceived with this new agent is that it can be a useful tool for a hospital with PET/CT but without a cyclotron, as it can be conveniently prepared from an onsite 68Ge/68Ga generator system. Moreover, the cost would be similar to that of other PET radiopharmaceuticals. It would also be a useful alternative to 99mTc-MDP in times of 99Mo-Molybdenum shortage [21]. Its favourable dosimetric data proves its utility from a radiation safety point of view. However, as the study was conducted in a small group of patients, further studies including a greater number of patients and with minor bias need to be conducted.
On a larger prospective, however, the main impact of the 68Ga-bisphosphonates will most probably consist of their theranostic potential. Once the chelated 68Ga-bisphosphonate has identified the patient with localisation of this tracer to nodal and bone metastases, a subsequent therapy with a structural analogue of that bisphosphonate, labelled with trivalent therapeutic radiometal such as 177Lu, will be feasible. This potential application is underway at our centre (FR). However, 177Lu-labelled compounds do not necessarily have biodistribution/pharmacokinetics as 68Ga-labelled radiopharmaceuticals.
Limitations of the study
The limitations of the study are small sample size and a possible referral bias.
Conclusion
Excellent lesion detection between 68Ga-NO2AP-BP PET/CT and 18F-NaF PET/CT with favourable dosimetric results of 68Ga-NO2AP-BP PET/CT suggests the utility of the former as a generator-based PET radiopharmaceutical for skeletal scintigraphy.
References
Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-P Planar bonescintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–97.
Yen RF, Chen CY, Cheng MF, Wu YW, Shiau YC, Wu K, et al. The diagnostic and prognostic effectiveness of F-18 sodium fluoride PET-CT in detecting bone metastases for hepatocellular carcinoma patients. Nucl Med Commun. 2010;31:637–45.
Fellner M, Biesalski B, Bausbacher N, Kubícek V, Hermann P, Rösch F, et al. 68Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter. Nucl Med Biol. 2012;39:993–9.
Kubícek V, Rudovský J, Kotek J, Hermann P, Vander Elst L, Muller RN, et al. A bisphosphonate monoamide analogue of DOTA: a potential agent for bone targeting. J Am Chem Soc. 2005;127:16477–85.
Holub J, Meckel M, Kubíček V, Rösch F, Hermann P. Gallium(III) complexes of NOTA-bis (phosphonate) conjugates as PET radiotracers for bone imaging. Contrast Media Mol Imaging. 2015;10:122–34.
Schirrmeister H, Guhlmann A, Kotzerke J, Santjohanser C, Kühn T, Kreienberg R, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol. 1999;17:2381–9.
Withofs N, Grayet B, Tancredi T, Rorive A, Mella C, Giacomelli F, et al. 18F-Fluoride PET/CT for assessing bone involvement in prostate and breast cancers. Nucl Med Commun. 2011;32:168–76.
Iagaru A, Mittra E, Dick DW, Gambhir SS. Prospective evaluation of 99mTc MDP scintigraphy, 18F NaF PET/CT, and 18F FDG PET/CT for detection of skeletal metastases. Mol Imaging Biol. 2012;14:252–9.
Damle NA, Bal C, Bandopadhyaya GP, Kumar L, Kumar P, Malhotra A, et al. The role of 18F-fluoride PET-CT in the detection of bone metastases in patients with breast, lung and prostate carcinoma: a comparison with FDG PET/CT and 99mTc-MDP bone scan. Jpn J Radiol. 2013;31:262–9.
Ibrahim T, Mercatali L, Amadori D. A new emergency in oncology: Bone metastases in breast cancer patients (Review). Oncol Lett. 2013;6:306–10.
Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48:1741–8.
Kumar R, Sonkawade RG, Tripathi M, Sharma P, Gupta P, Kumar P, et al. Production of the PET bone agent 18F-fluoride ion, simultaneously with 18F-FDG by a single run of the medical cyclotron with minimal radiation exposure- a novel technique. Hell J Nucl Med. 2014;17:106–10.
Basic Anatomical & Physiological Data for use in Radiological Protection - The Skeleton. ICRP Publication 70 - Ann. ICRP 25, 1995.
Passah A, Tripathi M, Kumar R, Das CJ, Goyal A, Bal CS. Brain metastasis in carcinoma breast demonstrated on 68Ga NOTA-bisphosphonate PET/CT. Clin Nucl Med. 2014;39:653–4.
Sty JR, Starshak RJ, Casper JT. Extraosseous accumulation of Tc-99m MDP. Metastatic intracranial neuroblastoma. Clin Nucl Med. 1983;8:26–7.
Tripathi M, Jaimini A, Singh N, Jain N, D’Souza M, Kaur P, et al. F-18 flurodeoxyglucose negative, F-18 fluoride accumulating in a brain metastasis in a treated case of carcinoma of the breast. Clin Nucl Med. 2009;34:287–9.
Fellner M, Baum RP, Kubícek V, Hermann P, Luke SL, Prasad V, et al. PET/CT imaging of osteoblastic bone metastases with (68)Ga-bisphosphonates: first human study. Eur J Nucl Med Mol Imaging. 2010;37:834.
Wong KK, Piert M. Dynamic bone imaging with 99mTc-labeled diphosphonates and 18F-NaF: mechanisms and applications. J Nucl Med. 2013;54:590–9.
Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51:1813–20.
Sánchez-Crespo A, Andreo P, Larsson SA. Positron flight in human tissues and its influence on PET image spatial resolution. Eur J Nucl Med Mol Imaging. 2004;31:44–51.
Gould P. Medical isotope shortage reaches crisis level. Nature. 2009;460:312–3.
Acknowledgement
We are grateful to Dr. Chandan J Das for his expertise in reporting the MRI brain scans and Dr. Ganesh Kumar M for keenly reviewing the manuscript. We acknowledge support from Prof. P. Hermann and Dr. V. Kubíček, Charles University in Prague, Czech Republic, for the preparation of NO2AP-BP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical clearance
Ethical clearance received Ref. No. IESC/T-236/15.06.2013
Informed consent
Informed consent was obtained from all patients.
Funding
None.
Rights and permissions
About this article
Cite this article
Passah, A., Tripathi, M., Ballal, S. et al. Evaluation of bone-seeking novel radiotracer 68Ga-NO2AP-Bisphosphonate for the detection of skeletal metastases in carcinoma breast. Eur J Nucl Med Mol Imaging 44, 41–49 (2017). https://doi.org/10.1007/s00259-016-3469-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3469-3