Abstract
Purpose
To investigate the role of 11C-choline PET/CT for evaluating the response to treatment in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with docetaxel in comparison with PSA response.
Methods
Inclusion criteria were (a) proven mCRPC, (b) docetaxel as first line of chemotherapy (docetaxel 75 mg/m2 + prednisone 5 mg), and (c) 11C-choline PET/CT and PSA values assessed before and after docetaxel administration. A total of 61 patients were retrospectively enrolled (mean age 68.9 years, range 57 – 84 years). 11C-Choline PET/CT was performed at baseline before docetaxel treatment (PET1) and after the end of treatment (PET2). PSA values were measured before treatment (PSA1) and after treatment (PSA2). PET2 was reported as complete response (CR), partial response (PR) or stable disease (SD). Progressive disease (PD) was considered if a new lesion was seen. PSA trend was calculated from the change in absolute values between PSA1 and PSA2. A decrease of ≥50 % between PSA1 and PSA2 was considered a PSA response. Clinical, radiological and laboratory follow-up ranged from 6 to 53 months (mean 13.5 months).
Results
Of the 61 patients, 40 (65.5 %) showed PD on PET2, 13 (21.3 %) showed SD, 2 (3.4 %) showed PR, and 6 (9.8 %) showed CR. An increasing PSA trend was seen in 29 patients (47.5 %) and a decreasing PSA trend in 32 patients (52.5 %). A PSA response of ≥50 % was seen in 25 patients (41 %). Radiological PD was seen in 23 of the 29 patients (79.3 %) with an increasing PSA trend, in 16 of the 32 patients (50 %) with a decreasing PSA trend, and in 11 of the 25 patients (44 %) with a PSA response of ≥50 %. In the multivariate statistical analysis, the presence of more than ten bone lesions detected on PET1 was significantly associated with an increased probability of PD on PET2. No association was observed between PSA level and PD on PET2.
Conclusion
Our results suggest that an increasing PSA trend measured after docetaxel treatment could be considered predictive of PD. In patients with decreasing PSA values (decreasing PSA trend and a PSA response of ≥50 %), 11C-choline PET/CT may be useful to identify those with radiological progression despite a PSA response. Finally, the tumour burden, expressed as number of bone lesions on PET1, is significantly associated with an increased probability of PD on PET2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PC) is the most common cancer and the second most common cause of cancer-related deaths in men [1]. The condition of castration-resistant prostate cancer (CRPC) when metastatic (mCRPC) is associated with a median overall survival of 2 to 3 years [2]. At this stage, palliation is the main target of any treatment. Nowadays, many different palliative therapies are available for these patients, including systemic chemotherapy and recently developed antiandrogenic therapies (ADT) [2, 3]. The optimal timing of different treatments has not yet been established, since biochemical markers and prognostic factors show a lack of accuracy in predicting the response to therapy in mCRPC [4, 5]. In patients with proven mCRPC, the most common treatment strategy proposed is docetaxel as the first line of chemotherapy (75 mg/m2 every 3 weeks + prednisone 5 mg twice daily for a maximum of ten cycles) [4, 5]. However, it is still not clear which prognostic factors (e.g. high PSA values, fast PSA kinetics) should be considered to select the most effective treatment strategy in patients with mCRPC [4, 5]. In this scenario, evidence of the presence of visceral or bone metastasis and the number of lesions (single lesion, or oligometastatic or multimetastatic disease) may help clinicians choose the most appropriate treatment strategy in a particular patient.
A lack of reliable response criteria is another limitation for clinicians, since variation in PSA serum levels shows a low clinical significance as a therapy response predictor [6–9]. Moreover, contrast-enhanced CT has a limited role, mainly due to the limited value of RECIST criteria in assessing the response of bone metastases [10]. Also bone scan has several limitations, including the flare phenomena that can affect patients after systemic therapy and limited accuracy in the presence of osteolytic lesions.
During the last decade, PET/CT with 11C-choline or 18F-choline has emerged as a useful tool for investigating patients with PC because of its ability to show the site of tumour recurrence earlier than other imaging methods in a single examination. Choline PET/CT detection rate has been found to be directly related to the PSA value and PSA kinetics [11–14]. Furthermore, it has shown optimal performance in mCRPC patients investigated during ongoing ADT, showing the lesions not responding to the hormone blockade [15]. However, at present, there are no precise indications about the proper use of choline PET/CT in the management of mCRPC patients [4, 5].
The aim of the present study was to evaluate the usefulness of 11C-choline PET/CT for therapy monitoring in mCRPC patients treated with docetaxel and to compare 11C-choline PET/CT response and PSA response. The secondary aim was to investigate if the extension of the disease demonstrated by 11C-choline PET/CT (expressed as the number of 11C-choline PET/CT-positive lesions detected before docetaxel treatment) could be related to the radiological and biochemical response to the first line of chemotherapy.
Materials and methods
Population selection and characteristics
This retrospective study was performed according to the principles of the Declaration of Helsinki and its subsequent amendments [16]. Written informed consent was obtained from all patients before the start of the study according to institutional guidelines, and all studies were performed in the course of diagnostic work-up. The clinical records of all mCRPC patients treated with docetaxel and referred to our centre in the course of clinical and diagnostic work-up for 11C-choline PET/CT between 2008 and 2014 were retrospectively analysed. Inclusion criteria were: (a) proven mCRPC (at least three rising PSA values during ADT and testosterone <50 ng/dL) [4]; (b) docetaxel as first line of chemotherapy in accordance with the therapy scheme proposed by the European Association of Urology (EAU) guidelines (docetaxel 75 mg/m2 + prednisone 5 mg) [4]; (c) 11C-choline PET/CT and PSA value assessments performed before and after docetaxel administration.
A total of 61 consecutive patients fulfilling all the inclusion criteria were retrospectively enrolled. 11C-Choline PET/CT was performed at baseline before administration of docetaxel (PET1) and after at least four cycles (range 4 – 12) of chemotherapy (PET2). Of the 61 patients, 53 were investigated after four cycles of docetaxel, 6 after eight cycles, and 2 after 12 cycles. The number of cycles of chemotherapy had already been decided before PET/CT by the referring oncologist on the basis of clinical and radiological data. All patients were investigated with 11C-choline PET/CT after the end of the docetaxel therapy scheme proposed. None of the patients enrolled was investigated during docetaxel administration. PET1 was performed a mean of 2.4 months before and PET2 a mean of 3 months after docetaxel administration. PSA values and dosage were available in all patients before (PSA1) and after (PSA2) administration of docetaxel. PSA1 was assessed a mean of 2.7 months before and PSA2 a mean of 2.8 months after treatment. Population characteristics are presented in detail in Table 1.
Radiopharmaceuticals
11C-Choline was synthesized according to the solid-phase method described by Pascali et al. [17] using a commercially available synthesis module (TracerLab; GE Medical Systems, Waukesha, WI). 11CO2, produced by a cyclotron (GE Healthcare), was converted into 11CH3I by the conventional LiAlH4/HI reaction. 11CH3I was used for the N-methylation of dimethylaminoethanol (60 μL) placed directly on a solid-phase support (C18 Sep-Pak Light; Waters). After washing with ethanol and water, 11C-choline retained on a cation exchange resin (Sep-Pak Accell Plus CM; Waters) was eluted with saline, sterilized by passing through a 0.22-μm filter and collected in a final volume of 8 mL. Radiochemical purity was evaluated by means of a high-performance liquid chromatography radiodetector equipped with a reverse-phase column, and the concentration of organic solvents was measured by gas chromatography. The endotoxin content was measured by the conventional lysosomal acid lipase method (Cambrex Bioscience).
Imaging protocols
Patients underwent a 11C-choline PET/CT scan following the standard procedure in our centre. All scans were obtained with a hybrid PET/CT tomograph (Discovery STE and Discovery 710; GE Medical Systems, Waukesha, WI). CT parameters were 120 kVp, 60 mA, 0.8 s per tube rotation, slice thickness 5 mm, pitch 1.5, and a table speed of 30 mm per rotation. Patients received an intravenous injection of 370 – 555 MBq of 11C-choline. PET/CT scans were started 3 – 5 min after radiotracer injection, and emission data were acquired for five or six bed positions from the mid-thigh to the base of the skull, taking 3 – 4 min for each bed position (in relation to body weight and volume).
Image analysis
All 11C-choline PET/CT images were analysed with dedicated software (eNTEGRA; GE Medical-Systems) which allowed review of the PET, CT and fused imaging data. PET images were first assessed visually using transaxial, sagittal and coronal displays and interpreted in consensus by two experienced nuclear medicine physicians aware of the clinical data. Visual interpretation was used as the main criterion to reach the final diagnosis. Any uptake higher than background was considered suspicious for malignancy. The maximum standardized uptake value (SUVmax) was measured for each lesion, but it was not used as the main criterion to reach the final diagnosis because a generally accepted cut-off value has not yet been established. Only in 4 (6.6 %) of the 61 11C-choline PET/CT scans was there disagreement between the readers. The final diagnosis was reached by consensus and by the opinion of a third reader.
Therapy response assessment
The responses to docetaxel treatment were assessed biochemically (change in PSA following chemotherapy) and radiologically (response vs. appearance of a new lesion on 11C-choline PET/CT). The change in PSA values from before treatment (PSA1) to after treatment (PSA2) were considered as the PSA trend. Any increase in absolute PSA values between PSA1 and PSA2 was considered an increasing PSA trend. Any decrease in absolute PSA values between PSA1 and PSA2 was considered a decreasing PSA trend. A decrease in absolute PSA value of ≥50 % between PSA1 and PSA2 was considered a PSA response. The PET2 findings were compared with the PET1 findings to assess the imaging response to treatment according to EORTC [18]. The appearance of a new PET-positive lesion on PET2 was considered to represent progressive disease (PD). An increase in SUVmax of <25 % or a decrease of <15 % in the lesions without further lesions was considered as stable disease (SD). A decrease in SUVmax of ≥25 % in the lesions on PET2 in relation to PET1 without further lesions was considered a partial response (PR). A PET2 scan reported as negative with no pathological choline uptake was considered to represent a complete response (CR). If diffuse metastatic spread of disease (more than ten lesions) was seen, SUVmax was determined in the ten lesions with the highest uptake.
Validation
All patients enrolled in the study were considered to have proven mCRPC on the basis of clinical and radiological evaluation. New lesions seen on PET2 (PD) were validated as new PC lesions by clinical follow-up in all patients (mean follow-up 13.5 months, range 6 – 53 months). Additional imaging confirmation of the progression of the disease was also obtained with a further 11C-choline PET/CT scan and/or additional imaging (contrast-enhanced CT, spinal MRI, bone scan) performed during follow-up.
Statistical analysis
All data are reported here as mean, standard deviation, median, range and individual values. PSA kinetics were calculated according to the method of Khan et al. [19]. ANOVA and t tests were used to compare continuous variables. The chi-squared test was used for categorical variables. In univariate and multivariate binary logistic regression analyses, PSA1 levels and SUVmax were coded as continuous variables. PSA trend (increase vs. decrease), PSA response (yes vs. no), PET response (CR/PR/SD vs. PD), number of positive lymph nodes (LNs) on PET1 (≤5 lesions, 5 – 10 lesions, >10 lesions), number of bone lesions on PET1 (≤5 lesions, 5 – 10 lesions, >10 lesions) were coded as categorical variables. Regression coefficients for each variable were determined. The Hosmer-Lemeshow test was used to assess the goodness-of fit in the multivariate analysis. Radiological progression on PET2 (PD) in relation to PSA1 values was assessed by receiver operating characteristic (ROC) curve analysis. The best PSA1 cut-off value for distinguishing between PD and no PD on PET2 was determined using Younden’s index. All tests were two-sided. Statistical significance was taken at p < 0.05. All statistical analyses were performed using the SPSS v.21 statistical software package (SPSS Inc., Chicago, IL).
Results
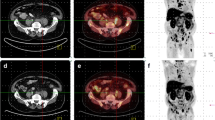
Of the 61 patients, 40 (65.5 %) showed PD on PET2, 13 (21.3 %) showed SD, 2 (3.4 %) showed PR, and 6 (9.8 %) showed CR. An increasing PSA trend was seen in 29 patients (47.5 %) and a decreasing PSA trend in 32 patients (52.5 %). A PSA response of ≥50 % was seen in 25 patients (41 %). Of the 29 patients with an increasing PSA trend, 23 (79.3 %) showed PD on PET2 and 6 (20.7 %) showed SD. Of the 32 patients with a decreasing PSA trend, 16 (50 %) showed PD on PET2, 8 (25 %) showed SD, 2 (6.2 %) showed PR and 6 (18.8 %) showed CR. Of the 25 patients with a PSA response of ≥50 %, 11 (44 %) showed PD on PET2, 6 (24 %) showed SD, 2 (8 %) showed PR, and 6 (24 %) showed CR. The results are summarized in Table 2, and 11C-choline PET/CT imaging in an example patient with a PSA response of ≥50 % following docetaxel treatment but PD on PET2 is shown in Fig. 1.
A 64-year-old patient with PC diagnosed in 2010 (PSA 12.1 ng/ml, Gleason score 5+5, T3N1M1) treated with external beam radiotherapy and ADT as primary treatment. Despite the primary treatment PSA had increased to 800 ng/ml 12 months later. The patient underwent the first 11C-choline PET/CT scan in September 2011, and was then treated with docetaxel (docetaxel 75 mg/m2 + prednisone 5 mg) until December 2011. After the first 3 months of treatment, PSA decreased from 800 to 100 ng/ml. A posttreatment 11C-choline PET/CT scan was performed in January 2012. a Pretreatment 11C-Choline PET/CT scan (PET1) shows multiple large lymph node lesions in the abdomen and in a left supraclavicular lymph node (maximum intensity projection). b Posttreatment 11C-choline PET/CT scan (PET2) confirms the presence of multiple lymph node lesions (increased uptake) (maximum intensity projection) and shows a new metastatic bone lesion in D4
On PET1, the SUVmax values for local lesions, LN lesions and skeletal lesions were 3.4 (range 3 – 4.2), 6 (range 2 – 14) and 7.9 (range: 3.4 – 17), respectively. On PET2, SUVmax values for LN lesions and skeletal lesions were 7.5 (range: 2 – 16) and 8.7 (range: 2.7 – 18), respectively. Data concerning all positive findings detected on PET1 and PET2 are presented in detail in Table 3.
A significant difference (t test and ANOVA) was observed for number of bone lesions (number of bone lesions >10) detected at PET1 between patients with radiological PD and patients without PD (higher number of skeletal lesions at PET1 in patients with PD; p < 0.05). No significant difference in PSA1 was observed between patients with PD and without PD on PET2 (p > 0.05). In the univariate and multivariate binary logistic regression analyses the number of skeletal lesions detected on PET1 was significantly associated with the probability of PD on PET2 (OR 1.627; 95 % CI 1.011 – 1.839; p = 0.046). Other factors included in the analysis (PSA1, number of LNs, PET1 SUVmax) were not significant predictors of radiological PD. None of the investigated factors (PSA1, number of bone lesions, number of LNs, PET1 SUVmax) was associated with the biochemical response to treatment (PSA response ≥50 %; p > 0.05). The univariate analysis demonstrated no change in the significance of the effects of the single variables. In the multivariate analysis, the Hosmer-Lemeshow test showed no lack of fit in the model. The ROC curve analysis was not able to provide a precise PSA1 cut-off value for distinguishing patients with PD from those without PD on PET2 (AUC 0.495; 95 % CI 0.342 – 0.647). Comparing different treatment schemes with the PET results, no significant differences in terms of the number of lesions and SUVmax were observed among patients who received different numbers of cycles.
Discussion
In the last decade, the use of choline PET/CT has been proposed in different stages of the PC natural history. However, the best results have been obtained in detecting the site of relapse in patients with biochemical relapse and in guiding salvage treatments [20–22]. Choline PET/CT has a significant impact on patient management, particularly if it is performed in selected populations and in a multidisciplinary clinical setting [23, 24]. Recently, it has also been demonstrated that choline PET/CT is a significant predictor of disease-free survival and overall survival in CRPC or castration-naive patients [25, 26]. Nevertheless, the proper use of choline PET/CT in mCRPC is not yet established and, considering the lack of evidence in the literature, the guidelines [4, 5] do not suggest the routine use of this imaging procedure at this stage of the disease. On the contrary, in mCRPC patients, radiological evaluation has recently been suggested by the European Expert Consensus Panel as the ideal method for predicting response to treatment [27].
De Giorgi et al. [28, 29] assessed the usefulness of 18F-choline PET/CT for assessing the early response to treatment with abiraterone and enzalutamide in two small cohorts of mCRPC patients. These authors concluded that a radiological response as assessed with 18F-choline PET/CT was associated with a more favourable overall survival than a PSA response of ≥50 %. Moreover, Kwee et al. [30] demonstrated that metastatic PC lesions detected by 18F-choline PET/CT can be quantified on the basis of volumetric measurements of tumour metabolic activity. They found that this factor, if measured in patients before the first line of chemotherapy or second line of ADT, has high value in predicting overall survival.
To the best of our knowledge, this is the first study designed to assess the usefulness of 11C-choline PET/CT for the monitoring of docetaxel treatment in mCRPC patients. Radiological evaluation with 11C-choline PET/CT was better for assessing the response to docetaxel than the biochemical response to treatment. In our series, disagreement between PSA and 11C-choline PET/CT response (in patients with PD) was seen in 32 of the 61 patients (52.5 %; Table 2), and it is noteworthy that the majority of disagreements between PSA and 11C-choline PET/CT response were in patients who showed a decreasing PSA trend. Of 32 patients with a decreasing PSA trend, 16 (50 %) showed PD on PET2. Within this group, considering the 25 patients with a PSA decrease of ≥50 % after docetaxel treatment, roughly 44 % (11/25) showed PD on PET2. On the contrary, considering the 29 patients with an increasing PSA trend, 79.3 % showed PD on PET2, while the remaining 20.7 % showed SD and none of them showed a response to treatment (Table 2). It therefore seems that the assessment of PSA is not an optimal predictor for assessing response to the first line of chemotherapy. Our results may indicate that a posttreatment 11C-choline PET/CT scan would be of value mostly in patients with decreasing PSA levels after treatment to identify radiological and metabolic progression of the disease.
Furthermore, this study also showed a significant association between the number of lesions detected by PET1 and the response to treatment: patients with more than ten bone lesions on PET1 had a higher risk of PD on PET2. On the contrary, no association was found between PSA1 and the risk of PD on PET2. Although investigation of the association between the number of lesions and response to treatment was a secondary aim of the study, the ability to predict the response to docetaxel before treatment could be considered useful for clinicians in planning the most appropriate therapy scheme in mCRPC patients.
Finally, preliminary data on new receptor radiopharmaceuticals for PET/CT imaging, such as 68Ga-labelled-PSMA [31–33], show a significant improvement in PET/CT performance in the detection of the site of recurrence in patients with biochemical relapse. However, it is still not clear if a receptor radiopharmaceutical, instead of a metabolic tracer, would have the same value for treatment response monitoring. Since dedicated studies designed to investigate CRPC patients with 68Ga-PSMA PET/CT have not yet been performed, comparison with choline PET/CT is not possible at present.
The retrospective design of the study could be considered its main limitation. A prospective trial specifically designed to evaluate treatment monitoring would be preferable. Such a study should enable the measurement of PSA levels and the scanning of patients at the same time before and after chemotherapy. Another limitation was the number of patients enrolled: a larger population would be preferable to produce a statistical analysis with higher predictive power.
Conclusion
Our data suggest that an increase in PSA values measured after the end of docetaxel treatment is predictive of radiological PD since PD was seen in 79.3 % of patients with an increasing PSA trend. On the other hand, when PSA values decreased after treatment (decreasing PSA trend and a PSA response of ≥50 %), 11C-choline PET/CT could be considered in selected patients as a promising imaging procedure to assess the risk of eventual radiological progression despite a PSA response. In our patient series, this occurred in the 44 % of the patients showing a PSA response of ≥50 %. Finally, the tumour burden, expressed as the number of bone lesions on PET1, is significantly associated with an increased probability of PD on PET2. Further prospective studies in larger populations are needed to confirm these preliminary results.
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403.
Sridhar SS, Freedland SJ, Gleave ME, Higano C, Mulders P, Parker C, et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment. Eur Urol. 2014;65:289–99.
Loblaw DA, Walker-Dilks C, Winquist E, Hotte SJ. Systemic therapy in men with metastatic castration-resistant prostate cancer: a systematic review. Clin Oncol (R Coll Radiol). 2013;25:406–30.
Mottet N, Bellmunt J, Briers E, van den Bergh RCN, Bolla M, van Casteren NJ, et al. Guidelines on prostate cancer. European Association of Urology; 2015.
Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D’Amico AV, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12(5):686–718.
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the PSA Working Group. J Clin Oncol. 1999;17:3461–7.
Mulders PF, Schalken JA. Measuring therapeutic efficacy in the changing paradigm of castrate-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2009;12:241–6.
Ryan CJ, Shah S, Efstathiou E, Smith MR, Taplin ME, Bubley GJ, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854–61.
Armstrong AJ, Garrett-Mayer E, de Wit R, Tannock I, Eisenberger M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16(1):203–11.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. doi:10.1016/j.ejca.2008.10.026.
Castellucci P, Fuccio C, Nanni C, Santi I, Rizzello A, Lodi F, et al. Influence of trigger PSA and PSA kinetics on 11C-choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med. 2009;50:1394–400.
Krause BJ, Souvatzoglou M, Tuncel M, Herrmann K, Buck AK, Praus C, et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35(1):18–23.
Castellucci P, Fuccio C, Rubello D, Schiavina R, Santi I, Nanni C, et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur J Nucl Med Mol Imaging. 2011;38(1):55–63.
Mamede M, Ceci F, Castellucci P, Schiavina R, Fuccio C, Nanni C, et al. The role of 11C-choline PET imaging in the early detection of recurrence in surgically treated prostate cancer patients with very low PSA level <0.5 ng/mL. Clin Nucl Med. 2013;38(9):e342–5.
Ceci F, Castellucci P, Mamede M, Schiavina R, Rubello D, Fuccio C, et al. (11)C-Choline PET/CT in patients with hormone-resistant prostate cancer showing biochemical relapse after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2013;40(2):149–55.
World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000;284:3043–5.
Pascali C, Bogni A, Iwata R. 11C-methylation on 18C SepPak cartridge: a convenient way to produce [N-methyl-11C]choline. J Labelled Comp Radiopharm. 2000;49:195–203.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35(13):1773–82.
Khan MA, Carter HB, Epstein JI, Miller MC, Landis P, Walsh PW, et al. Can prostate specific antigen derivatives and pathological parameters predict significant change in expectant management criteria for prostate cancer? J Urol. 2003;170(6 Pt 1):2274–8.
Castellucci P, Ceci F, Graziani T, Schiavina R, Brunocilla E, Mazzarotto R, et al. Early biochemical relapse after radical prostatectomy: which prostate cancer patients may benefit from a restaging 11C-choline PET/CT scan before salvage radiation therapy? J Nucl Med. 2014;55(9):1424–9.
Picchio M, Berardi G, Fodor A, Busnardo E, Crivellaro C, Giovacchini G, et al. (11)C-Choline PET/CT as a guide to radiation treatment planning of lymph-node relapses in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2014;41(7):1270–9.
Suardi N, Gandaglia G, Gallina A, Di Trapani E, Scattoni V, Vizziello D, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol. 2015;67(2):299–309.
Ceci F, Herrmann K, Castellucci P, Graziani T, Bluemel C, Schiavina R, et al. Impact of 11C-choline PET/CT on clinical decision making in recurrent prostate cancer: results from a retrospective two-centre trial. Eur J Nucl Med Mol Imaging. 2014;41(12):2222–31.
Soyka JD, Muster MA, Schmid DT, Seifert B, Schick U, Miralbell R, et al. Clinical impact of 18F-choline PET/CT in patients with recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2012;39(6):936–43.
Giovacchini G, Incerti E, Mapelli P, Kirienko M, Briganti A, Gandaglia G, et al. [11C]Choline PET/CT predicts survival in hormone-naive prostate cancer patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2015;42(6):877–84.
Giovacchini G, Picchio M, Garcia-Parra R, Briganti A, Abdollah F, Gianolli L, et al. 11C-choline PET/CT predicts prostate cancer-specific survival in patients with biochemical failure during androgen-deprivation therapy. J Nucl Med. 2014;55(2):233–41.
Fitzpatrick JM, Bellmunt J, Fizazi K, Heidenreich A, Sternberg CN, Tombal B, et al. Optimal management of metastatic castration-resistant prostate cancer: highlights from a European Expert Consensus Panel. Eur J Cancer. 2014;50(9):1617–27.
De Giorgi U, Caroli P, Burgio SL, Menna C, Conteduca V, Bianchi E, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget. 2014;5(23):12448–58.
De Giorgi U, Caroli P, Scarpi E, Conteduca V, Burgio SL, Menna C, et al. (18)F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur J Nucl Med Mol Imaging. 2015;42:1276–83.
Kwee SA, Lim J, Watanabe A, Kromer-Baker K, Coel MN. Prognosis related to metastatic burden measured by 18F-fluorocholine PET/CT in castration-resistant prostate cancer. J Nucl Med. 2014;55(6):905–10.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56(5):668–74.
Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. 68Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–94.
Compliance with ethical standards
Conflicts of interest
None.
Ethical approval
This study was performed according to the principles of the Declaration of Helsinki and its subsequent amendments.
Informed consent
Written informed consent was obtained from all patients before the start of the study according to institutional guidelines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceci, F., Castellucci, P., Graziani, T. et al. 11C-Choline PET/CT in castration-resistant prostate cancer patients treated with docetaxel. Eur J Nucl Med Mol Imaging 43, 84–91 (2016). https://doi.org/10.1007/s00259-015-3177-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3177-4