Abstract
Purpose
To determine the diagnostic efficacy of 11C-choline PET/CT in patients with prostate cancer (PC) after radical prostatectomy who presented with increasing PSA levels during follow-up in spite of being on hormone treatment (HT), and therefore showing HT resistance.
Methods
We evaluated a large series of 157 consecutive PC patients previously treated by radical prostatectomy who presented with biochemical recurrence with increasing PSA levels in spite of ongoing HT (HT-resistant patients). At the time of 11C-choline PET/CT, the mean value of trigger PSA level was 8.3 (range 0.2 – 60.6 ng/mL), the mean PSA doubling time (PSAdt) was 5.3 (range 0.4 – 35 months), and the mean PSA velocity (PSAvel) was 22.1 ng/mL/year (range 0.12 – 82 ng/mL/year). 11C-Choline PET/CT was performed following a standard procedure at our centre to investigate increasing PSA levels, either as the first imaging procedure or in patients with negative conventional imaging. At the time of 11C-choline PET/CT all patients were receiving HT (61 were receiving monotherapy and 96 multidrug therapy). PET-positive findings were validated by: (a) transrectal US-guided biopsy in patients with recurrence in the prostatic bed, (b) surgical pelvic lymphadenectomy, (c) other imaging modalities, including repeated 11C-choline PET/CT, performed during a minimum follow-up of 12-months.
Results
11C-Choline PET/CT showed positive findings in 104 of the 157 patients (66 %). 11C-choline PET/CT detected: a single lesion in 40 patients (7 in the prostate bed, 10 in lymph nodes, 22 in bone, 1 at another site); two lesions in 18 patients (7 in lymph nodes, 7 in bone, 4 in both lymph nodes and bone); three or four lesions in 7 patients (4 in lymph nodes, 2 in bone, 1 at another site); and more than four lesions in the remaining 39 patients (2 in the prostate bed, 12 in lymph nodes, 12 in bone, 11 in both lymph nodes and bone, 2 at other sites). In 11C-choline PET-negative patients, the mean values of trigger PSA, PSAdt and PSAvel were 3.8 ng/mL (range 0.2 – 11.9 ng/mL) 7.0 months (range 1.21 – 35 months) and 5.8 ng/mL/year (range 0.12 – 30.1) respectively, while in 11C-Choline-PET-positive patients they were 10.5 ng/mL (range 0.2 – 60.6), 4.4 months (range 0.4 – 19.7) and 15.9 ng/mL/year (range 0.5 – 82.0) respectively. The differences between PET-negative and PET-positive patients were statistically significant for all these parameters: trigger PSA, p < 0.01; PSAdt, p < 0.01; PSAvel, p = 0.03.
Conclusion
In our patient population, 11C-choline PET/CT was able to detect relapsed disease in a large proportion of HT-resistant PC patients during HT. These data, obtained in a large series, suggest that HT withdrawal before performing a 11C-choline PET/CT scan may not be necessary for the detection of recurrent disease if PSA levels are increasing and PSA kinetics are rapid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence of prostate cancer (PC) has greatly increased over recent years and it is now the second leading cause of cancer death in men over the age of 50 years. In the European Union the incidence rate of PC is 78.9 per 100,000 men per year, and the mortality rate is 30.6 per 100,000 men per year [1].
Recurrence following radical prostatectomy (RP) is a relatively common finding, occurring in 20 – 30 % of patients during a 10-year follow-up [2–4]. The monitoring of prostate-specific antigen (PSA) serum levels and its kinetic parameters PSA doubling time (PSAdt) and PSA velocity (PSAvel) is the most easy and cost-effective way to follow up PC patients after RP. Trigger PSA has been proven to be a highly sensitive marker for the early recognition of relapsing disease [5]. When biochemical relapse occurs after RP (trigger PSA >0.2 ng/mL), it is crucial to discriminate between the presence of local and distant metastatic spread in order to choose the most appropriate therapeutic approach [1, 2]. The sensitivity and accuracy of available imaging methods have greatly increased in the last few years. In particular, magnetic resonance (MR) imaging with spectroscopy or diffusion-weighted imaging has been reported to be the optimal modality for the detection of local relapse and as a guide for transrectal ultrasound-guided (TRUS-guided) biopsy [6, 7]. However, in the detection of distant metastases, different imaging modalities such as bone scan and contrast-enhanced CT, fail to detect tumoral deposits in most patients, in particular those with low trigger PSA values [8, 9].
This lack of sensitivity led the board of the European Urology Association to discourage the use of any imaging modality for PC restaging in patients who are asymptomatic and with trigger PSA values lower than 20 ng/mL [10]. As a consequence, in many centres, biochemically relapsed patients are either treated with radiotherapy to the prostatic fossa with or without confirmation of local recurrence by biopsy and with or without knowledge of the presence of distant metastases. However, it is reasonable to think that in the era of “personalized medicine and tailored therapy” this therapeutic strategy should be improved. This is particularly advisable considering that in recent years PET/CT with 11C-choline (or 18F-choline) has emerged as a promising molecular imaging tool for early detection of PC recurrence in patients with biochemical relapse [11–15]. However, the appropriate use of 11C-choline PET/CT during biochemical recurrence does not seem to be completely established. There are several open questions that need clarification. One of these concerns the necessity or not to withdraw hormone treatment (HT) before the performance of a 11C-choline PET/CT examination.
In this view, the main aim of our study was to determine the diagnostic value of whole-body 11C-choline PET/CT in PC patients previously treated with RP, who presented HT resistance during follow-up seen as an increase in trigger PSA levels in spite of ongoing HT. In particular, we wanted to determine if HT withdrawal before 11C-choline PET/CT is necessary to obtain a clinically useful PET/CT examination.
Materials and methods
Patient population
The study was performed in accordance with the principles of the Declaration of Helsinki and with national regulations. All patients provided informed consent for participation and publication of anonymized data.
We retrospectively analysed the clinical records of 3,721 consecutive patients referred to our centre for 11C-choline PET/CT examination from January 2007 to August 2011. Of these patients, 157 met the inclusion criteria for enrolment in the present study: (a) patients with previous RP, (b) patients receiving HT either with monotherapy or multidrug therapy, (c) patients with at least two consecutive PSA measurements showing increasing values in spite of ongoing HT (HT resistance), and (d) patients in whom 11C-choline PET/CT was the first imaging modality performed at diagnosis of biochemical relapse or in whom conventional imaging work-up did not reveal the presence of recurrent disease. The characteristics of the patient population are summarized in Table 1.
Radiopharmaceutical
11C-Choline was synthesized using a solid-phase method as described by Pascali et al. [14, 16], using a commercial synthesis module (TracerLab, GE Medical Systems, Waukesha, WI).
Imaging protocol
11C-Choline PET/CT was performed following a standard procedure in our centre. Briefly, all examinations were performed with a hybrid PET/CT tomograph (Discovery LS and Discovery STE; GE Medical Systems, Waukesha, WI). The patients fasted for at least 6 h before PET scanning and received an intravenous injection of 370 – 555 MBq of 11C-choline. The PET/CT scan started 3 – 5 min after radiotracer injection. Emission data were acquired with five or six bed positions from the mid-thigh to the base of the skull, taking 3 – 4 min for each bed position (in relation to body weight and volume). The low-dose CT parameters were 120 kVp, 60 mA, 0.8 s per tube rotation, slice thickness 5 mm, pitch 1.5, and table speed 30 mm per rotation. Low-dose CT images were used both for attenuation correction of the emission data and for image fusion.
Image analysis and validation criteria
All 11C-choline PET/CT images were analysed with dedicated software (eNTEGRA; GE Medical Systems, Waukesha, WI) which allowed PET, CT and fused imaging data to be reviewed. First, transaxial, sagittal and coronal PET images were assessed visually in consensus by two experienced nuclear medicine physicians aware of the clinical data. Maximum standardized uptake values (SUVmax) were also measured but not used to reach a final diagnosis. Patient in whom there was disagreement between the readers (15 out of 157) were re-examined and a consensus was reached. Criteria for validation of a positive PET/CT finding were: (a) TRUS-guided biopsy in patients with local recurrence in the prostatic bed, (b) histological findings obtained on re-operation to remove pelvic lymph node metastasis, (c) positive distant tumoral deposits on 11C-choline PET/CT that were confirmed by at least two targeted contrast-enhanced CT and/or MR and/or bone scan examinations, (d) prolonged (>12 months) clinical and imaging follow-up, including contrast-enhanced CT, MR, bone scan and repeated 11C-choline PET/CT that demonstrated the appearance of further metastatic lesions or, alternatively, the disappearance of metastatic deposits associated with normalization of PSA values (<0.2 ng/mL) under specific therapies.
Hormone treatment
At the time of PET/CT examination all patients had been taking HT from at least 6 months: 61 patients were receiving monotherapy and 96 were receiving multidrug therapy. Detailed data are presented in Table 2.
Statistical analysis
Continuous variables were compared between two groups using the t test. The chi-squared test was used for categorical variables. In the univariate analysis and multivariate binary regression analysis, age, PSA, PSAdt and PSAvel were coded as continuous variables; Gleason score (grouped as ≤7 vs. >7), initial T (T2 vs. T3/4), and any TN0 vs. any T, N1 and time to relapse, (≤24 vs. >24 months) as well as the type of ongoing HT (monotherapy vs. multidrug therapy) at the time of 11C-choline PET/CT scan were coded as categorical variables.
The relationship between clinical and pathological features and 11C-choline PET/CT findings was assessed using univariate and multivariate binary logistic analysis. Statistical significance was assumed at p < 0.05. All statistical analyses were performed using the SPSS v16 statistical software package (SPSS, Chicago, IL). As in our previous studies, PSAdt and PSAvel were calculated according to as described by Khan et al. [17].
Results
Overall, 11C-choline PET/CT showed positive findings in 104 of the 157 patients resistant to HT (66 %). In detail, 11C-Choline PET/CT detected a single lesion in 40 patients. two lesions in 18 patients, three or four lesions in 7 patients, and multiple lesions (more than four) in the remaining 39 patients. 11C-Choline PET/CT-positive findings are shown in detail in Table 3.
In PET-negative patients, the mean values of trigger PSA, PSAdt and PSAvel were 3.8 ng/mL (range 0.2 – 11.9 ng/mL), 7.0 months (range 1.21 – 35 months), and 5.8 ng/mL/year (range 0.12 – 30.1 ng/mL/year), respectively, while in PET-positive patients the mean values were 10.5 ng/mL (range 0,2 – 60.6 ng/mL), 4.4 months (range 0.4 – 19.7 months), and 15.9 ng/mL/year (range 0.5 – 82.0 ng/mL/year), respectively. These three parameters were significantly different between the PET-negative and PET-positive patients (trigger PSA p < 0.01, PSAdt p < 0.01, PSAvel p = 0.03). In contrast, age, TNM stage, Gleason score, time to relapse, previous radiotherapy, type of HT (monotherapy vs. multidrug therapy) were not significantly different in either the univariate or the multivariate analysis.
In the patients with a trigger PSA value <5 ng/mL (including 89 patients with a mean PSA value of 2.5 ng/mL), the rate of lesion detection by 11C-choline PET/CT was 52 % (46 of 89 patients). In 21 of these 46 patients, PET/CT showed a single lesion, in 11 two lesions, and in 14 multiple lesions. In the patients with a trigger PSA <2 ng/mL (including 32 patients with a mean PSA mean value of 0.97 ng/mL), the rate of lesion detection by 11C-choline PET/CT was 31 % (10 of 32 patients). In 7 of these 10 patients, PET/CT showed a single lesion, in 2 two lesions, and in 1 multiple lesions. The relationship between the PSA values and the number of positive findings detected by 11C-choline PET/CT is shown in Fig. 1.
Relationship between the rate of lesion detection by PET and PSA values in patients divided on the basis of trigger PSA level at the time of the PET scan: PSA ≤2 ng/mL (32 patients); PSA 2 – 5 ng/mL (57 patients); PSA >5 ng/mL (68 patients). The relationship between the detection rate in each patient group and the number of lesions (multiple lesions vs. 1 or 2 lesions) is also shown
Validation of findings
Positive findings were validated by TRUS-guided biopsy in 10 patients, surgery in 23 patients, other imaging-targeted modalities in 19 patients, and clinical and imaging follow-up of >12 months in 105 patients. According to these criteria, 11C-choline PET/CT did not show any false-positive findings.
Discussion
In recent years PET/CT with 11C-choline (or 18F-choline) has emerged as a promising molecular imaging tool for detection of PC recurrence in patients with biochemical relapse after RP [2, 3]. Even though the rate of lesion detection by 11C-choline PET/CT seems to be relatively poor in patients with low trigger PSA levels, it performs better than the other conventional imaging modalities [11, 15, 18, 19]. In a very recent study, Soyka et al. [20] investigated the clinical value of 18F-choline PET/CT in treatment decision making in 156 patients with recurrent PC, and confirmed the positive impact of this examination on planning of the optimal therapeutic strategy. Despite this, the use of 11C/18F-choline PET/CT in this field has not yet been completely established. In particular, the criteria to appropriately select the patients with PC to be scanned by 11C/18F-choline PET/CT, the best time to perform 11C/18F-choline PET/CT during the natural history of the disease, and whether or not HT should be withdrawn before performing 11C/18F-choline PET/CT, are issues still debated in the literature. In this work we focused on the relationship between HT administration and 11C-choline PET/CT. HT is largely used as adjuvant treatment in patients with biochemical recurrence after RP either using a continuous or intermittent administration scheme. Since 2001, it has been known that the administration of HT may cause a reduction in 18F-choline uptake by PC cells. [21]. Other studies have shown a significant influence of HT on 11C-choline uptake by PC [22, 23], and also in a recently study in our centre [24], we found an influence of HT on 11C-choline PET/CT sensitivity in a small population of 14 patients followed with sequential PET scans before and after HT administration. The above reported studies were performed in HT-sensitive patients or in mixed HT-sensitive and HT-resistant patients.
To the best of our knowledge, this is the first study specifically to include a large group of 157 HT-resistant patients only, that is patients who showed an increase in PSA levels during ongoing HT treatment. These patients were taken from large series of 3,721 consecutive PC patients referred to our centre for a 11C-choline PET/CT examination. In our study the rate of lesion detection by PET is similar (66 %) to that found in other studies performed in patient series with similar values of trigger PSA [12–14, 19, 25, 26] in which patients were not receiving HT or in which the patients investigated were mixed populations (patients receiving and not receiving HT). This finding confirms the observation that was first made by Giovacchini et al. [25] in a population of 358 patients. These authors found that the rate of lesion detection by11C-choline PET/CT in patients receiving HT at the time of the scan (155 patients) was even higher than in patients who were not receiving any therapy (203 patients): 56 % vs. 44 %, respectively. These findings were later confirmed, but in a very limited population of HT-resistant patients (14 patients) in a study conducted in our centre [24] with 11C-choline and in another study by Marzola et al. who recruited a large series of 233 patients with PC recurrence evaluated by 18F-choline PET/CT (Marzola MC et al, accepted for publication). In the latter study, at the time of PET investigation 103 patients were receiving HT and 163 were not. The detection rate of recurrent disease was 67 % and 44 %, respectively; the difference was significant (p < 0.001). These findings are very similar to the findings of Giovacchini et al. obtained using 11C-choline PET/CT [25].
The good detection rate with 11C-choline PET/CT observed in the present study suggests that 11C-choline PET/CT does not lose its sensitivity in detecting recurrent disease in PC patients, even in those receiving HT. Yet 11C-choline PET/CT may be able to detect tumour recurrence which has become HT-resistant. Targeted and aggressive therapy could be considered in HT-resistant patients as soon as the PSA level starts to increase. Moreover, if 11C-choline PET/CT is performed as soon as possible after the PSA level starts to increase, there is a higher possibility that only a few metastatic lesions will be present (Fig. 1).
As in previous studies, a limitation of the present study is its retrospective nature. Prospective studies performed in PC patients with recurrent disease and based on sequential PET/CT examinations performed before and during HT are desirable.
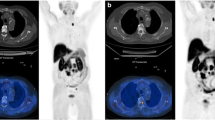
However, the administration of HT in PC patients who have developed hormone resistance and who show an increase in PSA levels does not seem to significantly influence the detection of recurrent disease by 11C-choline PET/CT. Moreover, the present study also showed that in patients receiving HT at the time of 11C-choline PET/CT there is also a significant relationship between PSA kinetics and 11C-choline PET/CT. Therefore, fast PSA kinetics appears to be the most relevant factor together with trigger PSA level in predicting a positive 11C-choline PET/CT independent of HT sensitivity or HT resistance. Furthermore, in a significant proportion of our patients a limited number of tumoral deposits were detected by 11C-choline PET/CT, in particular when PSA levels were low. The early diagnosis of limited disease relapse may be important from a therapeutic point of view. An example of early PET detection of a solitary recurrence in a PC patient receiving HT and completely cured by radiosurgery is shown in Fig. 2.
A 74-year-old man with PC treated by RP in June 2008. Biochemical recurrence was treated with HT (leuprorelin 375 mg + bicalutamide 50 mg daily) from September 2009. At the time of the 11C-choline PET/CT scan, the trigger PSA level was 1.6 ng/mL, the PSA doubling time was 4.1 months, and the PSA velocity was 16.2 ng/mL/year. a 11C-Choline PET/CT axial fused image shows a single sternal lesion that was treated with intensity modulated radiation therapy. b Maximum intensity projection anterior whole-body view shows the solitary sternal lesion. c 11C-Choline PET/CT fused image 8 months after treatment shows a complete response to the radiosurgery. The trigger PSA level had dropped to below the limit of detection (0.05 ng/mL). d Maximum intensity projection anterior whole-body view shows the absence of tumoral lesions
Finally, the problem of validation of positive findings is the main limitation of our study. Our validation, in the majority of our patients (105 of 157) was based on a longitudinal follow-up of each lesion. Histology would have been preferable, but it was not feasible for practical and ethical reasons.
Conclusion
In the present study, 11C-choline PET/CT detected recurrent disease in 66 % of a large series of PC patients in whom a progressive trigger PSA increase was observed in spite of ongoing HT (HT resistance). Moreover, the rate of lesion detection was higher in patients with faster PSA kinetics. On the basis of these findings, it is reasonable to state that HT withdrawal is not necessary before performing a 11C-choline PET/CT scan in HT-resistant PC patients. Furthermore, 11C-choline PET/CT may show the site of HT-resistant tissue that can be treated with a targeted therapy.
References
Kataja VV, Bergh J. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of prostate cancer. Ann Oncol. 2005;16 Suppl 1:i34–6.
Freedland SJ, Presti Jr JC, Amling CL, Aronson WJ, Dorey F, et al. Time trends in biochemical recurrence after radical prostatectomy: results of the SEARCH database. Urology. 2003;61:736–41.
Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–23.
Chism DB, Hanlon AL, Horwitz EM, Feigenberg SJ, Pollack A. A comparison of the single and double factor high-risk models for risk assignment of prostate cancer treated with 3D conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(20):380–5.
Roberts SG, Blute ML, Bergstralh EJ, Slezak JM, Zincke H. PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clin Proc. 2001;76:576–81.
Sella T, Schwartz LH, Swindle PW, Onyebuchi CN, Scardino PT, Scher HI, et al. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology. 2004;233:379–85.
Coakley FV, Teh HS, Qayyum A, Swanson MG, Lu Y, Roach 3rd M, et al. Endorectal MR imaging and MR spectroscopic imaging for locally recurrent prostate cancer after external beam radiation therapy: preliminary experience. Radiology. 2004;233:441–8.
Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008;179:906–10.
Okotie OT, Aronson WJ, Wieder JA, Liao Y, Freedland SJ, Dekernion JB, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol. 2004;171:2260–4.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, Mason MD, et al. Guidelines on prostate cancer. Eur Urol. 2011;59(1):61–71.
Cimitan M, Bortolus R, Morassut S, Canzonieri V, Garbeglio A, Baresic T, et al. 18Ffluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging. 2006;33:1387–98.
Husarik DB, Miralbell R, Dubs M, John H, Giger OT, Gelet A, et al. Evaluation of 18F-choline PET/CT for staging and restaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:253–63.
Giovacchini G, Picchio M, Scattoni V, Garcia Parra R, Briganti A, Gianolli L, et al. PSA doubling time for prediction of [(11)C]choline PET/CT findings in prostate cancer patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37:1106–16.
Castellucci P, Fuccio C, Nanni C, Santi I, Rizzello A, Lodi F, et al. Influence of trigger PSA and PSA kinetics on 11C-choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med. 2009;50:1394–400.
Picchio M, Messa C, Landoni C, Gianolli L, Sironi S, Brioschi M, et al. Value of 11Ccholine-positron emission tomography for re-staging prostate cancer: a comparison with 18Ffluorodeoxyglucose-positron emission tomography. J Urol. 2003;169:1337–40.
Pascali C, Bogni A, Itawa R, Cambiè M, Bombardieri E. [11C]Methylation on a C18 Sep-Pak cartridge: a convenient way to produce N-methyl-11Ccholine. J Labelled Comp Radiopharm. 2000;49:195–203.
Khan MA, Carter HB, Epstein JI, Miller MC, Landis P, Walsh PW, et al. Can prostate specific antigen derivatives and pathological parameters predict significant change in expectant management criteria for prostate cancer? J Urol. 2003;170:2274–8.
Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using carbon-11-choline. J Nucl Med. 1998;39:990–5.
Krause BJ, Souvatzoglou M, Tuncel M, Herrmann K, Buck AK, Praus C, et al. The detection rate of 11Ccholine-PET/TC depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:18–23.
Soyka JD, Muster MA, Schmid DT, Schick U, Miralbell R. Clinical impact of 18F-choline PET/CT in patients with recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2012;39:936–43.
DeGrado TR, Coleman RE, Wang S, Baldwin SW, Orr MD, Robertson CN, et al. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer. Cancer Res. 2001;61:110–7.
De Waele A, Van Binnebeek S, Mottaghy FM. Response assessment of hormonal therapy in prostate cancer by [11C]choline PET/CT. Clin Nucl Med. 2010;35:701–3.
Giovacchini G, Picchio M, Coradeschi E, Scattoni V, Bettinardi V, Cozzarini C, et al. [(11)C]choline uptake with PET/CT for the initial diagnosis of prostate cancer: relation to PSA levels, tumour stage and anti-androgenic therapy. Eur J Nucl Med Mol Imaging. 2008;35:1065–73.
Fuccio C, Schiavina R, Castellucci P, Rubello D, Martorana G, Celli M, et al. Androgen deprivation therapy influences the uptake of 11C-choline in patients with recurrent prostate cancer: the preliminary results of a sequential PET/CT study. Eur J Nucl Med Mol Imaging. 2011;38:1985–9.
Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, et al. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37:301–9.
Castellucci P, Fuccio C, Rubello D, Schiavina R, Santi I, Nanni C, et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5ng/ml? Eur J Nucl Med Mol Imaging. 2011;38:55–63.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceci, F., Castellucci, P., Mamede, M. et al. 11C-Choline PET/CT in patients with hormone-resistant prostate cancer showing biochemical relapse after radical prostatectomy. Eur J Nucl Med Mol Imaging 40, 149–155 (2013). https://doi.org/10.1007/s00259-012-2272-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2272-z