Abstract
Purpose
Despite good to excellent inter-reader agreement in the evaluation of amyloid load on PET scans in subjects with Alzheimer's disease, some equivocal findings have been reported in the literature. We aimed to describe the clinical characteristics of subjects with equivocal PET images.
Methods
Nondemented subjects aged 70 years or more were enrolled from the MAPT trial. Cognitive and functional assessments were conducted at baseline, at 6 months, and annually for 3 years. During the follow-up period, 271 subjects had 18F-AV45 PET scans. Images were visually assessed by three observers and classified as positive, negative or equivocal (if one observer disagreed). After debate, equivocal images were reclassified as positive (EP+) or negative (EP−). Scans were also classified by semiautomated quantitative analysis using mean amyloid uptake of cortical regions. We evaluated agreement among the observers, and between visual and quantitative assessments using kappa coefficients, and compared the clinical characteristics of the subjects according to their PET results.
Results
In 158 subjects (58.30 %) the PET scan was negative for amyloid, in 77 (28.41 %) the scan was positive and in 36 (13.28 %) the scan was equivocal. Agreement among the three observers was excellent (kappa 0.80). Subjects with equivocal images were more frequently men (58 % vs. 37 %) and exhibited intermediate scores on cognitive and functional scales between those of subjects with positive and negative scans. Amyloid load differed between the EP− and negative groups and between the EP+ and positive groups after reclassification.
Conclusion
Equivocal amyloid PET images could represent a neuroimaging entity with intermediate amyloid load but without a specific neuropsychological pattern. Clinical follow-up to assess cognitive evolution in subjects with equivocal scans is needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, many studies have demonstrated that amyloid biomarkers such as cerebrospinal fluid markers or specific ligands using PET can be useful in the accurate diagnosis of Alzheimer’s disease (AD) [1]. Numerous studies have involved the use of PET tracers that bind to amyloid plaques in vivo [2–4]. Among them 18F-AV45 (Amyvid™) is a radiopharmaceutical that shows cortical uptake well linked to AD pathology [5, 6]. This compound has been widely used with good performance in differentiating AD patients from cognitively normal subjects [7–9] and has recently been approved for use by the Food and Drug Administration (April 2012) and the European Medicines Agency (January 2013).
However, amyloid PET imaging suffers from some limitations. One of them is the high prevalence of amyloid positivity in normal older individuals. Population-based studies are only beginning to be reported, but estimates of age-specific positivity rates for amyloid PET are less than 5 % in subjects 50 to 60 years old, 10 % in those 60 to 70 years old, 25 % in those 70 to 80 years old, and more than 50 % in those 80 to 90 years old [10, 11]. This amyloid positivity linked to age is the subject of much work in progress and some authors have suggested that “amyloid positives" may be totally asymptomatic but could have an increased risk of developing symptomatic AD [12]. Another limitation of PET amyloid imaging is the variation reported in the level of inter-reader agreement in previous studies (with a Fleiss kappa coefficient between 0.68 and 0.98) [5, 7, 13, 14]. Such variability has led the radiopharmaceutical companies to introduce special educational reading programmes which have led to a great improvement of inter-rater agreement. However, some equivocal findings continue to be observed. It would be interesting to know if subjects’ cognitive performance associated with equivocal scans is similar to the cognitive pattern in amyloid-positive or amyloid-negative subjects. Amyloid imaging results alter physicians’ diagnostic thinking, intended testing and management of patients undergoing evaluation for cognitive decline [15]. No study has sought to determine if those equivocal scans are associated with a specific pattern of clinical signs.
The main objective of our study was to analyse the specific clinical patterns of such visual equivocal scans, based on a sample of older adults, aged 70 years and over, living in the community without any clinical sign of dementia. In addition, we aimed to assess interobserver and intercentre agreement with semiquantitative analysis.
Material and methods
MAPT trial
All subjects enrolled in this neuroimaging PET study came from the Multidomain Alzheimer Preventive Trial (MAPT) [16–18], a multicentre, randomized, placebo-controlled study. The MAPT study is a large long-term trial specifically designed to test whether omega-3 fatty acid supplementation in combination with a multidomain intervention consisting of nutritional counselling, physical exercise and cognitive stimulation, is effective in slowing cognitive decline in frail older adults at risk of cognitive decline. The protocol is registered on a public-access clinical trial database (www.clinicaltrials.gov, no. NCT01513252). The members of the MAPT study group are listed in the Appendix This ancillary MAPT study protocol was approved by the French Ethics Committee in Toulouse in December 2007.
Subjects
Included subjects were aged 70 years or older and fulfilled one of the three following clinical criteria: (1) spontaneous memory complaint expressed to a general practitioner, (2) limitation in one instrumental activity of daily living (e.g. the ability to use the telephone, shop, prepare meals, do housekeeping, do the laundry, use transportation, follow a medication schedule, or manage money [19, 20], (3) slow walking speed (speed ≤0.8 m/s, i.e. more than 5 s to walk 4 m). Subjects with dementia, cognitive impairment, limitation in basic activities of daily living (bathing, dressing, toileting, transferring, continence, eating) or suffering from severe depression were not included in the trial. Inclusion and noninclusion criteria for the MAPT study and PET ancillary studies are presented in Table 1. Written, informed consent was obtained from all participants.
The first randomization was on 30 May 2008, and the targeted number of randomized participants was reached on 24 February 2011. A total of 1,680 participants were recruited in the MAPT centres. The amyloid PET ancillary study was proposed to subjects enrolled in the ten centres close to a nuclear medicine department that could offer PET amyloid imaging (Bordeaux, Limoges, Montpellier, Nice and Toulouse).
Clinical data
Clinical visits were scheduled every 6 months to assess physical condition, diseases and corresponding treatments, and adherence to multidomain intervention. Cognitive and functional assessments were conducted at baseline, at 6 months, and annually at 1, 2 and 3 years by independent research staff blinded to the interventions. All the assessments were performed by hospital practitioner memory experts. A series of neuropsychological tests was administered for cognitive assessment. These included the Free and Cued Selective Reminding Test (FCRST, episodic memory/recall) [21], the Controlled Oral Word Association Test and Category Naming Test (COWAT and CNT, verbal fluency) [22], the Digit Symbol Substitution Subtest of the Wechsler Adult Intelligence Scale–Revised (attention and executive function) [23], the Trail-Making Test (TMT, switching) [24], the Mini-Mental State Examination (MMSE) [25], and the Clinical Dementia Rating Scale (CDR). Two visual analogue scales were also administered to assess memory functioning and the consequences of memory impairment in everyday life. In addition, functional assessment included the Alzheimer Disease Cooperative Study–Activities of Daily Living Prevention Instrument (ADCS-ADL, dependency) and the Short Physical Performance Battery (SPPB) to evaluate the functional capacities [26]. Frailty was evaluated using the classification system proposed by Fried et al. [27], based on assessments of grip strength, timed walking, unintentional weight loss, fatigue, and physical activity. Comorbid depression was assessed with the Geriatric Depression Scale-15 items (GDS) [28].
PET imaging
PET scans were performed as close as possible to a clinical visit during the 3 years of follow up in each patient. Subjects were examined using five different hybrid PET/CT scanners, including one PET/CT 690 (GE Healthcare), one Discovery RX VCT (General Electric), two True Point HiRez (Siemens Medical Solutions) and one Biograph 4 Emission Duo LSO (Siemens Medical Solutions). All tomographs operated in 3D detection mode. All PET sinograms were reconstructed with an iterative algorithm, with corrections for randomness, scatter, photon attenuation and decay, which produced images with an isotropic voxel of 2 × 2 × 2 mm and a spatial resolution of approximately 5-mm full-width at a half-maximum at the centre of the field of view. The acquisition data were processed using the standard package delivered with each acquisition system. All cerebral emission scans were begun 50 min after injection of a mean of 4 MBq/kg weight of 18F-AV45. In each subject, 10-min or 15-min frames were acquired to ensure movement-free image acquisition.
Image analysis
Visual reading
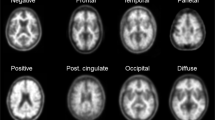
All amyloid scans were sent to the coordinator centre. 18F-AV45 PET images were visually assessed by a panel of three independent observers, who were specialists in molecular imaging and blinded to all clinical and diagnostic information. Each of the observers, who remained the same throughout the study, had interpreted more than 200 amyloid scans before this study. Prior to performing this assessment, the observers underwent a half-day training session on a training set provided by Avid Radiopharmaceuticals/Ely Lilly and Company. Briefly the observers used a binary scale to classify each scan as 0 if there was no significant florbetapir cortical retention (clear grey/white matter contrast) or as 1 if there was some significant florbetapir cortical retention (two or more brain areas in which there was reduced or absent grey/white matter contrast or one or more areas in which grey matter radioactivity was intense) as previously described [13].
Semiautomated quantitative analysis
In addition to the visual readings of scan images, semiautomated quantitative analysis (cortical to cerebellar regional mean standardized uptake values, SUVr) was applied using the mean signal of six predefined anatomically relevant cortical regions of interest (frontal, temporal, parietal, precuneus, anterior cingulate, and posterior cingulate) with the whole cerebellum used as the reference region. For this procedure, the 18F-AV45 PET images were coregistered to the 18F-AV45 template provided by Avid Radiopharmaceuticals and previously published [14]. A quality control based on a semiquantification process was also provided by Avid. Based on the literature, the positivity threshold for amyloid PET was set at SUVr >1.17 [8].
Data analysis
Classification of scans
The PET scan visual analysis results were classified into three classes: (1) “positive PET” if the three observers agreed there was some significant 18F-AV45 cortical retention, (2) “negative PET” if the three observers agreed there was no significant 18F-AV45 cortical retention, and (3) "equivocal PET" (EP) if there was no consensus among the three observers. After debate, the three observers reached a consensus and each EP scan was reclassified as positive (EP+) or negative (EP−).
Inter-rater agreement
We studied the 2 × 2 and overall concordances with kappa coefficients in the entire population and by PET system among the three observers and between visual analysis and quantitative analysis.
Clinical patterns according to PET scan result assessed by visual reading
We compared the clinical characteristics of subjects according to their visual PET scan result in three comparisons: (1) comparison of the three groups of subjects (negative PET, positive PET, EP), (2) comparison of the EP group with the negative PET and positive PET groups, (3) comparison of the EP subgroups after consensus reclassification with scans unanimously classified as positive or negative (i.e. EP− vs. negative PET, and EP+ vs. positive PET), (4) comparison of the EP subgroups (i.e. EP− vs. EP+)
We used the chi-squared test or Fisher’s exact test (for expected values <5) for categorical variables, one-way analysis of variance for quantitative variables with a normal distribution (Fisher tests), and nonparametric tests (Kruskal-Wallis test) for quantitative variables without a normal distribution. For variables that had a global comparison p value <0.05, we compared the characteristics of the EP group with the negative PET and positive PET groups using univariate polytomic regression for categorical variables and univariate linear regression or Kruskal-Wallis tests for continuous variables. In the absence of a normal distribution, variables were tested after transformation to square root or logarithmic values to obtain normal distributions. P values were based on two-sided tests and were considered statistically significant if <0.05, and <0.025 for subgroup analyses to take into account the multiple comparisons. Analyses were performed using SAS software version 9.3 (SAS institute, Cary, NC).
Results
Population
From the 1,680 participants in the MAPT study, 271 subjects were enrolled in this ancillary study at baseline (10 subjects), 6 months (51 subjects), 12 months (94 subjects), 24 months (111 subjects) and 36 months (5 subjects). The mean time between clinical evaluation and PET scan was 2.55 ± 1.42 (range 0.0 – 9.2). At baseline subjects who participated in the ancillary study were significantly younger (on average 74.74 ± 4.26 years vs. 75.46 ± 4.44, p = 0.010), had a slightly higher MMSE score (28.28 ± 1.50 vs. 28.03 ± 1.61, p = 0.018), had a lower TMT-B test score (112.93 ± 42.95 vs. 124.56 ± 66.53, p = 0.017), and had a lower total GDS score (2.75 ± 2.26 vs. 3.36 ± 2.68, p < 10−4) than subjects included in the MAPT study who did not participate in this ancillary study. The demographics of the included subjects and their cognitive performance at the time of the scan are presented in Table 2.
Agreement
Based on visual analysis, 77 subjects (28.41 %) had a positive scan for amyloid, 158 (58.30 %) a negative scan, and 36 (13.28 %) an equivocal scan. After reaching a consensus, these equivocal scans were rated EP+ in 14 subjects (38.89 %) and EP− in 22 subjects (61.11 %). The global agreement among the three observers was excellent (kappa 0.80, standard error 0.035). Kappa values for agreement among the three observers ranged from 0.42 to 0.87 in the five study centres.
Based on semiquantitative analysis (positive PET with cortical SUV >1.17), 103 subjects (38.01 %) had a positive PET scan and 168 (61.99 %) a negative PET scan. The agreement between semiquantification values (mean cortical SUVr) and the visual analysis of the three observers was substantial for all the observers (kappa 0.61, 0.63 and 0.64, respectively). The agreement between semiquantification values and the visual assessment by consensus was 0.63 and the proportion of scans with observer disagreement was 17 %.
Clinical patterns of equivocal scans
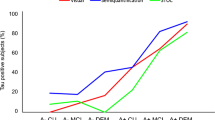
Subjects with positive PET, negative PET and EP scans significantly differed in terms of gender, intervention, verbal episodic memory assessment (FCRST total free recall score), activities of daily living functioning (ADCS-ADL score) and mean cortical SUVr (Table 2). Subjects with an EP scan were mostly men (58 %), younger than 75 years (53 %), had a high level of education (39 % had more than 12 years of formal education) and exhibited good physical performance (89 % had a SPPB score ≥10; Table 2). The subjects with an EP scan significantly differed from subjects in both the positive and negative PET groups in terms of gender and mean cortical SUVr. The subjects with an EP scan had intermediate scores between those of subjects in the positive and negative PET groups for MMSE, FCSRT, TMT-B, CNT and ADCS-ADL (Table 2).
The 158 subjects with negative PET scans (assessed by three raters) and the 22 subjects with an EP− scan significantly differed in terms of gender (p = 0.019) and SPPB score (p = 0.028). The characteristics of the subjects with an EP scan according to their PET classification after visual consensus (EP+ and EP−) are presented in Table 3. There was no significant clinical difference between the 77 subjects with positive PET scans (assessed by three raters) and subjects with an EP+ scan. However, mean cortical SUVr was significantly different between the negative and EP− groups (p = 0.006), and between the positive and EP+ groups (p = 0.007); Fig. 1).
Distributions of cortical SUVr (from semiautomated quantitative analysis) in the different visual analysis groups. Equivocal PET scans were reclassified as negative (EP−) or positive (EP+). Data from individual subjects are shown. Bottom of the boxes first quartile, top of the boxes third quartile, central lines second quartile (median), extremities minimum and maximum values. *p < 0.001, comparisons among the three groups of subjects; p = 0.006 and p = 0.007, 2 × 2 comparisons, Kruskal-Wallis test
The Z-values for cognitive performance using subjects with negative PET scans as the reference group are presented in Supplementary Table 1.
Discussion
This study explored for the first time the clinical patterns of “equivocal” amyloid PET scans in a large population of nondemented older adults aged 70 years and over. Our study had three main findings. First, 28 % of subjects had a positive amyloid scan based on visual analysis and 13 % had equivocal scans. Second, the global agreement among the three observers was excellent. Third, the clinical characteristics of subjects with equivocal scans did not significantly differ from those with positive or negative scans but seemed to be intermediate. Likewise, the amyloid load of subjects with equivocal scans was intermediate between the amyloid loads of subjects with positive and negative scans. After reclassification of equivocal scans, the mean cortical SUVr remained significantly different between the negative and EP− groups and between the positive and EP+ groups. A high prevalence of amyloid positivity as observed in this study has also been reported in the elderly. It reached 28 % in our population with a mean age of 75 years, and in previous studies the percentage was around 25 % in subjects aged 70 to 80 years [10, 11].
The global agreement among the three observers is in accordance with previously reported data and confirms the accuracy of our visual assessment methodology. Another interesting result was that the agreement varied with the PET centre (kappa varying between 0.42 and 0.87). The worst agreement was observed in the centre with the oldest PET scanner (installed in 2004). Image quality may then be a major factor explaining the misclassification in amyloid PET interpretation. The agreement between semiquantification (mean cortical SUVr, threshold set at 1.17) values and visual analysis of the three observers (kappa 0.61, 0.63 and 0.64) was not so good for all the observers or between the semiquantification value and the consensus visual assessment (kappa 0.63). This relative discordance between semiquantification and visual agreement may be explained by some limitations. First, in a recent communication based on 250 amyloid scans, Klein et al. have demonstrated that concordance between quantitative SUVr methods and visual assessment is highest for methods using MRI data and cerebral white matter reference regions [29]. Using the white matter or brainstem as a reference may provide superior performance. Those results are in line with those of a recent study by our group [30]. In this multicentre study, MRI of the subjects was not available and the semiquantification was based on the cerebellum. Another explanation may be the fact that semiquantification is based on a mean cortical value in contrast to visual assessment which is based on regional approaches [5]. These results suggest that quantification methods in development should involve a specific regional approach rather than a single global mean value.
Verbal episodic memory assessed by FCRST (free recall, delayed total recall) scores was associated with the results of visual assessment of scans, suggesting that cognitive function could be related to amyloid load in a nondemented population. Such a relationship between regional amyloid-beta deposition and episodic memory deficits in the presymptomatic stage of AD has been already described by the Australian Imaging, Biomarkers and Lifestyle (AIBL) group [31, 32]. The EP group significantly differed from both the positive and negative groups in terms of gender. There was no significant difference between the EP group and the other two groups in terms of functional and cognitive parameters. However, the EP group seemed to be an intermediate cognitive group (for episodic verbal memory and executive functions), and amyloid load based on mean SUVr was intermediate and corresponded to the threshold between positive and negative PET (1.17).
We sought to determine if the EP group corresponded to the combination of the two subgroups EP+ and EP−, similar to the positive and negative groups, respectively, or to one homogeneous group with its own specific cognitive patterns and mild amyloid load. After the consensus determination, the EP+ and positive groups and the EP− and negative groups did not differ from a neuropsychological point of view. In contrast, amyloid load, based on mean SUVr, differed among the groups. Subjects in the equivocal group had an intermediate amyloid load which could explain the difficulty in interpretation encountered by the three raters.
This study suffered from some limitations. First, the delay between clinical assessment and PET acquisition could have reached 3 months. However, a recent study has demonstrated that the kinetics of amyloid deposit are probably very slow [32], and this delay should not have interfered significantly with our results. Second, MRI scans were not available in this study. However, since PET template-based quantification seems adequate for clinical use to discriminate controls from patients with early AD, the use of MRI-based cortical quantification to avoid nonspecific white matter binding reported in healthy subjects as well as in patients with AD seemed not to be necessary [33]. Second, the population of this ancillary study was cognitively heterogeneous. The lack of accuracy in the diagnosis of mild cognitive impairment is a limitation. Last, the small number of subjects with equivocal scans prevented multivariate analysis of associated clinical factors and limited the comparison of subjects after reclassification of the equivocal scans (EP+ or EP−) with subjects of the other groups.
Equivocal PET scans could be a neuroimaging entity representing intermediate amyloid load and without any specific neuropsychological patterns. The results of this cross-sectional study could justify a clinical follow-up to assess the cognitive evolution of subjects with equivocal PET scans.
References
Risacher SL, Saykin AJ. Neuroimaging and other biomarkers for Alzheimer's disease: the changing landscape of early detection. Annu Rev Clin Psychol. 2013;9:621–48. doi:10.1146/annurev-clinpsy-050212-185535.
Tolboom N, Yaqub M, van der Flier WM, Boellaard R, Luurtsema G, Windhorst AD, et al. Detection of Alzheimer pathology in vivo using both 11C-PIB and 18F-FDDNP PET. J Nucl Med. 2009;50:191–7. doi:10.2967/jnumed.108.056499.
Kemppainen NM, Aalto S, Wilson IA, Nagren K, Helin S, Bruck A, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68:1603–6. doi:10.1212/01.wnl.0000260969.94695.56.
Wolk DA, Zhang Z, Boudhar S, Clark CM, Pontecorvo MJ, Arnold SE. Amyloid imaging in Alzheimer's disease: comparison of florbetapir and Pittsburgh compound-B positron emission tomography. J Neurol Neurosurg Psychiatry. 2012;83:923–6. doi:10.1136/jnnp-2012-302548.
Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–83. doi:10.1001/jama.2010.2008.
Choi SR, Schneider JA, Bennett DA, Beach TG, Bedell BJ, Zehntner SP, et al. Correlation of amyloid PET ligand florbetapir F18 binding with Abeta aggregation and neuritic plaque deposition in postmortem brain tissue. Alzheimer Dis Assoc Disord. 2012;26:8–16. doi:10.1097/WAD.0b013e31821300bc.
Camus V, Payoux P, Barre L, Desgranges B, Voisin T, Tauber C, et al. Using PET with 18F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment. Eur J Nucl Med Mol Imaging. 2012;39:621–31. doi:10.1007/s00259-011-2021-8.
Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68:1404–11. doi:10.1001/archneurol.2011.150.
Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18). J Nucl Med. 2010;51:913–20. doi:10.2967/jnumed.109.069088.
Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–31. doi:10.1002/ana.21843.
Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–83. doi:10.1016/j.neurobiolaging.2010.04.007.
Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–18.
Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9:S72–83. doi:10.1016/j.jalz.2012.10.007.
Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, et al. Performance characteristics of amyloid PET with florbetapir F18 in patients with Alzheimer's disease and cognitively normal subjects. J Nucl Med. 2012;53:378–84. doi:10.2967/jnumed.111.090340.
Grundman M, Pontecorvo MJ, Salloway SP, Doraiswamy PM, Fleisher AS, Sadowsky CH, et al. Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Dis Assoc Disord. 2013;27:4–15. doi:10.1097/WAD.0b013e318279d02a.
Carrie I, van Kan GA, Gillette-Guyonnet S, Andrieu S, Dartigues JF, Touchon J, et al. Recruitment strategies for preventive trials. The MAPT study (MultiDomain Alzheimer Preventive Trial). J Nutr Health Aging. 2012;16:355–9.
Gillette-Guyonnet S, Andrieu S, Dantoine T, Dartigues JF, Touchon J, Vellas B, et al. Commentary on "A roadmap for the prevention of dementia II. Leon Thal Symposium 2008." The Multidomain Alzheimer Preventive Trial (MAPT): a new approach to the prevention of Alzheimer's disease. Alzheimers Dement. 2009;5:114–21. doi:10.1016/j.jalz.2009.01.008.
Vellas B, Carrie I, Gillette-Guyonnet S, Touchon J, Dantoine T, Dartigues JF, et al. MAPT study: a multidomain approach for preventing Alzheimer’s disease: design and baseline data. J Prevent Alzheimer’s Dis. 2014;1:13–22.
Nourhashemi F, Andrieu S, Gillette-Guyonnet S, Vellas B, Albarede JL, Grandjean H. Instrumental activities of daily living as a potential marker of frailty: a study of 7364 community-dwelling elderly women (the EPIDOS study). J Gerontol A Biol Sci Med Sci. 2001;56:M448–53.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–3.
Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg. 1990;90:207–17.
Wechsler D. Manual for the Wechsler adult intelligence scale – revised. New York: Psychological Corporation; 1981.
Reitan R. Validity of the trail making test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–6.
Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56.
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49.
Klein G, Chiao P, Barakos J, Purcell D, Sampat M, Oh J, et al. Concordance of quantitative SUVr methods with visual assessment of florbetapir PET screening results. Alzheimers Dement. 2014;10(4 Suppl):P399
Nemmi F, Saint-Aubert L, Adel D, Salabert AS, Pariente J, Barbeau EJ, et al. Insight on AV-45 binding in white and grey matter from histogram analysis: a study on early Alzheimer's disease patients and healthy subjects. Eur J Nucl Med Mol Imaging. 2014;41:1408–18. doi:10.1007/s00259-014-2728-4.
Chetelat G, Villemagne VL, Pike KE, Ellis KA, Ames D, Masters CL, et al. Relationship between memory performance and beta-amyloid deposition at different stages of Alzheimer's disease. Neurodegener Dis. 2012;10:141–4. doi:10.1159/000334295.
Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi:10.1016/S1474-4422(13)70044-9.
Saint-Aubert L, Nemmi F, Peran P, Barbeau EJ, Payoux P, Chollet F, et al. Comparison between PET template-based method and MRI-based method for cortical quantification of florbetapir (AV-45) uptake in vivo. Eur J Nucl Med Mol Imaging. 2014;41:836–43. doi:10.1007/s00259-013-2656-8.
Compliance with ethical standards
ᅟ
Conflicts of interest
Prof. Payoux served on the scientific advisory board of Avid Radiopharmaceuticals and GEHC.
Dr. Delrieu served on the scientific advisory board of Avid Radiopharmaceuticals.
Dr. Gallini reports no conflicts of interest.
Dr. Adel reports no conflicts of interest.
Dr. Salabert reports no conflicts of interest.
Dr. Hitzel reports no conflicts of interest.
Dr. Cantet reports no conflicts of interest.
Dr. Tafani reports no conflicts of interest.
Dr. De Verbizier reports no conflicts of interest.
Prof. Darcourt served on the scientific advisory board of Avid Radiopharmaceuticals.
Prof. Fernandez reports no conflicts of interest.
Prof. Monteil reports no conflicts of interest.
Dr. Carrié reports no conflicts of interest.
Dr. Voisin T reports no conflicts of interest.
Dr. Gillette-Guyonnet reports no conflicts of interest.
Dr. Pontecorvo is an employee of Avid Radiopharmaceuticals.
Prof. Vellas served on the scientific advisory board of Avid Radiopharmaceuticals.
Prof. Andrieu reports no conflicts of interest.
Funding
Supported by MAPT study (NCT01513252), The Avid Company and Labex IRON.
This study was also supported by grants from the French Ministry of Health (PHRC 2008), and the Institut de Recherche Pierre Fabre (manufacturer of the omega-3 supplement). This study was supported by the University Hospital Center of Toulouse. Biological sample collection was supported by Exhonit Therapeutics. The AV45-MAPT study was supported by Avid Radiopharmaceuticals Inc. and this work has been supported in part by a grant from the French National Agency for Research called “Investissements d’Avenir” n°ANR-11-LABX-0018-01.
Human rights and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 16 kb)
Appendix: members of the MAPT study group
Appendix: members of the MAPT study group
Principal investigator: Bruno Vellas (Toulouse); Coordination: Sophie Gillette-Guyonnet; Project leader: Isabelle Carrié; CRA: Lauréane Brigitte; Investigators: Catherine Faisant, Françoise Lala, Julien Delrieu; Psychologists: Emeline Combrouze, Carole Badufle, Audrey Zueras; Methodology, statistical analysis and data management: Sandrine Andrieu, Christelle Cantet, Virginie Gardette, Christophe Morin; Multidomain group: Gabor Abellan Van Kan, Charlotte Dupuy, Yves Rolland (physical and nutritional components), Céline Caillaud, Pierre-Jean Ousset (cognitive component), Françoise Lala (preventive consultation) (Toulouse). The cognitive component was designed in collaboration with Sherry Willis from the University of Seattle, and Sylvie Belleville, Brigitte Gilbert and Francine Fontaine from the University of Montreal.
Co-Investigators in associated centres: Jean-François Dartigues, Isabelle Marcet, Fleur Delva, Alexandra Foubert, Sandrine Cerda (Bordeaux); Marie-Noëlle-Cuffi, Corinne Costes (Castres); Olivier Rouaud, Patrick Manckoundia, Valérie Quipourt, Sophie Marilier, Evelyne Franon (Dijon); Lawrence Bories, Marie-Laure Pader, Marie-France Basset, Bruno Lapoujade, Valérie Faure, Michael Li Yung Tong, Christine Malick-Loiseau, Evelyne Cazaban-Campistron (Foix); Françoise Desclaux, Colette Blatge (Lavaur); Thierry Dantoine, Cécile Laubarie-Mouret, Isabelle Saulnier, Jean-Pierre Clément, Marie-Agnès Picat, Laurence Bernard-Bourzeix, Stéphanie Willebois, Iléana Désormais, Noëlle Cardinaud (Limoges); Marc Bonnefoy, Pierre Livet, Pascale Rebaudet, Claire Gédéon, Catherine Burdet, Flavien Terracol (Lyon), Alain Pesce, Stéphanie Roth, Sylvie Chaillou, Sandrine Louchart (Monaco); Kristelle Sudres, Nicolas Lebrun, Nadège Barro-Belaygues (Montauban); Jacques Touchon, Karim Bennys, Audrey Gabelle, Aurélia Romano, Lynda Touati, Cécilia Marelli, Cécile Pays (Montpellier); Philippe Robert, Franck Le Duff, Claire Gervais, Sébastien
Gonfrier (Nice); Yves Gasnier and Serge Bordes, Danièle Begorre, Christian Carpuat, Khaled Khales, Jean-François Lefebvre, Samira Misbah El Idrissi, Pierre Skolil, Jean-Pierre Salles (Tarbes).
MRI group: Carole Dufouil (Bordeaux), Stéphane Lehéricy, Marie Chupin, Jean-François Mangin, Ali Bouhayia (Paris); Michèle Allard (Bordeaux); Frédéric Ricolfi (Dijon); Dominique Dubois (Foix); Marie Paule Bonceour Martel (Limoges); François Cotton (Lyon); Alain Bonafé (Montpellier); Stéphane Chanalet (Nice); Françoise Hugon (Tarbes); Fabrice Bonneville, Christophe Cognard, François Chollet (Toulouse).
PET scan group: Pierre Payoux, Thierry Voisin, Julien Delrieu, Sophie Peiffer, Anne Hitzel, (Toulouse); Michèle Allard (Bordeaux); Michel Zanca (Montpellier); Jacques Monteil (Limoges); Jacques Darcourt (Nice).
Medico-economics group: Laurent Molinier, Hélène Derumeaux, Nadège Costa (Toulouse).
Biological sample collection: Christian Vincent, Bertrand Perret, Claire Vinel (Toulouse).
Rights and permissions
About this article
Cite this article
Payoux, P., Delrieu, J., Gallini, A. et al. Cognitive and functional patterns of nondemented subjects with equivocal visual amyloid PET findings. Eur J Nucl Med Mol Imaging 42, 1459–1468 (2015). https://doi.org/10.1007/s00259-015-3067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3067-9