Abstract
Background
To date, the clinical significance of visually equivocal amyloid positron emission tomography (PET) has not been well established.
Objective
We studied the clinical significance of equivocal amyloid PET images from the Alzheimer’s Disease Neuroimaging Initiative (ADNI).
Methods
Subjects with F-18 florbetapir PET scans at baseline who were followed up for 4 years were selected. Clinical characteristics, imaging biomarkers, cognitive function, and rate of conversion to AD were compared in subjects with visually equivocal findings.
Results
Of 249 subjects who completed the follow-up, 153 (61.4%), 20 (8.0%), and 129 (30.5%) were F-18 florbetapir-negative, -equivocal, and -positive, respectively. The mean standardized uptake value ratios (SUVR) of F-18 florbetapir PET were 0.75 ± 0.04, 0.85 ± 0.10, and 1.00 ± 0.09 for each group (p <0.001 between groups), and 15.0%, 70.0%, and 98.7% of patients were quantitatively above the positive threshold. The change in the SUVR of F-18 florbetapir PET was higher in the equivocal (6.09 ± 3.61%, p <0.001) and positive (3.13 ± 4.38%, p <0.001) groups than the negative group (0.88 ± 4.28%). Among the subjects with normal or subjective memory impairment and mild cognitive impairment, 5.3% with negative amyloid PET and 37.5% with positive amyloid PET converted to AD over the 4-year period. None of the equivocal amyloid PET subjects converted to AD during this period.
Conclusion
Approximately 8% of subjects from the ADNI cohort showed visually equivocal amyloid PET scans with intermediate load and rapid accumulation of amyloid, but did not convert to AD during the 4-year follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) was initially defined as a clinical-pathologic entity and diagnosed as possible or probable AD using clinical criteria, including insidious onset and progressive impairment of memory and other cognitive functions. A definite diagnosis was provided following demonstration of plaques and tangles by autopsy [1]. However, postmortem validation of people clinically diagnosed with probable AD was shown to be diverse in relation to pathologic AD, tangle-only dementia, dementia with Lewy bodies, mixed AD, vascular dementia, and frontotemporal dementia [2]. This finding indicates that clinical diagnosis of AD is not an etiology, but rather the clinical consequences of one or more diseases. This made enrollment of patients with non-AD pathology in clinical trials that testing disease-modifying interventions for biologically defined targets [3]. Therefore, the need for biological diagnostic criteria has been raised and proposed as the National Institute on Aging and Alzheimer’s Association (NIA-AA) research framework [4]. This framework defines AD biologically by neuropathologic changes or biomarkers, which in turn provide a common language for researchers. However, it also raises questions regarding the definition of biomarker positivity. For amyloid-β (Aβ) peptides, there are several tracers for positron emission tomography (PET) that provide specific criteria for binary visual reading [5,6,7]. However, some patients showed visually equivocal PET findings, which commonly represent intermediate amyloid load; nevertheless, clinical follow-up to assess cognitive evolution in subjects with equivocal scans was needed [8,9,10,11]. We previously reported the clinical significance of patients with equivocal amyloid PET at the 2-year follow-up using the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort [12]. In this study, the results of 4 years of longitudinal observations have been reported.
Materials and Methods
Participants
We included subjects with cognitively normal (NC) or subjective memory impairment (SMI), mild cognitive impairment (MCI), and AD who had F-18 florbetapir PET scans at baseline and were followed up (F/U) for 4 years as of June 2017 as a part of the ADNI study. The criteria for classification of the subjects were described previously [13]. The ADNI study was approved by the Institutional Review Board of the respective institutions before beginning the study. Informed consent was obtained from all participants. The results of the 24-month follow-up have been reported previously [12]. All ADNI data are publicly available at http://www.loni.ucla.edu/ADNI.
F-18 Florbetapir PET Image Processing
ADNI PET image data were acquired as described online (adni.loni.ucla.edu/about-data-samples/image-data/) and processed as described previously [14]. Briefly, F-18 florbetapir PET image data were co-registered with 3T 3D MP-RAGE magnetic resonance imaging (MRI) and segmented and parcellated into individual cortical regions using FreeSurfer. The mean florbetapir uptake was extracted from the gray matter within the lateral and medial frontal anterior, posterior cingulate, lateral parietal, and lateral temporal regions. The standardized uptake value ratio (SUVR) relative to uptake in the composite region comprising the whole cerebellum and pons, with the eroded subcortical white matter as the reference region [15] and a positivity threshold of 0.79, was described previously. This ratio provides more accurate and conservative change measurements [15, 16]. The full protocols and data are available online (http://adni.loni.ucs.edu).
We conducted harmonization of different PET scanners to achieve the best visual analysis. Detailed descriptions of image processing and analysis have been reported in the previous study [12]. A panel of five independent board-certified nuclear medicine physicians that were blinded to all clinical information assessed all F-18 florbetapir PET images. Scans were considered 1 if there was some significant F-18 florbetapir cortical retention and 0 if there was no significant cortical retention according to previously defined criteria [17]. And the results were classified into three categories as follows: positive scan, if more than four observers rated the scan as 1; negative scan, if more than four observers rated the scan as 0; and equivocal scan, if there were no more than four observers with the same ratings.
F-18 Fluorodeoxyglucose (FDG) PET Image Processing
Each F-18 FDG PET image was spatially normalized to the standard O-15 H2O PET template using Statistical Parametric Mapping software (SPM5, Wellcome Department of Cognitive Neurology, UK; www.fil.ion.ucl.ac.uk/spm). The mean FDG uptake was extracted for each participant from a set of study-independent and previously validated regions of interest (metaROIs) located in the right and left inferior temporal and lateral parietal regions, and a bilateral posterior cingulate cortex region relative to the mean of the pons and cerebellar vermis reference regions [18].
Structural MRI Analyses
Cross-sectional structural differences were assessed using hippocampal volumes defined on MPRAGE images by FreeSurfer v5.1 and were divided by the total intracranial volume to adjust for head size.
Cerebrospinal Fluid (CSF) Analysis
CSF Aβ1–42 was concurrently measured using F-18 florbetapir scans at baseline and analyzed at the ADNI Biomarker core laboratory. We applied the autopsy-validated CSF Aβ1–42 positivity cut-off of 192 pg/mL, which was utilized in a previous study [19].
Clinical and Cognitive Measurements
We examined several clinical and cognitive performance measurements, including baseline and longitudinal performance, using the Mini-Mental State Examination (MMSE) [20], Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) [21], and clinical dementia rating-sum of boxes (CDR-SB) [22]. We also examined clinical profiles, including clinical diagnosis at baseline and 48-month F/U, to decide whether conversion to AD occurred during the F/U.
Statistical Analysis
Chi-squared test was used for categorical variables, and one-way analysis of variance for quantitative variables. P-values were two-sided and considered statistically significant at < 0.05 for global comparison and at < 0.05/3 for subgroup analyses to take into account multiple comparisons. Inter-observer agreement of the visual assessment was calculated on subject level using kappa values. Analyses were performed using SPSS® software (Statistical Package for the Social Science, version 18.0, Chicago, IL, USA).
Results
Demographic and Clinical Characteristics
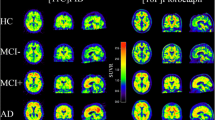
The demographics, clinical characteristics, and biomarker variables are summarized in Table 1. Among 249 subjects who completed the 4-year follow-up, the mean age of the patients was 71.6 ± 6.9 years, and 53.0% were men. The mean number of years of formal education was 16.5 ± 2.5 years. The numbers of F-18 florbetapir negative/equivocal/positive subjects were 153 (61.4%), 20 (8.0%), and 129 (30.5%), respectively. A total of 8% of subjects with NC or SMI (n = 8/100), 8.4% of patients with MCI (n = 12/143), and no patients with AD (n = 0/6) showed equivocal F-18 florbetapir PET findings. Among the 20 subjects in the equivocal group, 9 (45.0%) showed diffuse mild amyloid retention in the entire cerebral cortex. Among the remaining patients, retention was most common in the frontal cortex (n = 5, 20.0%), followed by the posterior cingulate gyrus (n = 3, 15.0%), temporal cortex (n = 2, 10.0%), and occipital cortex (n = 1, 5.0%). The types of equivocal F-18 florbetapir PET images are shown in Fig. 1. Among subjects with NC/SMI or MCI, 5.3% of subjects with negative amyloid PET and 37.5% of subjects with positive amyloid PET converted to AD during the 4-year follow-up. None of the subjects with equivocal amyloid PET converted to AD during the 4 years.
Biomarkers
At baseline, 32 (20.9%), 10 (50.0%), and 58 (76.4%) subjects with negative/equivocal/positive F-18 florbetapir PET were apolipoprotein E4 (ApoE4) positive. The mean SUVR of F-18 florbetapir PET was 0.75 ± 0.04, 0.85 ± 0.10, and 1.00 ± 0.09 for each group (p < 0.001 between groups). Among them, 15.0%, 70.0%, and 98.7% of the patients in each group were quantitatively above the positive threshold. The metaROI of FDG was significantly more hypometabolic in the positive group than those in the equivocal or negative groups (p = 0.010 and p < 0.001, respectively). Furthermore, the hippocampal volume normalized to the intracranial volume of the positive group was significantly lower than that of the negative group (p < 0.001). The CSF Aβ1–42 level was significantly lower in the positive group (133.43 ± 24.02) than that in the negative group (215.38 ± 38.56, p < 0.001), but was not significantly different to that in the equivocal group (174.24 ± 43.73, p = 0.270). Moreover, the CSF Aβ1–42 level was quantitatively positive in 35 (26.1%), 12 (70.6%), and 70 (97.2%) subjects in each group.
The changes in the SUVR of F-18 florbetapir PET were higher in the equivocal (6.09 ± 3.61%, p < 0.001) and positive (3.13 ± 4.38%, p < 0.001) groups than that in the negative group (0.88 ± 4.28%). Subject-specific time trajectories of amyloid burden measured by the SUVR of F-18 florbetapir PET in subjects with CN or SMI (Fig. 2a) and MCI (Fig. 2b) were presented. Changes in the SUVR, FDG metaROI, and hippocampal volume normalized to intracranial volume in each group during follow-up are shown in Table 1 and Fig. 3a–c.
Cognitive Function
The mean MMSE score at baseline in the positive group (27.7 ± 2.2) was lower than that in the negative group (28.6 ± 1.6, p = 0.001), and not significantly different in the equivocal group (27.8 ± 1.9, p = 1.000). Moreover, the MMSE score decreased significantly in the positive group compared with the other groups during the 4-year follow-up (p < 0.001). The baseline ADAS-cog score in the positive group (16.8 ± 8.1) was higher than that in the negative group (10.7 ± 5.4, p < 0.001) and not significantly different from that in the equivocal group (13.6 ± 5.5, p = 0.129). In addition, the ADAS-cog score increased significantly in the positive group during the 4-year follow-up (p < 0.001), as did the CDR-SB score. The changes in MMSE and ADAS-cog and CDR-SB scores in each group during follow-up are shown in Fig. 3d–f.
Discussion
The current study followed subjects from the ADNI cohort with visually equivocal amyloid PET findings for 4 years. We previously reported that the subjects showed intermediate amyloid load at baseline and rapid accumulation during the 2-year follow-up [12]. After the 4-year follow-up, changes of imaging biomarkers, including the degree of hypometabolism on FDG PET and hippocampal volume in MRI, were not significantly different, did not deteriorate cognitively, and did not convert to AD.
Two main neuropathologic hallmarks of AD are amyloid plaques and neurofibrillary tangles [23, 24]. It is considered to occur about 15 years prior to the onset of clinical symptoms of AD [25], Aβ peptides converted to β-sheet-enriched structures [26]. β-sheet structures aggregate into forms of toxic soluble oligomers, seeds, and, eventually, amyloid plaques, which related neurodegeneration in AD [27]. Therefore, Aβ accumulation in the brain is considered to be a spectrum.
Amyloid PET scans are rated as either positive or negative in clinical practice; however, equivocal ratings are often found in some patients. Although the definitions of equivocal amyloid PET vary in the respect of inter-rater agreement or degree of cortical retention, it has been known as intermediate amyloid load in previous reports, regardless of the type of tracers used [10, 15, 28,29,30,31]. Equivocal amyloid PET was first described by Hosokawa et al., as a quantitatively intermediate amyloid load, both regionally and globally, observed using C-11 Pittsburgh compound B (PIB). When the first F-18-labeled amyloid PET tracer, F-18 florbetapir was introduced, the FDA withheld approval until an interpretation training program was implemented to reduce inter-reader variability [32]. A portion of equivocal findings continue to be observed even after the approval for clinical use of F-18-labeled amyloid PET tracers, including F-18 florbetapir, F-18 flutemetamol, and F-18 florbetaben, which led to the development of special educational reading programs that improved inter-rater agreement in the early 2010s [33]. It could be a fundamental problem of F-18 amyloid PET tracers due to the higher white matter retention of F-18-labeled amyloid PET tracers than C-11 PIB [34], since almost all visual interpretation criteria is to determine whether the relative cortical uptake is increased in comparison to white matter retention.
It has been demonstrated that quantitative analysis using the SUVR of static images can reduce inter-rater variability and improve accuracy [30, 35]. To avoid individual variation in white matter retention of static images that acquire the recommended time frame, analysis of parametric images with dynamic acquisition has also been reported and shows overestimation of cortical uptake in static images [9]. Although these methods of quantitative analysis obviously compensate for the shortcomings of visual interpretation, they are disadvantageous in that they hinder convenience in clinical practice.
To date, the clinical significance of equivocal amyloid PET has not been well established due to the relative lack of long-term follow-up studies of current F-18 amyloid PET tracers, since they have only been introduced for a few years. We chose the ADNI cohort to show the clinical significance of equivocal amyloid PET because it is one of the oldest and longest cohorts to use F-18 florbetapir PET as a biomarker for Aβ.
Nevertheless, the majority of the subjects with equivocal amyloid PET were above the SUVR positive threshold, and none of the subjects converted to AD during the 4-year follow-up. These findings suggest that they are at the beginning of amyloid cascade, which is more than a decade prior to the appearance of the clinical symptoms of AD [36]. In anti-amyloid treatment of asymptomatic Alzheimer’s disease (A4 study), which is the first prevention trial in clinically normal older individuals identified as “at-risk” for progression to AD on the basis of evidence of brain amyloid accumulation on PET imaging, participants must show evidence of elevated amyloid accumulation on “both” a visual read and a quantitative measurement (SUVR) of the F-18 florbetapir PET scan [37]. Therefore, subjects with equivocal amyloid PET in this study were not the best candidates for the A4 study since they were not at increased risk for cognitive decline or for developing AD by the end of the study.
There are several limitations of the current study. First, the low rate of AD conversion in subjects with equivocal amyloid PET could be due to the small number of subjects. Since subjects with equivocal amyloid PET findings were usually less than 10%, further study with a larger cohort than the ADNI is required, which is usually not achievable practically. Second, we classified equivocal amyloid PET findings as diffuse and regional patterns but could not conclude whether their fate is different with the current sample size. Third, the number of subjects with AD was too small, and as a result, we were unable to decide the clinical significance of equivocal amyloid PET in AD.
References
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44.
Ranginwala NA, Hynan LS, Weiner MF, White CL 3rd. Clinical criteria for the diagnosis of Alzheimer disease: still good after all these years. Am J Geriatr Psychiatry. 2008;16:384–8.
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–33.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Avid Radiopharms inc. Amyvid (Florbetapir F 18 Injection). Highlights of prescribing information. In: Drugs@FDA: FDA-Approved Drugs. U.S. Food & Drug Administration. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202008s000lbl.pdf. Accessed 23 Nov 2020.
Healthcare G. VIZAMYL (flutemetamol F 18 injection) for intravenous use. Highlights of prescribing information. In: Drugs@FDA: FDA-Approved Drugs. U.S. Food & Drug Administration. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203137s000lbl.pdf. Accessed Nov 23 2020.
Life Molecular Imaging. NEURACEQ (florbetaben F 18 injection), for intravenous use. Highlights of prescribing information. In: Drugs@FDA: FDA-Approved Drugs. U.S. Food & Drug Administration. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204677s000lbl.pdf. Accessed Nov 23 2020.
Payoux P, Delrieu J, Gallini A, Adel D, Salabert AS, Hitzel A, et al. Cognitive and functional patterns of nondemented subjects with equivocal visual amyloid PET findings. Eur J Nucl Med Mol Imaging. 2015;42:1459–68.
Hosokawa C, Ishii K, Kimura Y, Hyodo T, Hosono M, Sakaguchi K, et al. Performance of 11C-Pittsburgh Compound B PET binding potential images in the detection of amyloid deposits on equivocal static images. J Nucl Med. 2015;56:1910–5.
Hosokawa C, Ishii K, Hyodo T, Sakaguchi K, Usami K, Shimamoto K, et al. Investigation of 11C-PiB equivocal PET findings. Ann Nucl Med. 2015;29:164–9.
Yamane T, Ishii K, Sakata M, Ikari Y, Nishio T, Ishii K, et al. Inter-rater variability of visual interpretation and comparison with quantitative evaluation of 11C-PiB PET amyloid images of the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) multicenter study. Eur J Nucl Med Mol Imaging. 2017;44:850–7.
Oh M, Seo M, Oh SY, Kim H, Choi BW, Oh JS, et al. Clinical significance of visually equivocal amyloid PET findings from the Alzheimer’s Disease Neuroimaging Initiative cohort. Neuroreport. 2018;29:553–8.
Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–9.
Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–86.
Landau SM, Fero A, Baker SL, Koeppe R, Mintun M, Chen K, et al. Measurement of longitudinal β-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J Nucl Med. 2015;56:567–74.
Blautzik J, Brendel M, Sauerbeck J, Kotz S, Scheiwein F, Bartenstein P, et al. Reference region selection and the association between the rate of amyloid accumulation over time and the baseline amyloid burden. Eur J Nucl Med Mol Imaging. 2017;44:1364–74.
Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9:S72–83.
Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–18.
Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–64.
O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s research consortium study. Arch Neurol. 2008;65:1091–5.
Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403.
Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016;17:5–21.
Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804.
Sarroukh R, Cerf E, Derclaye S, Dufrêne YF, Goormaghtigh E, Ruysschaert JM, et al. Transformation of amyloid β(1-40) oligomers into fibrils is characterized by a major change in secondary structure. Cell Mol Life Sci. 2011;68:1429–38.
Cavallucci V, D'Amelio M, Cecconi F. Aβ toxicity in Alzheimer’s disease. Mol Neurobiol. 2012;45:366–78.
Nayate AP, Dubroff JG, Schmitt JE, Nasrallah I, Kishore R, Mankoff D, et al. Use of standardized uptake value ratios decreases interreader variability of [18F] florbetapir PET brain scan interpretation. AJNR Am J Neuroradiol. 2015;36:1237–44.
Son HJ, Oh JS, Oh M, Kim SJ, Lee JH, Roh JH, et al. The clinical feasibility of deep learning-based classification of amyloid PET images in visually equivocal cases. Eur J Nucl Med Mol Imaging. 2020;47:332–41.
Harn NR, Hunt SL, Hill J, Vidoni E, Perry M, Burns JM. Augmenting amyloid PET interpretations with quantitative information improves consistency of early amyloid detection. Clin Nucl Med. 2017;42:577–81.
Pothier K, Saint-Aubert L, Hooper C, Delrieu J, Payoux P, de Souto BP, et al. Cognitive changes of older adults with an equivocal amyloid load. J Neurol. 2019;266:835–43.
Rey A. L’examen clinique en psychologie [The clinical examination of psychology]. 2nd ed. Paris: Presses universitaires de France; 1964. French
Filippi L, Chiaravalloti A, Bagni O, Schillaci O. 18F-labeled radiopharmaceuticals for the molecular neuroimaging of amyloid plaques in Alzheimer’s disease. Am J Nucl Med Mol Imaging. 2018;8:268–81.
Landau SM, Thomas BA, Thurfjell L, Schmidt M, Margolin R, Mintun M, et al. Amyloid PET imaging in Alzheimer’s disease: a comparison of three radiotracers. Eur J Nucl Med Mol Imaging. 2014;41:1398–407.
Chen YJ, Nasrallah IM. Brain amyloid PET interpretation approaches: from visual assessment in the clinic to quantitative pharmacokinetic modeling. Clin Transl Imaging. 2017;5:561–73.
Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16.
Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13.
Funding
This research was supported by grants of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI14C2768 and HI18C2383). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board of the respective institutions and with the Helsinki declaration as revised in 2013 and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all participants.
Conflict of Interest
Minyoung Oh, Minjung Seo, Sun Young Oh, Heeyoung Kim, Byung Wook Choi, Jungsu S. Oh, and Jae Seung Kim declare that they have no conflicts of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data used in the current study were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the study design and implementation of the database and/or provided data but did not participate in the analysis or writing of this manuscript. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp- content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Rights and permissions
About this article
Cite this article
Oh, M., Seo, M., Oh, S.Y. et al. A 4-Year Follow-Up of Subjects with Visually Equivocal Amyloid Positron Emission Tomography Findings from the Alzheimer’s Disease Neuroimaging Initiative Cohort. Nucl Med Mol Imaging 55, 71–78 (2021). https://doi.org/10.1007/s13139-021-00690-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-021-00690-x