Abstract
Purpose
The aim of this retrospective study was to evaluate the usefulness and the detection rate of 11C-choline PET/CT in a population of patients with prostate cancer (PC), exclusively treated with external beam radiotherapy (EBRT) as primary treatment, who showed biochemical relapse.
Materials and methods
We enrolled 140 patients showing a serum PSA level >2 ng/mL (mean 8.6 ng/mL, median 5 ng/mL, range 2 – 60 ng/mL). All patients had been treated with EBRT to the prostate gland and prostatic fossa with doses ranging from 70 to 76 Gy in low-risk patients (T1/T2 and/or serum PSA <10 ng/mL) and escalating to >76 Gy (range 76 – 81 Gy) in high-risk patients (T3/T4 and/or serum PSA >10 ng/mL). Of the 140 patients, 53 were receiving androgen deprivation therapy at the time of the scan. All positive 11C-choline PET/CT findings were validated by transrectal ultrasound-guided biopsy or at least 12 months of follow-up with contrast-enhanced CT, MR, bone scintigraphy or a repeated 11C-choline PET/CT scan. The relationships between the detection rate of 11C-choline PET/CT and the factors PSA level, PSA kinetics, Gleason score, age, time to relapse and SUVmax in patients with positive findings were analysed.
Results
11C-Choline PET/CT detected the site of relapse in 123 of the 140 patients with a detection rate of 87.8 % (46 patients showed local relapse, 31 showed local and distant relapse, and 46 showed only distant relapse). In patients with relapse the mean serum PSA level was 9.08 ng/mL (median 5.1 ng/mL, range 2 – 60 ng/mL), the mean PSA doubling time was 5.6 months (median 3.5 months, range 0.4 – 48 months), and the mean PSA velocity was 15 ng/mL/year (median 8.8 ng/mL/year, range 0.4 – 87 ng/mL/year). Of the 123 patients with relapse, 77 (62.6 %) showed distant relapse with/without local relapse, and of these 77, 31 (40.2 %) showed oligometastatic disease (one or two distant lesions: lymph node lesions only in 16, bone lesions only in 14, and lymph node lesions and bone lesions in 1). In univariate and multivariate analyses PSA kinetics was the only variable affecting 11C-choline PET/CT detection rate. A significant correlation between PSA kinetics and site of recurrence (local relapse only vs. distant metastasis) was also observed.
Conclusion
The detection rate of 11C-choline PET/CT in patients with PC showing biochemical recurrence after EBRT as primary treatment is relatively high (87.8 %). 11C-Choline PET/CT was able to detect extraprostatic disease in the 62.6 % of patients. Considering this high detection rate, 11C-choline PET/CT could have clinical usefulness in the management of these PC patients, but this should be confirmed in future studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PC) is the third leading cause of death in economically developed countries [1]. The diagnosis is based on digital rectal examination, serum level of prostate-specific antigen (PSA) and biopsy guided by transrectal ultrasound (TRUS) [2]. The most common approaches are radical surgery, external beam radiation therapy (EBRT), brachytherapy (without or in combination with EBRT) and/or androgen deprivation therapy (ADT) with adjuvant intent [2]. Cryosurgical ablation of the prostate gland and high-intensity focused ultrasound are also considered in patients with localized disease, but they are still under evaluation [3, 4].
Monitoring the serum PSA level is the most reliable and cost-effective way to follow patients after primary treatment. After radical prostatectomy (RP) as primary treatment, a confirmed serum PSA level >0.2 ng/mL is a sign of recurrent cancer, while a serum PSA level of 2 ng/mL above the nadir is the most accepted criterion of disease relapse after EBRT [5, 6]. Imaging has a limited role in the detection of the site of recurrence. Imaging modalities such as bone scintigraphy (BS) and CT may show the site of recurrence only in patients with very high PSA levels (>20 ng/mL) or with fast PSA kinetics [7, 8]. PET/CT using 11C-labelled or 18F-labelled choline may have a role in this scenario as shown in many studies in the last 10 years [9]. 11C-Choline PET/CT may show the site of tumour recurrence earlier than other imaging methods and in a single step examination [10, 11]. However, at present there are no precise indications about the proper use of this tool in patients showing biochemical relapse. It is already established that the detection rate of 11C-choline PET/CT is strictly related to PSA level [12] and PSA kinetics [13, 14]. However, most studies have evaluated a mixed patient population, indiscriminately enrolling patients showing biochemical recurrence after RP or EBRT, at the first biochemical relapse after primary treatment or already undergoing treatment with various therapies. For these reasons, it is difficult to deduce any conclusions about different risk factors, optimal timing for 11C-choline PET/CT and clinical impact and/or usefulness of this imaging technique.
The aim of our current study was to evaluate the potential role and the detection rate of 11C-choline PET/CT in a population of patients with PC exclusively treated with EBRT as primary treatment, who showed biochemical relapse.
Materials and methods
Patient population
This retrospective study was performed according to the principles of the declaration of Helsinki and to national regulations. A total of 183 consecutive PC patients who showed biochemical relapse after first-line treatment with EBRT were studied in our PET/CT centre from October 2009 to January 2013. This population constitutes an original series of patients who had never been enrolled in previous studies from the same group. European Association of Urology (EAU) guidelines specify that after radiation therapy, a rising serum PSA level of >2 ng/mL above the nadir PSA level, rather than a specific threshold value, is the most reliable sign of persistent or recurrent disease [5]. We therefore retrospectively enrolled 140 patients (mean age 73 years, range 54 – 87 years) who met our inclusion criteria: serum PSA >2 ng/mL, EBRT as primary treatment, and at least three PSA measurements in the last 6 months. At time of the PET/CT scan the mean serum PSA level (PSA trigger) was 8.6 ng/mL (median 5 ng/mL, range 2 – 60 ng/mL). PSA kinetics were calculated for each patient: the mean PSA doubling time (PSAdt) was 6.2 months (median 3.8 months, range 0.4 – 48 months), while the mean PSA velocity (PSAvel) was 13.6 ng/mL/year (median 8.2 ng/mL/year, range 0.4 – 48 ng/mL/year). The median Gleason score (GS) was 7. The characteristics of the enrolled patients are summarized in Table 1.
Primary radical treatment
All patients with PC enrolled had been treated exclusively with EBRT as primary treatment with a 3-D radiotherapy technique or with image-guided intensity-modulated radiotherapy (IG-IMRT). Low-risk patients (T1/T2 stage and/or PSA <10 ng/mL) received a dose range of 74 – 76 Gy to the prostate gland and 70 – 72 Gy to the prostatic fossa, while intermediate risk patients (T2b/T2c stage and/or PSA 10 – 20 ng/mL) and high-risk patients (T3/T4 stage and/or PSA >20 ng/mL) received an escalating dose of >76 Gy (range 76 – 81 Gy) to the prostate gland and 72 – 74 Gy to the prostatic fossa. Therapy was performed in accordance to EAU guidelines [2]. At staging, no patients showed lymph node (LN) positivity on CT, MRI or 11C-choline PET/CT so that, according to imaging, all patients were classified as N0 status. In spite of this, high-risk patients received additional nodal irradiation.
Of the 140 patients, 77 received ADT after EBRT with adjuvant intent or after biochemical relapse, and 53 were still receiving ADT at the time of the C-choline PET/CT scan (Table 1). As also shown in Table 1, 21 patients were receiving LH-RH antagonist, 17 were receiving antiandrogens (150 mg of bicatulamide) while 15 were receiving a combination of the two treatments.
Radiopharmaceuticals
11C-Choline was synthesized according to the solid-phase method described by Pascali et al. [15], using a commercial synthesis module (TRACERlab; GE Medical Systems, Waukesha, WI). 11C-CO2 produced by a cyclotron (GE Healthcare), was converted into 11C-CH3I by the conventional LiAlH4/HI reaction. 11C-CH3I was used for the N-methylation of dimethylaminoethanol (60 μl) placed directly on a solid-phase support (C18 Sep-Pak Light; Waters). After washing with ethanol and water, 11C-choline retained on a cation exchange resin (Sep-Pak Accell Plus CM; Waters) was eluted with saline, sterilized with a 0.22-μm filter and collected in a final volume of 8 mL. Radiochemical purity was evaluated by a high-performance liquid chromatography radiodetector equipped with a reversed phase column, and the concentration of organic solvents was measured by gas chromatography. Endotoxin content was measured using the conventional lysosomal acid lipase method (Cambrex Bioscience).
Imaging protocol
The 11C-choline PET/CT scan was performed following the standard procedure in our centre. All scans were obtained with a hybrid PET/CT tomograph (Discovery LS and Discovery STE; GE Medical Systems, Waukesha, WI). CT parameters were 120 kVp, 60 mA, 0.8 s per tube rotation, slice thickness 5 mm, pitch 1.5, and table speed 30 mm/rotation. CT images were used both for image fusion and for attenuation correction of emission data. The patients fasted for at least 6 h before PET scanning and received an intravenous injection of 370 – 555 MBq of 11C-choline. The PET/CT scan was started 3 – 5 min after radiotracer injection. Emission data were acquired for five or six bed positions from the mid-thigh to the base of the skull, taking 3 – 4 min for each bed position (in relation to body weight and volume).
Image analysis and validation criteria
All 11C-choline PET/CT images were analysed with dedicated software (eNTEGRA; GE Medical Systems, Waukesha, WI), which allowed review of the PET, CT and fused-imaging data. PET images were first assessed visually using transaxial, sagittal and coronal displays and interpreted in consensus by two experienced nuclear medicine physicians with more than 5 years 11C-choline PET/CT reading who were aware of clinical data. Visual interpretation was used as the main criterion to reach the final diagnosis. Any uptake higher than background was considered as tissue suspicious of malignancy. The SUVmax (maximum standardized uptake values) was measured for each lesion, but it was not used as the main criterion to reach the final diagnosis because an accurate cut-off value has not yet been established. The readers disagreed in only 5 % (7 out of 140) of the 11C-choline PET/CT scans. The final diagnoses were reached by consensus and by the opinion of a third reader.
Criteria for validation of a PET-positive finding were: (1) TRUS-guided biopsy in patient with local recurrence; (2) 11C-choline PET/CT-positive findings that were confirmed by a targeted contrast-enhanced CT, MR or BS scans; or (3) clinical follow-up (FU) of more than 12 months, including contrast-enhanced CT, MR or BS, and repeated 11C-choline PET/CT scans revealing further metastatic lesions or alternatively the disappearance of metastatic lesions associated with normalization of PSA level (PSA <2 ng/mL) following systemic therapy.
Statistical analysis
All data reported are expressed as mean, median and range for each value. PSA kinetics (PSAdt and PSAvel) were calculated according to the method of Khan et al. [16]. Continuous variables were compared between two groups using the t test. Continuous variables were compared among three groups using one-way analysis of variance. The chi-squared test was used for categorical variables. In univariate and multivariate binary logistic analyses, trigger PSA levels, PSAdt and PSAvel and SUVmax were coded as continuous variables; GS (<7 vs. ≥7) and age (<65 vs. >65 years) were coded as categorical variables. The associations between clinical and pathological features and 11C-choline PET/CT findings were assessed using univariate and multivariate binary logistic analyses. Odds ratios (OR) computed by logistic regression together with their 95 % confidence intervals (CI) are reported. The regression coefficients of each variable are also provided. The Hosmer-Lemeshow test was used to assess the goodness-of-fit in the multivariate analysis. All tests were two-sided. Statistical significance was taken at p < 0.05. All statistical analyses were performed using the SPSS v. 21 statistical software package (SPSS Inc., Chicago, IL).
Results
11C-Choline PET/CT was positive in 123 of the 140 patients with an overall detection rate of 87.8 %. Local relapse was detected in 46 patients showing 11C-choline uptake in the prostate gland only. Local and systemic disease was found in 31 patients (17 with local relapse and involved LNs; 6 with local relapse and involved bone; and 8 with local relapse, and involved LNs and bone), while only systemic disease diffusion was found in 46 patients (21 with involved LNs only; 20 with involved bone only; 4 with involved LNs and bones; and 1 with involved lung). In the 123 patients with at least one site of pathological choline uptake, the mean PSA level was 9.08 ng/mL (median 5.1 ng/mL, range 2 – 60 ng/mL) while in the 17 PET/CT-negative patients, the mean PSA level was 5.54 ng/mL (median 3.4 ng/mL, range 2 – 12 ng/mL, p < 0.05). In PET/CT-positive patients the mean PSAdt was 5.6 months (median 3.5 months, range 0.4 – 48 months) but was 10.2 months (median 7.2 months, range 1.2 – 48 months, p < 0.05) in PET/CT-negative patients. The mean PSAvel was 15 ng/mL/year (median 8.8 ng/mL/year, range 0.4 – 87 ng/mL/year) in PET/CT-positive patients but was 5.9 ng/mL/year (median 3.7 ng/mL/year, range 0.8 – 25.6 ng/mL/year, p < 0.05) in PET/CT-negative patients.
The univariate and multivariate binary logistic analyses showed a significant correlation between PSA kinetics and 11C-choline PET/CT results. PSAdt (OR 0.94, CI 95 % 0.89 – 0.99, p = 0.029) and PSAvel (OR 1.101, CI 95 % 1 – 1.2, p = 0.039) were associated with a significantly increased risk of positive 11C-choline PET/CT. No significant association was found for other factors evaluated including PSA level, age, GS, time to biochemical relapse (TTR), ADT and SUVmax). In particular, the PSA level at the time of the scan (OR 1.077, CI 95 % 0.96 – 1.2 p = 0.200) was found not to be a significant factor predicting a positive scan. The results of the logistic regression analyses are summarized in Table 2.
A site-by-site analysis in the whole population showed that 11C-choline PET/CT detected extraprostatic involvement of disease in 77 of the 123 of positive patients (62 % of positive patients; 55 % of the whole population) with or without evidence of local deposit; 31 of 77 showed local and distant involvement and 46 of 77 showed distant spread only. 11C-choline PET/CT also demonstrated the presence of oligometastatic disease (one or two choline deposits) in 31 of the 77 patients (40 %) with evidence of distant recurrence: in LNs only in 16 patients, in bone only in 14 patients, and in LNs and bone in 1 patient. Statistical analysis showed a significant correlation between PSA kinetics and the site of relapse: local relapse only vs. distant relapse (PSAdt OR 0.904, CI 95 % 0.82 – 1.03, p = 0.009; PSAvel OR 1.065, CI 95 % 0.83 – 0.97, p = 0.001; Table 3). In particular, PSAdt and PSAvel were correlated with the detection of positive LNs (PSAdt OR 0.876, CI 95 % 0.793 – 0.968, p = 0.009: PSAvel OR 1.022, CI 95 % 0.99 – 1.03, p = 0.033) and with the detection of bone metastasis (PSAdt OR 0.968, CI 95 % 0.906 – 1.034, p = 0.032:; PSAvel OR 1.043, CI 95 % 1.01 – 1.07, p = 0.01). Figure 1 shows the relationship between PSAdt and detection of local vs. distant relapse (a PSAdt cut-off of 6 months was taken in accordance with EAU guidelines for defining low-risk vs. high-risk patients).
Relationship between PSAdt and the detection of local only and distant relapse using an arbitrary PSAdt cut-off of 6 months in accordance with EAU guidelines for defining low-risk vs. high-risk patients. Note that with a PSAdt of <6 months, there is a relatively high probability (65.1 %) of detecting a distant site of recurrence
The binary logistic regression analysis, with positive PET coded as a variable of selection, showed a significant correlation between PSAdt only and the number of positive LNs detected (OR 1.055, CI 95 % 0.51 – 1.11, p = 0.032). No significant relationships were observed for the other predictive factors investigated (PSA level, PSAvel, GS, TTR, age, SUVmax; p > 0.05). Furthermore, no significant relationship was found between these predictive factors (also including PSAdt) and the number of bone metastases detected (p > 0.05).
In relation to the administration of ADT at the time of the 11C-choline PET/CT scan, 48 of 53 patients (90.5 %) receiving ADT had a scan, while 75 of 87 patients (86 %) not receiving ADT had a positive scan. Otherwise, no significant correlation between ADT and 11C-choline PET/CT was observed in univariate or multivariate binary logistic regression (OR 1.536, CI 95 % 0.3 – 0.47, p = 0.44).
In an analysis based on a trigger PSA level, we investigated a subcohort of 71 patients with a serum PSA level in the range >2 to ≤ 5 ng/mL. This division was related to the median PSA level (5 ng/mL) observed in our population of positive patients. The detection rate was approximately the same as that in the whole population (88 % vs. 85.9 %), with evidence of local relapse only in 33 of the 71 patients (Fig. 2). Among the remaining 28 of the 71 patients, an essentially similar percentage with distant findings (with or without evidence of local relapse) was seen as among the whole population (40 % vs. 55 %). Statistical analysis confirmed that even in this subcohort PSA kinetics were the only variable significantly related to the 11C-choline PET/CT detection rate. Again, no relationship was found between the 11C-choline PET/CT findings and PSA level (OR 0.995, CI 95 % 0.364 – 1.876, p = 0.649).
Validation
In the 46 patients showing only local recurrence, positive deposition of choline in the prostate gland was validated using TRUS-guided biopsy. The biopsy result was decisive in 18 and indecisive in 28 of the 46 patients who were later investigated by MRI. There were no false-positive findings. In the remaining 77 patients with evidence of at least one site of distant relapse, 11C-choline PET/CT findings were confirmed by targeted contrast-enhanced CT and/or MR and/or BS scans in 52 patients, while in 25 patients the PET results were validated by clinical FU of more than 12 months (mean FU 23 months, median FU 19 months), including contrast-enhanced CT and/or MR and/or BS and/or repeated 11C-choline PET/CT scans which revealed further metastatic lesions or alternatively the disappearance of metastatic lesions associated with normalization of PSA levels (<2 ng/mL) following systemic therapy.
Discussion
Biochemical recurrence after EBRT as a primary treatment is relatively frequent and ranges from 10 % to 60 % depending on patient risk and on the radiotherapy technique used (standard EBRT vs. new approaches such as IG-IMRT) [17]. In the largest randomized study published so far, the rate of recurrence in high-risk patients was 50 % [18]. The site of PC disease relapse may be classified into four main groups: (1) PSA-only relapse, (2) local recurrence only, (3) distant metastases only (nodal or osseous disease are the most common sites), and (4) both local and distant relapse. Otherwise, the low sensitivity in detecting the site or sites of relapse still remains a main limitation of many imaging procedures. For this reason the site of relapse is rarely detected. This explains why none of the main international guidelines at present recommend any imaging procedure if: (a) PSA does not reach high levels (e.g. PSA level >20 ng/mL) and/or (b) if there is evidence of a clinically detectable lesion (pain, fracture, etc.) that can be treated with palliative intent [5, 19]. Based on T and N status, GS, PSA level, PSA kinetics (PSAdt and PSAvel), TTR, many risk tables and predictive nomograms have been generated to assist the clinicians in the diagnosis of the site of recurrence (local vs. distant) [5]. However, in clinical practice, it is not easy to identify the origin of an increase in PSA level and the number and the sites of metastatic deposits due to the well-known lack of accuracy of conventional imaging [7, 8]. At the present time, most patients receive ADT with different regimens soon after PSA relapse without any effort to localize the real site or sites of relapse.
For these reasons and in view of the discussion above, we are led to wonder as to which patients would benefit the most from a positive 11C-choline PET/CT. Considering the high cost of this investigation and its limited availability, optimal selection of patients is essential. Proper detection of the disease may direct patients to personalized and tailored approaches such as: (1) the inclusion of local PET-positive LNs in patients already scheduled for salvage treatment in the prostatic fossa, (2) the treatment of few LN lesions (oligometastatic disease) with curative intent with salvage radiotherapy or salvage extended peritoneal lymph node dissection, or (3) the treatment of metastatic lesions at risk of complications (bone, joint) before the appearance of any clinical symptoms that may be detected early by PET and may lead to palliative treatment with radiotherapy and/or diphosphonate therapy. Furthermore, a relevant distinction should be made between salvage therapies with “palliative” intent (such as ADT and bisphosphonates) and salvage therapies with “curative” intent (such as salvage radiotherapy, bone irradiation, tomotherapy, high-intensity focused ultrasound, pelvic or retroperitoneal LN dissection and salvage prostatectomy). Considering that a curative approach is the best option for patients, early detection of the site of relapse is crucial. Indeed, only patients showing oligometastatic spread of disease would have real benefit from such aggressive treatments. In the last few years, a few but well-designed studies have drawn attention to the potential usefulness of 11C-choline PET/CT for guiding these salvage therapies with curative intent using surgery [20–22] or EBRT [23–25]. These results are encouraging and, despite some obvious limitations, for example the low sensitivity in detection of small metastatic LNs [20–22], they prove that salvage therapy with curative intent is feasible, providing a relapse-free survival of approximately 2 to 3 years in half of the patients studied [20, 24]. Examples of this approach are provided in Figs. 3 and 4.
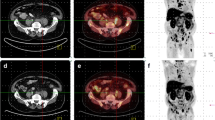
Complete response in a 78-year-old patient with PC (GS 3 + 3, staging PSA level 11.3 ng/mL) treated with IG-IMRT with 74 Gy to the prostate gland in June 2010. In April 2011 the patient showed biochemical relapse (TTR <12 months). The patient underwent a 11C-choline PET/CT scan in May 2011 (PSA level 2.4 ng/mL, PSAdt 2.1 months, PSAvel 8 ng/mL/year). The images show a choline deposit on the paraaortic LN (a, b). The patient was treated with IG-IMRT with salvage intent. Images obtained in May 2012 (c, d) show a complete response after therapy
A 75-year-old patient with PC (GS 4 + 3, staging PSA level 8.9 ng/mL) who received EBRT as first-line treatment in September 2010. In October 2012 the patient showed a biochemical response (TTR >12 months). ADT was not given. The patient underwent a 11C-choline PET/CT scan in January 2013 (PSA level 2.2 ng/mL, PSAdt 1.5 months, PSAvel 6 ng/mL/year) that revealed a pathological deposit on the prostate gland. This finding was confirmed by TRUS-guided biopsy
The three main results of our study are as follows:
-
1.
We showed that 11C-choline PET/CT is able to detect the sites of relapse in the majority of patients with rising PSA levels after EBRT as first primary treatment. To the best of our knowledge, this is the largest study published so far including a population of patients exclusively treated with EBRT. These results confirm those recently reported by Chondrogiannis et al. [26] who found a detection rate of 80 % in 46 patients showing biochemical relapse after EBRT or brachytherapy as primary treatment and studied with 18F-choline PET/CT.
-
2.
Surprisingly, in this patient population, the influence of the absolute value of PSA (trigger PSA) on the relapse detection rate of 11C-choline PET/CT was not statistically significant (p = 0.221). This finding is in contrast to those of all previously published studies which included only patients treated with RP [10, 27] or mixed populations treated with RP or EBRT or ADT [11, 12, 28, 29]. This result could probably be related to the presence of viable prostatic tissue that produces PSA. Moreover, this nonneoplastic PSA production could vary from patient to patient in terms of amount and percentage. The presence of such nonneoplastic tissue producing PSA may have influenced the statistical relationship between the trigger PSA level and the 11C-choline PET/CT detection rate. In contrast, in patients showing biochemical relapse after RP any increase in PSA level above 0.2 ng/mL could be related to the presence of disease relapse, and this may explain the linear correlation between the value of PSA and the detection rate of PET/CT [10, 27]. In the end, we have to consider that in only 17 of 140 patients was the 11C-choline PET/CT scan negative; this could even have affected the detection rate and could explain why no statistical significance was observed. Also in a subcohort of 71 patients with a PSA level lower than 5 ng/mL no significant correlation was observed.
-
3.
In our selected patient population, PSA kinetics was the most important factor predicting a positive 11C-choline PET/CT scan and was the only statistically significant factor in univariate and multivariate analyses (p < 0.05). More interesting, our results show that there could be a statistically significant difference in PSA kinetics between patients with local relapse only and distant relapse (with or without local relapse). In our study, local relapse only was found in 46 patients (32 % of the whole population), distant relapse only in 46 (32 %) and local with distant relapse in 31 (22 %). The relationship between PSA kinetics and the site of relapse is in accordance with the findings reported by Giovacchini et al. [27], in a population showing biochemical recurrence after RP. Otherwise, this is the first time that a statistically significant correlation between the site of relapse and PSA kinetics has been assessed in a population who received EBRT as primary treatment. We also assessed whether other factors could affect the relapse detection rate of 11C-choline PET/CT, including age, TTR, GS, ADT and SUVmax resulted, but found no statistical significance in predicting a positive study and, as a consequence, the site and number of lesions.
This study had some limitations. Validation of the positive findings detected by 11C-choline PET/CT was mostly based on a longitudinal FU of each lesion after therapy. Histology in each patient would have been preferable but was not feasible for practical and ethical reasons. Moreover, the sensitivity of TRUS-guided biopsy in an irradiated prostate is low and this procedure is eventually required only if salvage treatment is planned [30]. Furthermore, the number of patients enrolled in the study was small and consequently there were only 17 patients with a negative 11C-choline PET/CT scan patients and data on such patients are therefore lacking. Finally, the study was based on a retrospective analysis of the data.
Conclusion
The relapse detection rate of 11C-choline PET/CT in patients with PC showing biochemical recurrence after EBRT as primary treatment was high (87 %). 11C-Choline PET/CT was able to detect extraprostatic disease in 62 % of the patients. In patients showing biochemical relapse after EBRT, the influence of PSA level on 11C-choline PET/CT detection rate was not statistically significant. In contrast, PSA kinetics were not only the main statistically significant factor predicting a positive 11C-choline PET/CT scan, but also a factor able to discriminate between local and distant relapse. Considering this high detection rate, 11C-choline PET/CT could have clinical usefulness in the management of these PC patients, but these findings should be confirmed in controlled clinical trials.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason MD, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71.
Babaian RJ, Donnelly B, Bahn D, Baust JG, Dineen M, Ellis D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180:1993–2004.
Warmuth M, Johansson T, Mad P. Systematic review of the efficacy and safety of high-intensity focussed ultrasound for the primary and salvage treatment of prostate cancer. Eur Urol. 2010;58:803–15.
Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–83.
Freedland SJ, Presti Jr JC, Amling CL, Kane CJ, Aronson WJ, Dorey F, et al. Time trends in biochemical recurrence after radical prostatectomy: results of the SEARCH database. Urology. 2003;61:736–41.
Kane CJ, Amling CL, Johnstone PAS, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–11.
Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008;179(3):906–10.
Fuccio C, Rubello D, Castellucci P, Marzola MC, Fanti S. Choline PET/CT for prostate cancer: main clinical applications. Eur J Radiol. 2011;80(2):e50–6.
Castellucci P, Fuccio C, Nanni C, Santi I, Rizzello A, Lodi F, et al. Influence of trigger PSA and PSA kinetics on 11C-choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med. 2009;50:1394–400.
Picchio M, Messa C, Landoni C, Gianolli L, Sironi S, Brioschi M, et al. Value of 11C-choline-positron emission tomography for re-staging prostate cancer: a comparison with 18F-fluorodeoxyglucose-positron emission tomography. J Urol. 2003;169:1337–40.
Krause BJ, Souvatzoglou M, Tuncel M, Herrmann K, Buck AK, Praus C, et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35(1):18–23.
Castellucci P, Fuccio C, Rubello D, Schiavina R, Santi I, Nanni C, et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur J Nucl Med Mol Imaging. 2011;38(1):55–63.
Mamede M, Ceci F, Castellucci P, Schiavina R, Fuccio C, Nanni C, et al. The role of 11C-choline PET imaging in the early detection of recurrence in surgically treated prostate cancer with very low PSA level <0.5 ng/mL. Clin Nucl Med. 2013;38(9):e342–5.
Pascali C, Bogni A, Iwata R. 11C-methylation on 18C SepPak cartridge: a convenient way to produce [N-methyl-11C]choline. J Labelled Compds Radiopharm. 2000;49:195–203.
Khan MA, Carter HB, Epstein JI, Miller MC, Landis P, Walsh PW, et al. Can prostate specific antigen derivatives and pathological parameters predict significant change in expectant management criteria for prostate cancer? J Urol. 2003;170:2274–8.
Khuntia D, Reddy CA, Mahadevan A, Klein EA, Kupelian PA. Recurrence-free survival rates after external-beam radiotherapy for patients with clinical T1–T3 prostate carcinoma in the prostate-specific antigen era: what should we expect? Cancer. 2004;100:1283–92.
Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–73.
Mohler J, Bahnson RR, Boston B, Busby JE, D’Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8(2):162–200.
Rigatti P, Suardi N, Briganti A, Da Pozzo LF, Tutolo M, Villa L, et al. Pelvic/retroperitoneal salvage lymph node dissection for patients treated with radical prostatectomy with biochemical recurrence and nodal recurrence detected by [11C]choline positron emission tomography/computed tomography. Eur Urol. 2011;60(5):935–43.
Tilki D, Reich O, Graser A, Hacker M, Silchinger J, Becker AJ, et al. 18F-Fluoroethylcholine PET/CT identifies lymph node metastasis in patients with prostate-specific antigen failure after radical prostatectomy but underestimates its extent. Eur Urol. 2013;63(5):792–6.
Passoni NM, Suardi N, Abdollah F, Picchio M, Giovacchini G, Messa C, et al. Utility of [11C]choline PET/CT in guiding lesion-targeted salvage therapies in patients with prostate cancer recurrence localized to a single lymph node at imaging: results from a pathologically validated series. Urol Oncol. 2013. doi:10.1016/j.urolonc.2013.03.006
Souvatzoglou M, Krause BJ, Pürschel A, Thamm R, Schuster T, Buck AK, et al. Influence of (11)C-choline PET/CT on the treatment planning for salvage radiation therapy in patients with biochemical recurrence of prostate cancer. Radiother Oncol. 2011;99(2):193–200. doi:10.1016/j.radonc.2011.05.005.
Würschmidt F, Petersen C, Wahl A, Dahle J, Kretschmer M. [18F]fluoroethylcholine-PET/CT imaging for radiation treatment planning of recurrent and primary prostate cancer with dose escalation to PET/CT-positive lymph nodes. Radiat Oncol. 2011;6:44.
Berkovic P, De Meerleer G, Delrue L, Lambert B, Fonteyne V, Lumen N, et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer. 2013;11(1):27–32.
Chondrogiannis S, Marzola MC, Ferretti A, Maffione AM, Rampin L, Grassetto G, et al. Role of 18F-choline PET/CT in suspicion of relapse following definitive radiotherapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40(9):1356–64.
Giovacchini G, Picchio M, Scattoni V, Garcia Parra R, Briganti A, Gianolli L, et al. Doubling time for prediction of [(11)C]choline PET/CT findings in prostate cancer patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37(6):1106–16.
de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. 11C-choline positron emission tomography for the evaluation after treatment of localized prostate cancer. Eur Urol. 2003;44(1):32–8.
Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, et al. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37(2):301–9.
Martino P, Scattoni V, Galosi AB, Consonni P, Trombetta C, Palazzo S, et al. Role of imaging and biopsy to assess local recurrence after definitve treatment for prostate carcinoma (surgery, radiotherapy, cryotherapy, HIFU). World J Urol. 2011;29(5):595–605. doi:10.1007/s00345-011-0687-y.
Acknowledgments
We would like to thank Marcelo Mamede, MD PhD, for his contribution to the final review of the statistical analysis of data and Joshua James Morigi, MD, for his contribution to the language editing.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceci, F., Castellucci, P., Graziani, T. et al. 11C-Choline PET/CT detects the site of relapse in the majority of prostate cancer patients showing biochemical recurrence after EBRT. Eur J Nucl Med Mol Imaging 41, 878–886 (2014). https://doi.org/10.1007/s00259-013-2655-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2655-9