Abstract
Purpose
Myocardial ischaemia is frequently silent in patients with type 2 diabetes. Although it has been proposed as a potential screening tool, the role of myocardial perfusion single photon emission computed tomography (MPS) has recently been questioned, due to the low prevalence of positive scans and the low rate of cardiac events. The aim of this study was to assess if pretest clinical variables can identify a subgroup of asymptomatic patients with type 2 diabetes at risk of silent myocardial ischaemia and a subsequent poor outcome
Methods
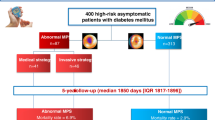
This prospective study included 77 patients (50 men, mean age 63 ± 9 years) with type 2 diabetes and no known coronary artery disease (CAD) or angina pectoris who underwent gated MPS to screen for CAD between March 2006 and October 2008. MPS images were interpreted using a semiquantitative visual 20-segment model to define summed stress, rest and difference scores. Ischaemia was defined as a sum difference score (SDS) ≥2. Patients were followed-up (median 4.1 years, range 0.8 – 6.1 years) and cardiac hard events (cardiac death or nonfatal myocardial infarction) were recorded.
Results
Silent ischaemia was detected in 25 of the 77 patients (32 %). Specifically, 10 patients (13 %) had mild ischaemia (SDS 2 to ≤4) and 15 patients (19 %) had severe ischaemia (SDS >4). In univariate binary logistic analysis, microalbuminuria was the only significant predictor of silent ischaemia on MPS (odds ratio 4.42, 95 % CI 1.27 – 15.40; P = 0.019). The overall accuracy of microalbuminuria for predicting silent ischaemia was 71.4 % and was 89.6 % for predicting severe ischaemia. Kaplan-Meier curves showed no significant group differences in 5-year cardiac event-free survival between patients with and those without microalbuminuria, or between patients with SDS ≥2 and those with SDS <2. In contrast, 5-year event-free survival was significantly lower in patients with SDS >4 than in patients with SDS ≤4: 55.6 % (95 % CI 39.0 – 72.2 %) vs. 94.5 % (95 % CI: 91.4 – 97.6 %), respectively (Breslow test, chi-square 20.9, P < 0.001). Median cardiac event-free survival was not observed in the whole group, while the 25th percentile of cardiac event-free survival was reached only in patients with SDS >4 (2.3 years). In univariate Cox regression analysis, SDS >4 predicted cardiac event-free survival (hazard ratio 12.87, 95 % CI 2.86 – 27.98; P = 0.001), while SDS ≥2 did not (hazard ratio 2.78, 95 % CI 0.62 – 12.46, P = 0.16).
Conclusion
In this group of patients with type 2 diabetes, microalbuminuria was the only predictor of silent ischaemia on MPS. Assessment of microalbuminuria should be routinely considered among the first risk stratification steps for CAD in patients with type 2 diabetes, even though severe ischaemia on MPS is a major predictor of cardiac event-free survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes is a growing health problem associated with high rates of cardiovascular morbidity and mortality [1]. Individuals with diabetes have a higher prevalence of silent coronary artery disease (CAD) than those without diabetes, and diabetic patients without prior myocardial infarction have as high a risk of death from CAD as nondiabetic patients with previous myocardial infarction [1, 2]. In comparison to nondiabetic patients, diabetic patients have a lower incidence of one-vessel disease and a higher incidence of three-vessel/left main artery disease and left ventricular dysfunction [3]. Most importantly, myocardial ischaemia is often asymptomatic in patients with diabetes until the onset of myocardial infarction or cardiac death [4, 5]. Although it has been proposed as a potential screening tool, the role of myocardial perfusion single photon emission computed tomography (MPS) has recently been questioned due to the low prevalence of positive results and the low rate of cardiac events [5–8].
Risk stratification is of primary importance for identification of asymptomatic patients who have a higher risk of developing symptomatic cardiovascular disease and who might benefit from testing by MPS [6, 7]. Risk factors associated with detection of myocardial ischaemia have varied across studies depending on study design, inclusion criteria and data analysis [5, 9, 10]. Thus, further studies demonstrating an association between CAD risk factors and silent myocardial ischaemia are warranted to identify patients who may benefit most from screening procedures [6, 11]. The aim of this study was to identify clinical predictors of silent ischaemia in patients with type 2 diabetes using MPS.
Materials and methods
Patients
This prospective study included 77 patients with type 2 diabetes who were referred for MPS from March 2006 to October 2008 from the Diabetes Center to the Nuclear Medicine Department of the S. Andrea Hospital, La Spezia, Italy. These patients were referred for MPS as part of a screening programme for CAD offered to all patients receiving therapy at the Diabetes Center of the S. Andrea Hospital. Study exclusion criteria were: (1) angina pectoris, (2) previous coronary angiography or coronary artery revascularization, (3) history of myocardial infarction or heart failure, (4) electrocardiographic evidence of Q-wave myocardial infarction, ischaemic ST-segment or T-wave changes, (5) history of ketoacidosis or dialysis, (6) macroalbuminuria, and (7) inability to provide signed informed consent [12].
In each patient a medical history was obtained and physical examination performed. Blood and urine samples were obtained for laboratory testing, including dyslipidaemia, microalbuminuria and HbA1c concentration. Hypertension was defined as a blood pressure >140/90 mmHg or normal blood pressure values on antihypertensive drug treatment. Dyslipidaemia was defined as total cholesterol >240 mg/dl, low-density lipoproteins >100 mg/dl or HDL cholesterol <35 mg/dl [5, 9]. Microalbuminuria was defined as albumin urine excretion between 20 and 200 µg/min. Microalbuminuria was measured within 1 month of MPS. Pathological HbA1c urine concentration was set at >7 %. Family history of CAD was defined as diagnosis of CAD in parents or siblings under 50 years of age. Autonomic nervous function was assessed in terms of changes in heart rate during deep breathing and after postural change, and variation in systolic blood pressure during postural change. The thresholds adopted for positive tests were those recommended by Ewing et al. [13]. The definitive presence of diabetic autonomic neuropathy was established if one or more of the above tests were positive [13]. The characteristics of the patients are presented in Table 1.
MPS protocol
Patients underwent gated single photon emission computed tomography (SPECT) imaging according to a 2-day protocol. A bicycle exercise stress test was performed with a standard Bruce protocol using a 12-lead electrocardiogram (ECG). Exercise endpoints included physical exhaustion, ECG ischaemic changes, angina, sustained ventricular tachycardia, haemodynamically significant supraventricular arrhythmias, or exertional hypotension. Blood pressure was measured and recorded at rest, at the end of each exercise stage and at peak exercise. Horizontal or downsloping ST segment depression ≥1 mm or upsloping ≥1.5 mm was considered positive for inducible ischaemia. MPS was performed using a 2-day protocol. At maximum stress, 740 MBq of 99mTc-tetrofosmin was injected intravenously. After tracer injection, exercise was continued at the maximum workload for at least 1 min. MPS imaging was started within 30 min of tracer injection. Patients who failed to perform maximal exercise underwent a pharmacological stress test with dipyridamole and were excluded from this analysis. Rest imaging was performed within 1 week of stress imaging.
SPECT acquisition protocol
Gated SPECT was performed with a double-head gamma camera (E-Cam Dual Head; Siemens Medical Solutions) equipped with a high-resolution collimator. Raw data were collected in a 64 × 64 matrix, with a zoom of 1.45, using 32 projections over a 180° semicircular orbit for each detector, with an acquisition time of 45 s for each step. The energy setting was centred on the 140 keV photopeak of 99mTc with a 20 % window. Activity counts were gated over 12 frames per cycle. Images were reconstructed with filtered back-projection using a Butterworth filter, with an order of 5 and a cut-off frequency of 0.4 cycles per pixel. No attenuation correction was performed.
Data analysis
Fixed and reversible perfusion defects were automatically quantified using the 20-segment model of the Quantitative Perfusion SPECT (QPS) software (Cedars-Sinai Medical Center, Los Angeles, CA). Each myocardial segment was visually scored using a five-point scoring system: 0 normal, 1 mildly reduced, 2 moderately reduced, 3 severely reduced, and 4 absent. Total scores in 20 segments were calculated as the sum of stress, rest and difference scores (SSS, SRS and SDS) [14]. SDS ≥2 was used as the threshold for defining myocardial ischaemia and SDS >4 was used to define severe ischaemia [15–18]. SSS ≥4 was considered pathological [15–18]. Four SSS categories were used for risk-based stratification: SSS <4 was considered normal, 4 – 8 mildly abnormal, 9 – 13 moderately abnormal, and ≥14 severely abnormal [15–18]. Based on SRS and SDS scores, myocardial perfusion defects were classified as reversible (SRS <4 and SDS ≥2), fixed (scar; SRS ≥4 and SDS <2), and mixed defects (SRS ≥4 and SDS ≥2) [16, 19]. End-diastolic volume, end-systolic volume and ejection fraction for stress and rest were computed with the Quantitative Gated SPECT (QGS) software.
Patient follow-up
To assess the value of the examined variables in predicting cardiac event-free survival, patients were followed-up by periodic examinations every 3 – 6 months at the Diabetes Center of the S. Andrea Hospital. None of the patients was lost to follow-up. Endpoints of the survival analysis were cardiac hard events, defined as either cardiac death or nonfatal myocardial infarction. Cardiac death was defined as death due to any cardiovascular cause, and was confirmed by review of the death certificate, hospital chart, or physician’s record. Nonfatal myocardial infarction was documented by a consistent history accompanied by elevation of cardiac enzymes and/or new Q waves on the ECG. The study was approved by the ethics committee of S. Andrea Hospital, La Spezia, Italy. All patients gave written informed consent to participation in the study.
Statistical analysis
Patient groups were compared using unpaired Student’s t test for continuous variables and the chi-square test for categorical variables. Normality of the distribution of continuous variables was assessed with the Kolmogorov-Smirnov test (all variables showed a normal distribution). Factors predicting silent myocardial ischaemia were assessed using binary logistic analysis. The independent variable in the binary logistic analysis was SDS ≥2 (independent of SSS). The odds ratios (ORs) computed by logistic regression, together with their 95 % confidence intervals (CIs), are reported. Because of the small sample size, only the following dependent variables were considered for logistic regression analysis: family history of CAD, microalbuminuria, hypertension, dyslipidaemia, autonomic nervous function and smoking habit [9].
Cardiac event-free survival was estimated using Kaplan-Meier curves. Since cardiac hard events occurred early during follow-up (all events occurred before the median follow-up), differences between survival curves were evaluated using the Breslow test. Patients were censored from Kaplan-Meier cardiac event-free survival curves at timing of elective revascularization (i.e. revascularization performed >6 months after MPS in patients who did not experience a hard event). The association with cardiac event-free survival was assessed using Cox proportional hazard regression models. Hazard ratios (HRs) are reported together with their 95 % CIs.
Results
On the basis of SDS score, 25 of the 77 patients (32 %) had ischaemia. Specifically, 15 patients (19 %) had mild ischaemia and 10 (13 %) had severe ischaemia. On the basis of SSS scores, perfusion defects were abnormal in 17 patients (22 %). Specifically, stress perfusion defects were mildly abnormal in 14 patients (18 %), moderately abnormal in 1 (1 %) and severely abnormal in 2 (3 %). Thus, in the whole population, moderate to severe stress perfusion defects were found in 3 patients (4 %). In patients with ischaemia, SSS was only slightly higher than SDS (5.5 ± 4.6 vs. 4.5 ± 3.1, P = 0.017, paired t test). On the basis of SRS and SDS, 20 patients (26 %) had only ischaemia, 5 (6 %) had both ischaemia and fixed defects, and only 1 (1 %) had only a fixed defect. Eight patients (10 %) had ischaemic ECG abnormalities during exercise.
Among clinical variables, patients with silent ischaemia significantly differed from patients without ischaemia only for the higher rate of microalbuminuria (32.0 % vs. 9.6 %, respectively; P = 0.014; Table 2). The rate of ischaemia did not significantly differ between patients treated with insulin and patients treated with metformin and/or sulphonylureas (34.6 % vs. 31.4 %; P = 0.77).
In univariate binary logistic analysis (Table 3), only microalbuminuria was a significant predictor of myocardial ischaemia (OR 4.42, 95 % CI 1.27 – 15.40; P = 0.019). No statistically significant trend (P < 0.1) was observed for any other variable, and therefore multivariate analysis was not performed. Similar results were obtained using SSS ≥4 as independent variable (independent of SDS) (OR 4.13, 95 % CI 1.16 – 14.69; P = 0.029), but not using SRS ≥4 (OR 0.98, 95 % CI 0.10 – 9.19; P = 0.99).
The classification table for microalbuminuria is shown in Table 4. Silent ischaemia was present in 61.5 % of patients with microalbuminuria, while there was no evidence of silent ischaemia in 73.4 % of patients without microalbuminuria. Overall accuracy of microalbuminuria for predicting silent ischaemia was 71.4 %. Overall accuracy of microalbuminuria was higher for prediction of severe ischaemia (89.6 %).
The median follow-up was 4.1 years (range 0.8 – 6.1 years). During follow-up there were five cardiac deaths and two nonfatal myocardial infarctions. Medical treatment (continuation and/or intensification) was adopted in all 15 patients with mild ischaemia and in six of ten patients with severe ischaemia. Elective coronary angiography with revascularization was adopted in four of ten patients with severe ischaemia.
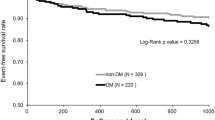
Kaplan-Meier cardiac event-free survival curves are shown in Fig. 1. There was no significant group difference in 5-year cardiac event-free survival between patients with and without microalbuminuria: 85.7 % (95 % CI 72.5 % – 98.9 %) vs. 90.2 % (95 % CI 86.4 – 94.0 %), respectively (Breslow test, chi-square 0.3; P = 0.61). Similarly, there was no significant group difference in 5-year cardiac event-free survival between patients with SDS ≥2 and those with SDS <2: 84.0 % (95 % CI 76.7 – 91.3 %) vs. 92.5 % (95 % CI 89.5 – 95.5 %), respectively (Breslow test, chi-square 2.7; P = 0.10). In contrast, 5-year event-free survival was significantly lower in patients with SDS >4 than in patients with SDS ≤ 4: 55.6 % (95 % CI 39.0 – 72.2 %) vs. 94.5 % (95 % CI 91.4 – 97.6 %), respectively (Breslow test, chi-square 20.9; P < 0.001). The median cardiac event-free survival was not reached in the whole group. The 25th percentile of cardiac event-free survival was reached only in patients with SDS >4 (2.3 years).
The results of Cox regression analysis for cardiac event-free survival are shown in Table 5. In univariate analysis, SDS significantly predicted cardiac event-free survival (HR 1.18, 95 % CI 1.03 – 1.36; P = 0.021). However, when the variable was dichotomized to distinguish according to grade of ischaemia, SDS >4 predicted cardiac event-free survival (HR 12.87, 95 % CI 2.86 – 27.98; P = 0.001), while SDS ≥2 did not (HR 2.78, 95 % CI 0.62 – 12.46; P = 0.16). Significant results were also obtained using SSS (HR 1.14, 95 % CI 1.04 – 1.25; P = 0.004) and SRS (HR 1.60, 95 % CI 1.21 – 2.11; P = 0.001).
Discussion
Over recent decades there has been increasing interest in the use of imaging techniques to detect subclinical CAD and improve risk stratification in patients with type 2 diabetes [5–9, 20, 21]. Currently, the American Diabetes Association guidelines recommend screening for CAD in asymptomatic patients with diabetes who have an abnormal resting ECG suggesting prior myocardial infarction or ischaemia, peripheral arterial disease, or two or more additional CAD risk factors among dyslipidaemia, hypertension, smoking, family history of CAD, and micro- or macroalbuminuria [9]. Screening of diabetic patients has been advocated for many years as a potential tool for identifying patients at high cardiovascular risk and for reducing the likelihood of cardiovascular events through aggressive treatment [9]. Several retrospective studies have shown that the prevalence of myocardial perfusion defects in diabetic patients is highly dependent on the characteristics of the population, and that perfusion defects are associated with a worse outcome [18, 22–27]. Therefore, cardiac imaging was thought to have the potential for risk stratification of diabetic patients. However, the value of such screening has long been debated in the absence of prospective studies.
In 2004, the first report of the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study was published. The study was a controlled trial in which patients were randomly assigned either to systematic screening with MPS and 5-year clinical follow-up or to follow-up only [5]. In the initial DIAD report 22 % of patients had silent ischaemia [5]. All patients underwent a second MPS study after intensification of medical therapy. The vast majority (79 %) of patients with baseline perfusion defects had resolution of perfusion defects while only a minor proportion (10 %) of patients with normal baseline MPS developed new ischaemia [12]. Longitudinal clinical evaluation showed that nonfatal and fatal cardiac events were similar in the screened group and in the nonscreened group. Overall cardiac events and revascularizations were much lower than anticipated on the basis of the MPS results and their occurrence was not modified by the screening procedure [8]. In the present investigation, silent ischaemia was found in 32 % of patients. This value is higher than that found in the prospective DIAD study and this can be attributed to the less conservative definition of ischaemia (SDS ≥2). Indeed, only a minor proportion of patients (12 %) displayed severe ischaemia (SDS >4).
The patients in our selected population were receiving multiple drug treatment and were under good metabolic control. Great care was taken to exclude diabetic patients with symptomatic disease, those with previous infarctions or those with previous revascularization procedures. In fact, in patients with ischaemia, SSS was only slightly higher than SDS (Table 2). We found that microalbuminuria was the only predictor of silent ischaemia on MPS. The overall accuracy of microalbuminuria for predicting silent ischaemia was 71.4 % and was 89.6 % for predicting severe ischaemia.
The frequency of perfusion defects in asymptomatic diabetic patients varied considerably across studies and ranged between 12 % and 51 % [26, 28, 29]. Generally, the prevalence of silent ischaemia increased in the presence of one or more cardiovascular risk factors [6, 7]. However, in the DIAD study the frequency of silent ischaemia was the same in patients with two or more CAD risk factors an in patients with fewer than two risk factors [5]. In our series, we also did not find a significant difference in the frequency of silent ischaemia between patients with two or more CAD risk factors in comparison to patients with fewer than two risk factors. The frequency of abnormal results and of large perfusion defects is higher in retrospective than in prospective studies [11, 26, 28–33].
Many studies that addressed the role of clinical factors in predicting MPS in diabetic patients did not include microalbuminuria [5, 10, 20, 24–26, 29, 32–34], while in other studies patients with microalbuminuria were not distinguished from patients with macroalbuminuria [18, 22, 35]. Nakajima reported significantly higher urine albumin levels in patients with SSS >8 than in patients with SSS <8 [19]. In the Milan study, proteinuria, but not microalbuminuria, predicted MPS findings [22]. Giugliano et al. reported a higher prevalence of microalbuminuria in patients with positive MPS than in patients with negative MPS [35]. However, in that study patients presenting with chest pain and ischaemic ECG changes were also included [35]. Rutter et al. found that in multivariate analysis microalbuminuria was the strongest independent predictor of silent ischaemia [36]. In that study, patients with macroalbuminuria were excluded but ischaemia was assessed by ECG changes on exercise stress testing without MPS [36]. In the current study patients with macroalbuminuria were excluded because we wanted to evaluate whether early kidney dysfunction could be used for identification of patients at higher risk of silent ischaemia.
Recent studies have indicated that insulin resistance, as quantified by the homeostasis model (HOMA-IR), predicts silent ischaemia in prediabetic adults [37, 38]. In patients without obstructive CAD receiving haemodialysis, low HOMA-IR predicted earlier cardiac death [39]. Unfortunately, HOMA-IR could not be quantified in the current study because insulin is not routinely measured in our department in the follow-up of patients with type 2 diabetes. This is acknowledged as a limitation of the study. In our study population, we did not find any significant difference in the prevalence of silent ischaemia between patients treated with insulin and patients treated with metformin or sulphonylureas. Moreover, BMI, which is related to insulin resistance [40], did not differ between patients with and without ischaemia. However, we would expect HOMA-IR to be a more sensitive index for assessing the relationship between insulin resistance and silent ischaemia on MPS.
Emerging evidence indicates that measurements of high-sensitivity C-reactive protein and fibrinogen improve stratification of cardiovascular risk and could be helpful in reducing cardiovascular events in the screened population [41]. In patients with type 2 diabetes, high-sensitivity C-reactive protein, which is closely linked to the HOMA-IR [42], was associated with greater risk of silent ischaemia [43, 44]. However, in the current study, we found no significant differences in levels of high-sensitivity C-reactive protein between patients with and without ischaemia.
According to the traditional, simplified concept of ischaemia pathogenesis, myocardial perfusion defects may be due either to atherosclerotic obstruction of the epicardial coronary arteries or to endothelial dysfunction [20, 45]. Endothelial dysfunction would account for the fact that in 20 – 40 % of diabetic patients myocardial perfusion defects are found in the absence of angiographic obstruction of the coronary arteries [20, 45]. It has been hypothesized that microalbuminuria and cardiovascular disease may be linked by a common pathophysiological process (the Steno hypothesis), i.e. dysfunction of the vascular endothelium [46]. Microalbuminuria in type 2 diabetes is accompanied by impairment of several endothelial functions, including endothelium-dependent vasodilation and nitric oxide synthesis [47]. Thus, it is conceivable that the association that we detected between microalbuminuria and silent ischaemia may be partially attributed to endothelial dysfunction.
Diabetic patients with myocardial ischaemia had shorter survival than nondiabetic patients [48]. Diabetic patients with ischaemia had an annual cardiac hard event rate 2.4 times higher than diabetic patients with normal MPS and the rate of objective evidence of CAD and annual critical events were similar to those found in diabetic patients with angina [26]. A multicentre study showed that patients with type 2 diabetes with positive MPS had shorter cardiac event-free survival than patients with normal MPS for all clinical risk categories. Addition of MPS imaging data to a model based on traditional risk factors and ECG stress testing significantly improved coronary heart disease risk classification [30]. In the current study, SDS >4 predicted cardiac event-free survival, while SDS ≥2 did not. SSS >4 and SRS >4 also predicted cardiac event-free survival, confirming previous results in patients with type 2 diabetes as well as in nondiabetic patients [8, 15, 17, 18, 26, 30, 49].
Previous studies with larger patient samples have found an association between microalbuminuria and cardiovascular disease and mortality [31, 50–57]. For example, individuals with microalbuminuria and type 2 diabetes have a total annual mortality of approximately 8 % and a cardiovascular disease mortality of 4 % [55]. These values are up to four times higher than in patients without microalbuminuria [55]. In the current study, we failed to show a significant association between microalbuminuria and cardiovascular hard events. We also failed to show associations between low-density lipoprotein concentrations and various risk factors and cardiovascular hard events that in other studies have been found to be associated with either myocardial ischaemia or cardiac event-free survival [10, 58–60]. Because of the low number of events and limited follow-up in our small sample, these findings could be attributed to a type II error.
Other limitations of this study need to be acknowledged. No attenuation correction was performed and we cannot exclude the possibility that attenuation artifacts could have led to partial overestimation of the observed perfusion defects. Quantification of endothelial dysfunction was not performed, even though indirect, noninvasive measurement of coronary endothelial dysfunction is feasible [20]. In the absence of such information, the hypothesis that the association between microalbuminuria and perfusion defects is mirrored by endothelial dysfunction remains under evaluation.
In summary, in a small but carefully selected sample of asymptomatic patients with type 2 diabetes screened for CAD by MPS, silent ischaemia was detected in 32 % of patients, but only 12 % of patients had severe ischaemia. Microalbuminuria was the only predictor of silent ischaemia. Assessment of microalbuminuria should be routinely considered among the first risk stratification steps for CAD in patients with type 2 diabetes, even though severe ischaemia on MPS is a major predictor of cardiac event-free survival.
References
Bonow RO, Bohannon N, Hazzard W. Risk stratification in coronary artery disease and special populations. Am J Med. 1996;101:4A17S–22S.
Bax JJ, Inzucchi SE, Bonow RO, Schuijf JD, Freeman MR, Barrett EJ. Cardiac imaging for risk stratification in diabetes. Diabetes Care. 2007;30:1295–304.
Kang X, Berman DS, Lewin H, Miranda R, Erel J, Friedman JD, et al. Comparative ability of myocardial perfusion single-photon emission computed tomography to detect coronary artery disease in patients with and without diabetes mellitus. Am Heart J. 1999;137:949–57.
BARI Investigators. Influence of diabetes on 5-year mortality and morbidity in a randomized trial comparing CABG and PTCA in patients with multivessel disease: the Bypass Angioplasty Revascularization Investigation (BARI). Circulation. 1997;96:1761–9.
Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–61.
Miller TD, Redberg RF, Wackers FJ. Screening asymptomatic diabetic patients for coronary artery disease: why not? J Am Coll Cardiol. 2006;48:761–4.
Bax JJ, Young LH, Frye RL, Bonow RO, Steinberg HO, Barrett EJ. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729–36.
Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–55.
American Diabetes Association. Consensus development conference on the diagnosis of coronary heart disease in people with diabetes: 10–11 February 1998, Miami, Florida. Diabetes Care. 1998;21:1551–9.
Zellweger MJ, Hachamovitch R, Kang X, Hayes SW, Friedman JD, Germano G, et al. Threshold, incidence, and predictors of prognostically high-risk silent ischemia in asymptomatic patients without prior diagnosis of coronary artery disease. J Nucl Cardiol. 2009;16:193–200.
Bax JJ, Bonow RO, Tschope D, Inzucchi SE, Barrett E. The potential of myocardial perfusion scintigraphy for risk stratification of asymptomatic patients with type 2 diabetes. J Am Coll Cardiol. 2006;48:754–60.
Wackers FJ, Chyun DA, Young LH, Heller GV, Iskandrian AE, Davey JA, et al. Resolution of asymptomatic myocardial ischemia in patients with type 2 diabetes in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study. Diabetes Care. 2007;30:2892–8.
Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8:491–8.
Berman DS, Hachamovitch R, Kiat H, Cohen I, Cabico JA, Wang FP, et al. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: a basis for optimal utilization of exercise technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. 1995;26:639–47.
Zellweger MJ, Weinbacher M, Zutter AW, Jeger RV, Mueller-Brand J, Kaiser C, et al. Long-term outcome of patients with silent versus symptomatic ischemia six months after percutaneous coronary intervention and stenting. J Am Coll Cardiol. 2003;42:33–40.
Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93:905–14.
Berman DS, Abidov A, Kang X, Hayes SW, Friedman JD, Sciammarella MG, et al. Prognostic validation of a 17-segment score derived from a 20-segment score for myocardial perfusion SPECT interpretation. J Nucl Cardiol. 2004;11:414–23.
Nakajima K, Kusuoka H, Nishimura S, Yamashina A, Nishimura T. Prognostic value of myocardial perfusion and ventricular function in a Japanese multicenter cohort study (J-ACCESS): the first-year total events and hard events. Ann Nucl Med. 2009;23:373–81.
Nakajima K, Yamasaki Y, Kusuoka H, Izumi T, Kashiwagi A, Kawamori R, et al. Cardiovascular events in Japanese asymptomatic patients with type 2 diabetes: a 1-year interim report of a J-ACCESS 2 investigation using myocardial perfusion imaging. Eur J Nucl Med Mol Imaging. 2009;36:2049–57.
Djaberi R, Roodt J, Schuijf JD, Rabelink TJ, de Koning EJ, Pereira AM, et al. Endothelial dysfunction in diabetic patients with abnormal myocardial perfusion in the absence of epicardial obstructive coronary artery disease. J Nucl Med. 2009;50:1980–6.
Schuijf JD, Shaw LJ, Wijns W, Lamb HJ, Poldermans D, de Roos A, et al. Cardiac imaging in coronary artery disease: differing modalities. Heart. 2005;91:1110–7.
Milan Study on Atherosclerosis and Diabetes (MiSAD) Group. Prevalence of unrecognized silent myocardial ischemia and its association with atherosclerotic risk factors in noninsulin-dependent diabetes mellitus. Am J Cardiol. 1997;79:134–9.
Gazzaruso C, Garzaniti A, Giordanetti S, Falcone C, De Amici E, Geroldi D, et al. Assessment of asymptomatic coronary artery disease in apparently uncomplicated type 2 diabetic patients: a role for lipoprotein(a) and apolipoprotein(a) polymorphism. Diabetes Care. 2002;25:1418–24.
De Lorenzo A, Lima RS, Siqueira-Filho AG, Pantoja MR. Prevalence and prognostic value of perfusion defects detected by stress technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography in asymptomatic patients with diabetes mellitus and no known coronary artery disease. Am J Cardiol. 2002;90:827–32.
Berman DS, Kang X, Hayes SW, Friedman JD, Cohen I, Abidov A, et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared with men. Impact of diabetes mellitus on incremental prognostic value and effect on patient management. J Am Coll Cardiol. 2003;41:1125–33.
Zellweger MJ, Hachamovitch R, Kang X, Hayes SW, Friedman JD, Germano G, et al. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J. 2004;25:543–50.
Rajagopalan N, Miller TD, Hodge DO, Frye RL, Gibbons RJ. Identifying high-risk asymptomatic diabetic patients who are candidates for screening stress single-photon emission computed tomography imaging. J Am Coll Cardiol. 2005;45:43–9.
Moralidis E, Didangelos T, Arsos G, Athyros V, Mikhailidis DP. Myocardial perfusion scintigraphy in asymptomatic diabetic patients: a critical review. Diabetes Metab Res Rev. 2010;26:336–47.
Petretta M, Acampa W, Evangelista L, Daniele S, Ferro A, Cuocolo A. Impact of inducible ischemia by stress SPECT in cardiac risk assessment in diabetic patients: rationale and design of a prospective, multicenter trial. J Nucl Cardiol. 2008;15:100–4.
Acampa W, Petretta M, Evangelista L, Daniele S, Xhoxhi E, De Rimini ML, et al. Myocardial perfusion imaging and risk classification for coronary heart disease in diabetic patients. The IDIS study: a prospective, multicentre trial. Eur J Nucl Med Mol Imaging. 2012;39:387–95.
Vanzetto G, Halimi S, Hammoud T, Fagret D, Benhamou PY, Cordonnier D, et al. Prediction of cardiovascular events in clinically selected high-risk NIDDM patients. Prognostic value of exercise stress test and thallium-201 single-photon emission computed tomography. Diabetes Care. 1999;22:19–26.
Miller TD, Rajagopalan N, Hodge DO, Frye RL, Gibbons RJ. Yield of stress single-photon emission computed tomography in asymptomatic patients with diabetes. Am Heart J. 2004;147:890–6.
Scholte AJ, Schuijf JD, Kharagjitsingh AV, Dibbets-Schneider P, Stokkel MP, van der Wall EE, et al. Prevalence and predictors of an abnormal stress myocardial perfusion study in asymptomatic patients with type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging. 2009;36:567–75.
Nishimura T, Nakajima K, Kusuoka H, Yamashina A, Nishimura S. Prognostic study of risk stratification among Japanese patients with ischemic heart disease using gated myocardial perfusion SPECT: J-ACCESS study. Eur J Nucl Med Mol Imaging. 2008;35:319–28.
Giugliano D, Acampora R, De Rosa N, Quatraro A, De Angelis L, Ceriello A, et al. Coronary artery disease in type-2 diabetes mellitus: a scintigraphic study. Diabete Metab. 1993;19:463–6.
Rutter MK, McComb JM, Brady S, Marshall SM. Silent myocardial ischemia and microalbuminuria in asymptomatic subjects with non-insulin-dependent diabetes mellitus. Am J Cardiol. 1999;83:27–31.
Nasr G, Sliem H. Silent myocardial ischemia in prediabetics in relation to insulin resistance. J Cardiovasc Dis Res. 2010;1:116–21.
Nasr G, Sliem H. Silent ischemia in relation to insulin resistance in normotensive prediabetic adults: early detection by single photon emission computed tomography (SPECT). Int J Cardiovasc Imaging. 2011;27:335–41.
Nishimura M, Tsukamoto K, Tamaki N, Kikuchi K, Iwamoto N, Ono T. Risk stratification for cardiac death in hemodialysis patients without obstructive coronary artery disease. Kidney Int. 2011;79:363–71.
Kawamori R. Insulin resistance seen in non-insulin dependent diabetes mellitus and hypertension. Hypertens Res. 1996;19 Suppl 1:S61–4.
Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20.
Pfutzner A, Schondorf T, Hanefeld M, Forst T. High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: effects of insulin-sensitizing treatment with pioglitazone. J Diabetes Sci Technol. 2010;4:706–16.
Hsieh MC, Tien KJ, Chang SJ, Perng DS, Hsiao JY, Chen YW, et al. High-sensitivity C-reactive protein and silent myocardial ischemia in Chinese with type 2 diabetes mellitus. Metabolism. 2008;57:1533–8.
Lombardi F, Tundo F, Terranova P, Battezzati PM, Ramella M, Bestetti A, et al. Prognostic value of C-reactive protein in patients with stress induced myocardial ischemia. Int J Cardiol. 2005;98:313–7.
Scholte AJ, Schuijf JD, Kharagjitsingh AV, Dibbets-Schneider P, Stokkel MP, Jukema JW, et al. Different manifestations of coronary artery disease by stress SPECT myocardial perfusion imaging, coronary calcium scoring, and multislice CT coronary angiography in asymptomatic patients with type 2 diabetes mellitus. J Nucl Cardiol. 2008;15:503–9.
Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–26.
Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17:2106–11.
Weiner DA, Ryan TJ, Parsons L, Fisher LD, Chaitman BR, Sheffield LT, et al. Significance of silent myocardial ischemia during exercise testing in patients with diabetes mellitus: a report from the Coronary Artery Surgery Study (CASS) Registry. Am J Cardiol. 1991;68:729–34.
Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–43.
Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374:543–50.
Gerstein HC, Mann JF, Pogue J, Dinneen SF, Halle JP, Hoogwerf B, et al. Prevalence and determinants of microalbuminuria in high-risk diabetic and nondiabetic patients in the Heart Outcomes Prevention Evaluation Study. The HOPE Study Investigators. Diabetes Care. 2000;23 Suppl 2:B35–9.
Borch-Johnsen K, Feldt-Rasmussen B, Strandgaard S, Schroll M, Jensen JS. Urinary albumin excretion. An independent predictor of ischemic heart disease. Arterioscler Thromb Vasc Biol. 1999;19:1992–7.
Beijers HJ, Ferreira I, Bravenboer B, Dekker JM, Nijpels G, Heine RJ, et al. Microalbuminuria and cardiovascular autonomic dysfunction are independently associated with cardiovascular mortality: evidence for distinct pathways: the Hoorn Study. Diabetes Care. 2009;32:1698–703.
Rutter MK, Wahid ST, McComb JM, Marshall SM. Significance of silent ischemia and microalbuminuria in predicting coronary events in asymptomatic patients with type 2 diabetes. J Am Coll Cardiol. 2002;40:56–61.
Schmitz A. Microalbuminuria, blood pressure, metabolic control, and renal involvement: longitudinal studies in white non-insulin-dependent diabetic patients. Am J Hypertens. 1997;10:189S–97S.
Rein P, Vonbank A, Saely CH, Beer S, Jankovic V, Boehnel C, et al. Relation of albuminuria to angiographically determined coronary arterial narrowing in patients with and without type 2 diabetes mellitus and stable or suspected coronary artery disease. Am J Cardiol. 2011;107:1144–8.
Deveci OS, Kabakci G, Tulumen E, Okutucu S, Aksoy H, Kaya EB, et al. The relationship between microalbuminuria and the presence and extent of coronary atherosclerosis. Angiology. 2010;61:184–91.
Elhendy A, Tsutsui JM, O'Leary EL, Xie F, McGrain AC, Porter TR. Noninvasive diagnosis of coronary artery disease in patients with diabetes by dobutamine stress real-time myocardial contrast perfusion imaging. Diabetes Care. 2005;28:1662–7.
Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care. 1999;22:1396–400.
Gimelli A, Rossi G, Landi P, Marzullo P, Iervasi G, L'Abbate A, et al. Stress/rest myocardial perfusion abnormalities by gated SPECT: still the best predictor of cardiac events in stable ischemic heart disease. J Nucl Med. 2009;50:546–53.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giovacchini, G., Cappagli, M., Carro, S. et al. Microalbuminuria predicts silent myocardial ischaemia in type 2 diabetes patients. Eur J Nucl Med Mol Imaging 40, 548–557 (2013). https://doi.org/10.1007/s00259-012-2323-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2323-5