Abstract
Purpose
To determine whether stress–rest myocardial perfusion single-photon emission (MPS) computed tomography improves coronary heart disease (CHD) risk classification in diabetic patients.
Methods
In 822 consecutive diabetic patients, risk estimates for a CHD event were categorized as 0% to <3%, 3% to <5%, and ≥5% per year using Cox proportional hazards models. Model 1 used traditional CHD risk factors and electrocardiography (ECG) stress test data and model 2 used these variables plus MPS imaging data. We calculated the net reclassification improvement (NRI) and compared the distribution of risk using model 2 vs. model 1. CHD death, myocardial infarction and unstable angina requiring coronary revascularization were the outcome measures.
Results
During follow-up (58 ± 11 months), 148 events occurred. Model 2 improved risk prediction compared to model 1 (NRI 0.25, 95% confidence interval, CI, 0.15-0.34; p < 0.001). Overall, 301 patients were reclassified to a higher risk category, with an event rate of 28%, and 26 to a lower risk category, with an event rate of 15%. Among patients at 3% to <5% risk, 53% were reclassified at higher risk and 25% at lower risk (NRI 0.42, 95% CI 0.07–0.76; p < 0.05). The cost per NRI was $880.80 for MPS imaging as compared to an outpatient visit with an ECG stress test.
Conclusion
The addition of MPS imaging data to a prediction model based on traditional risk factors and ECG stress test data significantly improved CHD risk classification in patients with diabetes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary heart disease (CHD) is the leading cause of morbidity and mortality in patients with diabetes mellitus [1–4]. The value of stress–rest myocardial perfusion single-photon emission (MPS) computed tomography in the evaluation of diabetic patients has been widely investigated [5–8]. The American Diabetes Association guidelines recommend testing patients with symptoms suspicious of CHD and asymptomatic patients with two or more risk factors [9]. However, the rates of MPS scans indicating a high risk of CHD in patients with two or more risk factors and in those with one risk factor have been reported to be similar [10]. It has also been suggested that diabetic patients with multiple risk factors have an incidence of cardiac events of about 3% per year even in the absence of symptoms [9]. The Impact of inDucible Ischemia by Stress MPS (IDIS) trial [11] was designed to evaluate if, in patients with diabetes, the use of an aggregate score incorporating and weighting multiple risk factors could be superior to an approach based on the number of risk factors to define the patient’s risk. The concept of net reclassification improvement (NRI), as a method for quantifying the enhancement in risk estimation when an additional test is added to a standard risk prediction model, has recently been introduced [12]. This approach is rapidly being accepted for evaluating the clinical utility of risk markers [13, 14]. However, at this time limited data are available on risk reclassification by MPS imaging in patients with suspected or known CHD [15]. The aim of the present study was to evaluate the extent to which adding MPS imaging data to a model based on traditional risk factors and electrocardiography (ECG) stress test data correctly reclassifies the risk of subsequent CHD events during a long-term follow-up in diabetic patients participating in the IDIS trial.
Materials and methods
Study population
The study population included 996 consecutive diabetic patients referred for MPS imaging as part of the IDIS investigation [11]. All patients had a history of type 2 diabetes of at least 5 years. Patients were excluded from study enrolment for: (1) recurrent chest pain unresponsive to anti-ischaemic medications; (2) recent acute coronary syndrome, stroke or transient ischaemic attack (during the previous 3 months); (3) uncompensated congestive heart failure (New York Heart Association class III or IV) or recent admission for congestive heart failure (during the previous 3 months); (4) prior myocardial revascularization procedures; (5) in patients unable to exercise, an absolute contraindication to dipyridamole defined as ongoing wheezing, greater than first-degree atrioventricular block without a pacemaker, systolic blood pressure lower than 90 mmHg or recent (<24 h) use of dipyridamole or xanthines (e.g. aminophylline, caffeine); or (6) a concomitant noncardiac illness that would limit follow-up for at least 1 year. Also excluded were premenopausal women, unless it could be documented that they were not pregnant or lactating, and any patients unable to provide signed informed consent. The ethics committees of the different institutions approved the protocol and all patients provided informed consent.
Risk factors
As part of the baseline examination [11], besides diabetes and its complications (including neuropathy, nephropathy, peripheral vascular disease and retinopathy), clinical teams collected information on traditional cardiovascular risk factors, including age, gender, presenting symptom, history of CHD, blood pressure, smoking history, serum cholesterol, family history of CHD, rest ECG characteristics and the results of ECG stress testing (heart rate, blood pressure, duration, magnitude and slope of ST segment changes and exertional hypotension). From these clinical variables the ratio of the annual expected mortality to the annual non-coronary mortality was calculated for each patient as an aggregate descriptor of the patient’s clinical CHD risk [16].
MPS imaging
All patients underwent same-day sestamibi stress–rest gated MPS imaging under exercise or dipyridamole stress, according to the recommendations of the European Association of Nuclear Medicine and European Society of Cardiology [17] as previously described in detail [11]. For both types of stress, heart rate, blood pressure and 12-lead ECG data were recorded at rest, at the end of each stress stage, at peak stress and in the delay phases at rest. Gated MPS acquisition was performed using a dual head rotating gamma camera equipped with a low-energy high-resolution collimator and connected to a dedicated computer system. No attenuation or scatter correction was used. Relative perfusion distribution was analysed semiquantitatively using standardized segmentation of 17 myocardial segments [18]. An automated software program (Cedars-Sinai Medical Center, Los Angeles, CA) was used to calculate left ventricular ejection fraction (LVEF) and the variables incorporating both the extent and severity of perfusion defects [19]. A scan was considered normal if the summed stress score (SSS) was 3 or lower, mildly to moderately abnormal if the SSS was between 4 and 10, and severely abnormal if the SSS was more than 10, as previously reported [20].
Follow-up
Patients were followed up by way of a questionnaire that was completed by a phone call to all patients and/or general practitioners or cardiologists, and by review of hospital or physicians’ records by individuals blinded to the patients’ test results. The end-point was the first occurrence of a major adverse cardiac event, including cardiac death, nonfatal myocardial infarction and unstable angina requiring coronary revascularization. Patients undergoing revascularization within 60 days of MPS imaging were excluded from the analysis. Cardiac death, defined as due to acute myocardial infarction, ventricular arrhythmias, refractory heart failure or cardiogenic shock, was confirmed by review of the death certificate, hospital chart or physician’s records. Nonfatal myocardial infarction was defined based on the criteria including typical chest pain, elevated cardiac enzyme levels and typical alterations of the ECG. Patients experiencing noncardiac death during follow-up were censored at the time of death. The date of the last examination or consultation was used to the determine follow-up period.
Statistical analyses
Continuous variables are expressed as means±SD and categorical data as percentages. Groups were compared using Student’s t-test, the chi-squared test or Fisher’s exact test, as appropriate. A p value <0.05 was considered statistically significant. Differences in annual event rate were evaluated using a score test for trend using STATA version 11.1 for Windows (Stata Corporation, College Station, TX). The effects of variables on event-free survival were evaluated using the stratified Cox proportional hazards survival model, and the 95% confidence interval (CI) was calculated. Stress type was treated as the stratification variable. The proportional hazard assumption of the Cox model was checked separately for the other covariates using a graphical and analytical method before performing the regression analysis. The proportional hazard assumption was not rejected for any covariate included in the Cox model. The 5-year estimated risk of a CHD event was calculated for each participant using two Cox models. Model 1 considered for analysis the patient’s clinical CHD risk. Model 2 used the patient’s clinical CHD risk plus the MPS imaging data. The annual risk estimates were categorized as 0% to less than 3%, 3% to less than 5%, and 5% or more. Reclassification of a patient’s risk was defined as correct or incorrect according to patient outcome, and the NRI, defined as the difference between correct and incorrect reclassification, was calculated [12].
Assuming independence between events and non-events and following McNemar’s logic for significance testing in correlated proportions, a simple asymptotic test for the null hypothesis of NRI = 0 was used (z test). Survival curves were constructed using the Kaplan-Meier method to account for censored survival times, and were compared with the log-rank test. The incremental prognostic value of prescan risk, LVEF and MPS imaging findings was assessed considering these variables in hierarchical order. Harrell’s C concordance statistic, which is defined as the probability that predictions and outcomes are concordant, was estimated to assess the predictive accuracy of different models [21]. To estimate the cost per NRI, the incremental cost of MPS imaging over clinical evaluation during an outpatient visit with an ECG stress test was calculated as proposed by Shaw et al. [15]. Procedural costs were determined from the 2010 Medicare Physician Fee Schedule for Current Procedural Terminology codes 93015 and 78452 and from the Medicare Outpatient PC Pricer System using APC code 601 for a midlevel clinic visit.
Results
Study cohort and events
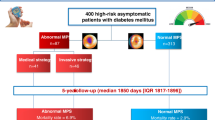
MPS imaging was performed in 996 patients. Follow-up was successful in 952 patients (96%). Of these, 66 were excluded due to early revascularization, leaving 822 patients available for analysis. During the follow-up (58 ± 11 months), 148 major cardiac events occurred (18% cumulative event rate). The events were cardiac death in 31 patients, myocardial infarction in 30 and revascularization for unstable angina in 87. The characteristics of patients with and without events are presented in Table 1. Patients with events had higher clinical CHD risk, lower LVEF and a higher prevalence of an abnormal MPS scan.
Survival analysis
The results of the univariate and multivariate Cox analyses are presented in Table 2. In the multivariate analysis, clinical CHD risk and an abnormal MPS scan were independent predictors of major cardiac events. In the incremental analysis, the addition of perfusion findings significantly improved the prognostic power of the model including clinical CHD risk and LVEF (Fig. 1), increasing the global chi-squared value from 27 to 52 (p < 0.001). The Harrell’s C concordance statistic of the final model was 0.78 (95% CI 0.73 to 0.82). The annual event rate according to the clinical CHD risk and MPS imaging is shown in Fig. 2. As shown, the event rate progressively increased as SSS increased (p for trend <0.001) in all risk categories. Figure 3 shows the event-free survival curves in the three different clinical CHD risk categories according to the MPS imaging results. A separate analysis was performed considering only cardiac death or nonfatal myocardial infarction as end-points (Table 3). Clinical CHD risk, LVEF and an abnormal MPS scan showed a significant association with events in both the univariate and multivariate analysis.
Risk reclassification
A crosstabulation of the estimated risk for major cardiac events using the models with and without MPS imaging data is shown in Table 4. The addition of MPS imaging data resulted in reclassification of 40% of the patients with a NRI of 0.25 (95% CI 0.15–0.34; p < 0.001). Overall, 301 patients were reclassified to a higher risk category, with an event rate of 28%, and 26 patients to a lower risk category, with an event rate of 15%. When MPS imaging data were added to the predictive model, 120 were reclassified as ≥5% risk and 38 (32%) of them experienced events. Conversely, 23 patients were reclassified as 0% to <3% risk, of whom 4 (17%) had events. Considering the 91 patients at 3% to <5% clinical CHD risk, 48 (53%) were reclassified as higher risk, while 23 (25%) were reclassified as lower risk (NRI 0.42, 95% CI 0.07 to 0.76; p < 0.05). Of the 28 events that occurred in patients at 3% to <5% clinical CHD risk, 71% were among those reclassified as higher risk, whereas 14% were among those reclassified as lower risk.
The risk stratification capacity of a MPS-adjusted model is shown in Fig. 4. Including MPS in the model places 45% of the overall population into either 3% to <5% or ≥5% risk categories, compared with 17% with clinical CHD risk alone. Figure 5 shows the event rate for each risk category before and after reclassification. When only clinical CHD risk was considered, there was no correlation between event rate and risk category (p for trend nonsignificant). After reclassification, the event rate progressively increased with worsening of risk category (p for trend <0.001).
Incremental cost effectiveness analysis
For each patient, the incremental cost for a stress-rest MPS study was an added $220.20 when compared to an outpatient visit with an ECG stress test (i.e. $298.83 – $78.63). The cost per NRI for a MPS was $880.80 in the overall study population and $524.28 in patients at 3% to <5% clinical CHD risk (Table 5).
Discussion
The main finding of this prospective multicentre trial was that in diabetic patients referred for MPS imaging, the addition of perfusion data to clinical risk factors resulted in a significant improvement in the classification of risk for the prediction of cardiac events during a long-term follow-up. The patients in the 3% to <5% clinical risk category achieved a substantially higher NRI than the overall cohort, and therefore patients in this category appear to be those who would benefit the most from a strategy that includes MPS data.
CHD risk in diabetic patients
According to the recommendations of the American Heart Association, diabetic patients should be considered as at equivalent risk to CHD patients [22]. However, recent published data do not support this hypothesis [23, 24]. In particular, the findings of the Detection of Ischemia in Asymptomatic Diabetics (DIAD) outcome studies indicate that a substantial proportion of the population can be defined as having intermediate/high baseline cardiovascular risk, but their annual cardiac event rate is low and not altered by routine screening for inducible ischaemia [25, 26]. It has also been demonstrated that patients with diabetes without prior myocardial infarction have a 43% lower risk of developing total cardiovascular events compared to patients without diabetes with previous myocardial infarction [23]. Thus, published data demonstrate that diabetic patients show widely varying cardiovascular risk. In fact, while many patients with diabetes have increased cardiovascular risk compared to the general population, there is a subset that have a very low cardiovascular risk and an large subset that have an extremely high risk [24]. These findings suggest that an individual approach seems necessary in determining CHD risk in diabetic patients.
In the present investigation we used an aggregate descriptor incorporating and weighting multiple risk factors, as well as the results of ECG stress testing, to categorize the patients’ clinical risk. In our study population, the prevalence of patients showing perfusion abnormalities was 50% and the MPS imaging results provided incremental prognostic information over clinical CHD risk and LVEF. The presence of perfusion abnormalities and the extent and severity of these abnormalities were strong predictors of subsequent cardiac events. MPS imaging data provided independent prognostic information even when only cardiac death or nonfatal myocardial infarction was considered as the end-point. These results seem to indicate that many diabetic patients may benefit from risk assessment by MPS imaging and are in agreement with those of previous studies [6–8, 27]. On the other hand, the DIAD study showed a much lower percentage of abnormal MPS images (16%) in a selected population of asymptomatic diabetic patients [25].
Risk reclassification
In clinical prognostic models, risk stratification is important for advising patients and making treatment decisions. The distribution of predicted values for each model separately, or the marginal distribution, may indicate how many patients should be classified into each risk category but not whether this is done correctly [28]. It is also not possible to determine which of the two models is better at classifying individuals, or if individual risk estimates differ between the two models. One way of evaluating this is to examine the joint distribution through risk reclassification as a novel marker of a test’s incremental value. Although net reclassification in risk has been applied in the setting of cardiovascular screening in apparently healthy subjects [29, 30] and in patients with suspected or known CHD [15], previous studies have not utilized this approach for the evaluation of risk in diabetic patients. In the present study, diabetic patients were classified according to pretest clinical variables and reclassified on the basis of MPS findings. The difference between correct and incorrect reclassification according to patients outcome was defined as the net change in reclassifications. Our findings demonstrate that the addition of MPS imaging data resulted in reclassification of 40% of the sample with a NRI of 0.25. A substantial number of patients (37%) were reclassified to a higher risk category and experienced a high event rate (28%). In the subset of patients in the 3% to <5% clinical risk category, the NRI was higher than the overall cohort and of the 28 events that occurred among these patients the majority (71%) were among those reclassified as higher risk. Therefore, patients in this category appear to be those who would benefit the most from a strategy that includes MPS data.
Cost-effectiveness analysis
The cost per NRI for an MPS scan as compared to an outpatient visit with ECG stress test was $880.80 in the overall study population and $524.28 in patients with a CHD risk of 3% to <5%. Although this novel cost-effectiveness methodology has not yet been standardized, this approach may provide evidence for assessing the value or discriminating appropriate utilization of clinical resources. It should be considered that induced costs are important in developing any cost-effectiveness metric. However, employing the calculation of cost per NRI in diverse patient populations and across multiple modalities would help validate this metric and would allow comparison of the cost effectiveness of risk assessment strategies in different study populations [21]. The results of the present study could also be useful to guide resource utilization in a “real-world” population of diabetic patients referred for MPS imaging such as that prospectively enrolled in the IDIS trial. The relative contribution of MPS imaging variables in step-wise modelling reveals that improvement in CHD risk prediction also remains possible in a population at high cardiovascular risk such as diabetic patients.
Study limitations
In our study population about 35% of patients had previous myocardial infarction. However, it should be considered that the aim of this study was to determine whether adding MPS imaging data to a prediction model based on traditional risk factors improves classification of risk in a large prospective registry of consecutive diabetic patients undergoing MPS imaging. Moreover, autopsy studies have shown a prevalence of CHD in decedents with diabetes but without antemortem evidence of CHD ranging from 50% to 75% [31]. Thus, the convenient epidemiological separation between primary and secondary prevention has significant limitations in people with diabetes [32]. Another potential limitation is that in the IDIS trial, the MPS imaging results were revealed to participants and their physicians. This could have affected our results. Patients with a high SSS on MPS imaging may have had more intensive medical treatment, thereby reducing the number of events and decreasing the NRI. It is also possible that with a longer follow-up and additional events, our results could change.
Conclusion
This prospective multicentre study demonstrated that the addition of MPS imaging data to a prediction model based on traditional risk factors and ECG stress test data significantly improves the classification of risk in diabetic patients during a long-term follow-up and places more individuals in higher risk categories. Thus, a strategy that includes MPS data would appear to be useful to refine risk estimation in diabetic subjects referred for MPS imaging.
References
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary artery disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34.
Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes. Diabetes Care. 1999;22:1396–400.
May O, Arildsen H, Damsgaard EM, Mickley H. Prevalence and prediction of silent ischemia in diabetes mellitus: a population-based study. Cardiovasc Res. 1997;34:241–7.
Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–7.
Kang X, Berman DS, Lewin H, Miranda R, Erel J, Friedman JD, et al. Comparative ability of myocardial perfusion single-photon emission computed tomography to detect coronary artery disease in patients with and without diabetes mellitus. Am Heart J. 1999;137:949–57.
Giri S, Shaw LJ, Murthy DR, Travin MI, Miller DD, Hachamovitch R, et al. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation. 2002;105:32–40.
Shaw LJ, Berman DS, Hendel RC, Alazraki N, Krawczynska E, Borges-Neto S, et al. Cardiovascular disease risk stratification with stress single-photon emission computed tomography technetium-99m tetrofosmin imaging in patients with the metabolic syndrome and diabetes mellitus. Am J Cardiol. 2006;97:1538–44.
Miller TD, Rajagopalan N, Hodge DO, Frye RL, Gibbons RJ. Yield of stress single-photon emission computed tomography in asymptomatic patients with diabetes. Am Heart J. 2004;147:890–6.
American Diabetes Association. Consensus development conference on the diagnosis of coronary heart disease in people with diabetes: 10–11 February 1998, Miami, Florida. Diabetes Care. 1998;21:1551–9.
Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–61.
Petretta M, Acampa W, Evangelista L, Daniele S, Ferro A, Cuocolo A. Impact of inducible ischemia by stress SPECT in cardiac risk assessment in diabetic patients: rationale and design of a prospective, multicenter trial. J Nucl Cardiol. 2008;15:100–4.
Pencina MJ, D’Agostino RB, D’Agostino Jr RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72.
Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121:1768–77.
Shaw LJ. The new era of risk reclassification in cardiovascular imaging. J Nucl Cardiol. 2011;18:536–7.
Shaw LJ, Wilson PWF, Hachamovitch R, Hendel RC, Borges-Neto S, Berman DS. Improved near-term coronary artery disease risk classification with gated stress myocardial perfusion SPECT. JACC Cardiovasc Imaging. 2010;3:1139–48.
Diamond GA, Staniloff HM, Forrester JS, Pollok BH, Swan HJ. Computer assisted diagnosis in the noninvasive evaluation of patients with suspected coronary artery disease. J Am Coll Cardiol. 1983;1:444–55.
Hesse B, Tägil K, Cuocolo A, Anagnostopoulos C, Bardiés M, Bax J, et al. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur J Nucl Med Mol Imaging. 2005;32:855–97.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42.
Germano G, Kavanagh PB, Waechter P, Areeda J, Van Kriekinge S, Sharir T, et al. A new algorithm for the quantitation of myocardial perfusion SPECT. I: Technical principles and reproducibility. J Nucl Med. 2000;41:712–9.
Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–43.
Harrell Jr FE. Predicting outcomes: applied survival analysis and logistic regression. Charlottesville, VA: University of Virginia; 2000.
Grundy SM, Pasternak R, Greenland P, Smith Jr S, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–92.
Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. 2009;26:142–8.
Saely CH, Aczel S, Koch L, Schmid F, Marte T, Huber K, et al. Diabetes as a coronary artery disease risk equivalent: before a change of paradigm? Eur J Cardiovasc Prev Rehabil. 2010;17:94–9.
Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–55.
Bansal S, Wackers FJ, Inzucchi SE, Chyun DA, Davey JA, Staib LH, et al. Five-year outcomes in high-risk participants in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study: a post hoc analysis. Diabetes Care. 2011;34:204–9.
Zellweger MJ, Hachamovitch R, Kang X, Hayes SW, Friedman JD, Germano G, et al. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J. 2004;25:543–50.
Cook NR. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem. 2008;54:17–23.
Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–9.
Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;30:1610–6.
Miller TD, Redberg RF, Wackers FJ. Screening asymptomatic diabetic patients for coronary artery disease: why not? J Am Coll Cardiol. 2006;48:761–4.
Winocour PH, Fisher M. Prediction of cardiovascular risk in people with diabetes. Diabet Med. 2003;20:515–27.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wanda Acampa and Mario Petretta contributed equally to this work
Rights and permissions
About this article
Cite this article
Acampa, W., Petretta, M., Evangelista, L. et al. Myocardial perfusion imaging and risk classification for coronary heart disease in diabetic patients. The IDIS study: a prospective, multicentre trial. Eur J Nucl Med Mol Imaging 39, 387–395 (2012). https://doi.org/10.1007/s00259-011-1983-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1983-x